Abstract
Hypercholesterolemia, high serum cholesterol in the form of LDL, is a major risk factor for atherosclerosis. LDL is mostly degraded in the liver after its cellular internalization with the LDL receptor (LDLR). This clathrin-mediated endocytosis depends on the protein autosomal recessive hypercholesterolemia (ARH), which binds the LDLR cytoplasmic tail. Mutations in either the LDLR tail or in ARH lead to hypercholesterolemia and premature atherosclerosis. Despite the significance of this interaction for cholesterol homeostasis, no structure of either ARH or the LDLR tail is available to determine its molecular basis. We report the crystal structure at 1.37-Å resolution of the phosphotyrosine-binding (PTB) domain of ARH in complex with an LDLR tail peptide containing the FxNPxY0 internalization signal. Surprisingly, ARH interacts with a longer portion of the tail than previously recognized, which extends to I-7xF-5xNPxY0QK+2. The LDLR tail assumes a unique “Hook”-like structure with a double β-turn conformation, which is accommodated in distinctive ARH structural determinants (i.e., an extended backbone hydrogen-bonding platform, three hydrophobic helical grooves, and a hydrophobic pocket for Y0). This unique complementarity differs significantly in related PTB proteins and may account for the unique physiological role of these partners in the hepatic uptake of cholesterol LDL. Moreover, the unusual hydrophobic pocket for Y0 explains the distinctive ability of ARH to internalize proteins containing either FxNPxY0 or FxNPxF0 sequences. Biophysical measurements reveal how mutations associated with hypercholesterolemia destabilize ARH and its complex with LDLR and illuminate LDL internalization defects seen in patients.
Keywords: lipoprotein, crystallography, trafficking, lipid metabolism
Excess serum levels of cholesterol, mainly in the form of cholesteryl-ester LDL, promote atherosclerosis and coronary heart disease, currently the leading causes of death in the United States (1). The majority of circulating LDL is degraded in the liver after its cellular uptake by the LDL receptor (LDLR) and clathrin-mediated endocytosis of the LDL–LDLR complex (2).
A number of inheritable genetic lesions that perturb LDL uptake in the liver lead to hypercholesterolemia. Not surprisingly, most of the highly frequent mutations identified in familial hypercholesterolemia (FH) are located in the gene for LDLR, and the resulting loss of function leads to elevation of plasma LDL and to the development of atherosclerosis at an early age (3). Mutations in the ectodomain of LDLR disrupt the binding of LDL to the cell surface, whereas mutations in the cytoplasmic tail of LDLR retain LDL binding but lead to internalization defects by precluding clustering of the receptor in clathrin-coated pits and its subsequent endocytosis (4–6).
Despite the early identification of the tail FxNPxY sequence motif as the internalization signal (7), the mechanism for the interaction of LDLR with the clathrin machinery had been largely unknown. The first significant clue came a decade later from studies of patients with autosomal recessive hypercholesterolemia (ARH), who were described as clinically similar to FH patients but carried no LDLR mutations (8). The genetic defects in ARH patients were mapped to a single gene that codes for a 308-amino acids protein, named ARH, which was required for the function of LDLR in hepatocytes (9).
ARH was found to contain a central phosphotyrosine-binding (PTB) domain (residues 40–180), a module for interaction with the FxNPxY motif on the LDLR tail (9). This domain can also simultaneously interact with cell membrane phosphoinositides (10) while specific sequences within the C-terminal region of ARH bind clathrin and its adaptor AP2 (10, 11). Together, this set of interactions enables ARH to function as an endocytic adaptor for the clathrin-mediated endocytosis of LDLR in the liver. Accordingly, ARH has been classified as a clathrin-associated sorting protein (CLASP) (12), a group of proteins that serve as a molecular bridge between receptors and the clathrin machinery for their endocytic internalization.
Intriguingly in the absence of ARH, disabled-2 (Dab2), another PTB domain protein and a CLASP, was also found to internalize LDLR in certain cell types [e.g., fibroblasts (13, 14)]. However, the physiological importance of this interaction has yet to be understood because, at the level of the whole organism, Dab2 is unable to compensate for LDL uptake in either ARH patients (9) or ARH−/− mice (15). This could be because Dab2 is not sufficiently expressed in liver cells (14), the predominant site of internalization of plasma LDL.
The crystal structures of the PTB domains of Dab1 and Dab2 in complex with NPxY peptides have been reported (16, 17); however, their low sequence identity with the PTB domain of ARH (≈18%) limits their extrapolative value for ARH and its complex with LDLR. Moreover, so far only the ectodomain of LDLR has received structural attention (18–24), and no structural data for the LDLR tail are available to establish the molecular basis for its interaction with ARH and for hypercholesterolemia resulting from internalization defects.
To gain insight into this unique and indispensible role of ARH, we studied the ARH–LDLR molecular interface by solving a high-resolution crystal structure of the PTB domain of ARH in complex with a peptide corresponding to the cytosolic tail of LDLR and by analyzing the effect of naturally occurring mutations on formation of this complex.
We found that the ARH–LDLR complex is structurally distinct from previously reported structures of PTB–NPxY complexes. The unique features revealed for both ARH and LDLR explain the seemingly exclusive pairing of these proteins for cholesterol homeostasis and establish the molecular basis for interactions of ARH with other proteins.
Results
Structure of the PTB Domain of ARH.
The PTB domain (residues 43–174) of rat ARH (rARH) produced in bacteria showed significant propensity to aggregate and did not crystallize. However, mixing it with a synthetic peptide corresponding to the LDLR cytoplasmic tail significantly improved its solubility and permitted crystallization of the complex. To explore the extent of the molecular signal recognized by ARH, the designed synthetic LDLR peptide had four additional residues on both sides of the known internalization FxNPxY0 motif (i.e., the sequence NSINFDNPVY0QKTT) (hereafter, Tyr is chosen as an arbitrary reference position).
The PTB domain of ARH crystallized as a monomer, consistent with its elution by size-exclusion chromatography. The structure, as revealed by the outstanding electron density maps at 1.37-Å resolution (Table 1 and Fig. S1), shows a seven-stranded β-sandwich fold flanked by two α-helices (Fig. 1A), which is similar to the fold of the PTB domains of Dab proteins (16, 17), despite their low sequence similarity with ARH (Fig. S2). Sequence analysis reveals that the PTB domain of ARH is highly conserved; human ARH is 86% identical to zebra fish, 94% to rat, and 100% to chimpanzee ARH, and all of the amino acids in contact with the LDLR peptide are completely invariant (Fig. S3). Therefore, the reported structure of rARH in complex with the conserved LDLR tail is very applicable to all of the above-mentioned species, including human. This extremely high conservation of the ARH–LDLR interface indicates that this interaction did not tolerate sequence variation during evolution, presumably owing to its vital role in LDL internalization.
Table 1.
Data collection and refinement statistics for the structure of ARH–LDLR peptide complex
| Space group | P212121 |
| Cell dimensions | |
| a, b, c (Å) | 29.10, 59.10, 77.08 |
| Resolution (Å) | 46.9–1.37 (1.44–1.37) |
| Rsym | 0.054 (0.360) |
| Mean((I)/σ(I)) | 13.7 (3.4) |
| Completeness (%) | 96.5 (98.9) |
| Redundancy | 4.8 (4.6) |
| No. reflections | 27,539 (4062) |
| Refinement | |
| Resolution (Å) | 46.9–1.37 (1.44–1.37) |
| No. reflections/5.2% free | 26,101/1,437 |
| Rwork/Rfree | 0.152/0.206 |
| No. total atoms | 1,402 |
| No. water | 185 |
| B-factors | |
| Protein | 17.2 |
| Water | 32.7 |
| Estimated overall coordinate error | |
| Based on free-R value | 0.068 Å |
| Based on R value | 0.069 Å |
| Based on maximum likelihood | 0.039 Å |
| rmsd | |
| Bond lengths (Å) | 0.023 |
| Bond angles (°) | 2.05 |
| Rammachandran analysis | 98.5% preferred, 1.5% allowed, 0 outliers |
Values in parentheses are for the highest-resolution shell.
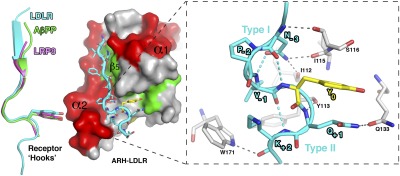
Fig. 1.
The structure of the ARH–LDLR tail complex. (A) 3D structure of the PTB domain of ARH showing β-strands (β1–β7) as yellow and green arrows and α-helices (α1, α2) as red ribbons. The LDLR tail is drawn in cyan and the tyrosine (Y0) of its FxNPxY0 motif in yellow. H-bonds between the NH3-terminal β-strand of LDLR and the β5 strand of ARH are indicated as dashed lines. (B) Surface representation of the PTB structure and the LDLR tail (cyan sticks) emphasizing the FxNPxY residues (yellow). The portion of the tail that interacts specifically with ARH, namely the sequence INFDNPVY0QKT starting from its NH3-terminus on the right, is very well defined in the 2Fo-Fc electron density (gray mesh contoured at 1.6 σ). For orientation the α1 and α2 helices along with the β5 strand and the pocket for Y0 (dashed circle) are labeled.
Crystal structures of PTB domains of Dab proteins explained their simultaneous ability to interact with receptor tail peptides and with the phosphoinositide phosphatidylinositol 4,5-biphosphate (PIP2), which binds away from the peptide binding site and likely facilitates their interaction with membranes (16, 17). Similarly, ARH was shown to bind PIP2 independent of its ability to interact with the LDLR tail (10). We tried to obtain structural data on the complex of ARH with PIP2; however, because of the specific crystal contacts of this structure, neither PIP2 nor its sugar head group could bind ARH. Nevertheless, we identified clusters of lysine residues for potential interaction with phosphate groups, which although they do not exactly correspond to the identified sites on Dab proteins are located away from the LDLR binding site and on the homologous side of the ARH structure (Fig. S4), suggesting that the general mechanism of association and orientation of these domains with respect to the cell membrane is similar.
Unique “Hook”-Like Structure of the LDLR Tail.
The structure shows very clear electron density for the LDLR tail, indicative of its highly ordered binding mode, in a shallow cleft formed along the foothills of the α2 helix in the direction of the β5 strand of ARH (Fig. 1B). Surprisingly, the portion of LDLR that interacts specifically with ARH (i.e., INFDNPVY0QKT) extends well beyond the characterized FxNPxY motif (Fig. 1B) for internalization of LDLR (7).
The N-terminal portion of the LDLR tail (i.e., residues INFDN) assumes a β-strand conformation by forming main-chain hydrogen bonds (H-bonds) with the β5 strand of ARH (Fig. 1A). Immediately downstream of this β-strand, the tail adopts a type I β-turn facilitated by an intra main-chain H-bond between the Asn at position −3 and Y0. Together the β-strand and the type I β-turn form a “Hook”-like structure with the Y0 at its tip (Fig. 2). Interestingly, although this β-turn conformation is supported by multiple H-bonds with ARH, it is at least partially inherent to the NPxY sequence because a similar turn conformation, referred to as a “reverse-turn,” was suggested by NMR studies for the tetra-peptide NPvY in the unbound state (25).
Fig. 2.
LDLR adopts a unique “Hook”-like structure. Left: Structure of the ARH–LDLR peptide complex. The LDLR receptor tail adopts a “Hook” shape formed by an N-terminal β-strand followed by a type I β-turn. This turn is characterized by an intra H-bond between the asparagine (N-3) and the tyrosine (Y0) as seen for other, albeit shorter, NPxY receptor tails overlaid on the LDLR tail on the left (LRP8 in magenta and AβPP in green; PDB codes 1NU2 and 1M7E, respectively). However, a close-up view (Right) shows that the type I β-turn of LDLR is followed by a consecutive type II β-turn, and each turn is supported by multiple intra- (cyan) and inter- (gray) hydrogen bonds, indicated as dashed lines.
Intriguingly, the bound tail assumes an additional consecutive tight turn in the reversed direction (i.e., type II β-turn) from position −1 (Val) to position +2 (Lys), which to our knowledge has not been reported for other NPxY tails. Moreover, the sequence of the second β-turn (VYQK) is not frequent in β-turns (26), and the turn is supported by two specific inter H-bonds with the ARH side-chains of W171 and Q133, which are unique in their positions among other PTB domain structures and likely contribute to the shaping of its final conformation (Fig. 2). Together, these observations suggest that the first (type I) β-turn is inherent to the NPxY sequence but that the overall double β-turn conformation of the LDLR tail is unique to its ARH-bound state.
Specificity of ARH for Tails as Encoded on Its α2 Helix.
The interactions that ARH forms with the LDLR tail fall into two groups: interactions that are not specific for the tail sequence and mediated by backbone H-bonds, such as between the β5 strand of ARH and the N-terminal β-strand of the LDLR peptide, or interactions that play a significant role in the specificity of ARH for receptors, such as those between the α2 helix and the side-chains of LDLR (Fig. 3A).
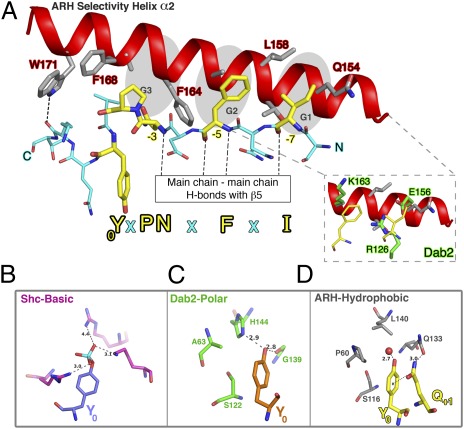
Fig. 3.
Specificity for receptor tails as encoded by ARH. (A) The N-terminal portion of the LDLR peptide (cyan sticks) forms a β-strand conformation by interacting with the β5 strand of ARH, which is located directly underneath the LDLR peptide in this view (Fig. 1) and represented as a dashed rectangle. This interaction is independent of the sequence and mediated by main-chain H-bonds. The extended conformation of the β-strand allows the tail side-chains to alternate from one side of the binding cleft, in which they are mostly solvent exposed (in the direction of the side-chains at positions −4 and −6), to the opposite side, where they make specific interactions with the α2 helix. Thus, residues in positions −7, −5 and both −3 and −2 of the tail dock into the three equally spaced hydrophobic grooves of α2 (G1–G3, gray shaded). The side-chains on the ARH α2 helix (gray sticks) facing the LDLR peptide are either hydrophobic or expose their hydrophobic portions for interaction with the receptor tail. Inset: Contrasting environment (highly polar) of the structurally related PTB domain of Dab2 (green) in the positions corresponding to the ARH hydrophobic grooves for F-5 and I-7 of the LDLR tail. (B–D) Y0 pocket classes among PTB domains. (B) Binding of Tyr-phosphorylated NPxY0 sequences, such as shc (PDB ID 1SHC), that contain a basic pocket. (C) Binding of nonphosphorylated Y0 is mediated by polar pockets, represented here by Dab2 (PDB ID 1M7E). (D) ARH represents a new class of hydrophobic pockets that favors phenylalanine but can also accommodate tyrosine (Y0) through H-bond with a water molecule (red sphere).
These interactions with α2 are primarily hydrophobic, except for one specific H-bond formed between W171 and the backbone carbonyl of K+2 of LDLR that stabilizes its unique C-terminal type II β-turn (Fig. 2). Elegantly, the tail residues I-7, F-5, and N-3P-2 intercalate into three helical grooves equally spaced along the α2 helix and formed by the Q154-L158, L158-F164, and F164-F168 intervals (shaded G1, G2, and G3 areas in Fig. 3A), whereas the noncontacting residues (N-6 and D-4) point to the solvent area. Thus, the sequence pattern recognized by ARH has an alternating nature that extends back to the −7 position (Ι−7xF-5xNPxY0).
These structural features distinguish the PTB domain of ARH from that of Dab2, which has a more polar α2 surface for interaction with receptor tails, and a shorter site for recognition (limited to the F-5 position) because the position in Dab2 corresponding to the G1 groove of ARH is occluded by a salt-bridge (Fig. 3A, Inset).
ARH Has an Unusual Hydrophobic Pocket for Y0 Diverged to Accommodate F0.
The characteristic type I β-turn of bound NPxY0 tails points the Y0 in the opposite direction, away from the α2 helix, where it is accommodated in a pocket (dashed circle, Fig. 1B) whose specificity varies among different PTB domains.
The originally characterized PTB domains (e.g., 1shc or 1irs involved in signaling) were found to be specific for Tyr-phosphorylated NPxY sequences (27, 28). PTB domains of such proteins contain a highly positively charged pocket (Fig. 3B) for interaction with phosphoryl-tyrosines of receptor tails (e.g., EGF or insulin receptors). Other PTB proteins involved in endocytosis of cell surface receptors, such as the CLASP proteins Dab1 and Dab2, favor interaction with nonphosphorylated tyrosines (29) and, accordingly, possess a hydrophilic pocket (Fig. 3C).
In contrast, the CLASP protein ARH has an unusual hydrophobic pocket for Y0 (Fig. 3D). This unique pocket architecture renders ARH suitable for interaction not only with Tyr but also with a Phe at position 0 (NPxF0), as carried by ROMK and cubam, which ARH internalizes (30, 31). In the case of NPxY0, a water molecule is recruited to the ARH pocket to form an H-bond with the hydroxyl group of Y0 of LDLR (Fig. 4D). This water molecule is not directly bound to ARH and thus is expected to be released in the presence of Phe at position 0 and to give rise to an entropic contribution to the binding energy, which would make Phe even more favorable than Tyr at position 0. Additional enhancement of the anchoring of LDLR in the ARH pocket for Y0 may be provided by the consecutive LDLR tail residue (Q+1), which seems to support the Y0 in the pocket via stacking interaction (possibly π-π), and via H-bonding with Q133, which lines this pocket (Fig. 3D). In essence, to secure itself in the hydrophobic F/Y0 pocket of ARH, LDLR uses a “thicker” “Hook” with an additional specific attachment point provided by its Gln residue at position +1. The significance of this position is supported by mutational analysis of the LDLR tail, showing that substitution of Cys or Pro for Q+1 slowed down the internalization of LDL, whereas introduction of a stop codon in place of Q+1 dramatically reduced LDL internalization to the same extent as a stop codon at Y0 (32).
Fig. 4.
Mutations associated with hypercholesterolemia destabilize the ARH–LDLR complex. The PTB domain of rARH was injected at various concentrations (indicated in μM) over a CAP Biacore sensor chip coated with either WT LDLR (A) or the J.D. Y807C mutated LDLR (B) peptide. One representative sensorgram is shown for each interaction (Materials and Methods). The affinity (KD) ± SD values are shown for each interaction. In the case of the mutated LDLR peptide, saturation of binding has not been reached even at 300 μM ARH, beyond which inconsistency arises owing to experimental limitation, and thus the calculated average KD value likely represents an underestimation of the true value. Most of the reduction in affinity was attributable to the kinetic on rate. (C) The sensorgram for T55M rARH shows significantly reduced responses for the same series of concentrations as in A and correspondingly reduced affinity for LDLR. (D) Possible structural implications for the T55M mutation. R72 seems to anchor the α1 helix to the second the β-sheet (green) of ARH by interacting with two consecutive residues on β1b strand, T55 and L56 (gray spheres). The loop between these structural elements (containing P60) lines the pocket for Y0 (Inset). Thus, it is likely that T55M perturbs the proper orientation of the α1 helix, which in turn disturbs the integrity of the pocket for Y0, leading to the observed reduction in the on-rate and affinity.
Hypercholesterolemia-Causing Mutations Destabilize the ARH–LDLR Complex.
The biological significance of the ARH pocket for Y0 is perhaps best demonstrated by the J.D. genetic lesion of a single amino acid substitution at Y0 on the LDLR tail (Y807C) that reduces binding (11) and leads to FH (6).
To address the extent of the destabilization of the ARH–LDLR complex due to the J.D. mutation, the binding of the WT and the Y0(C) mutant LDLR peptides by ARH was studied in real time by surface plasmon resonance (SPR). ARH has a moderate affinity, KD, of ≈4 μM, for the LDLR peptide characterized by fast kinetic rates (Fig. 4A). The affinity measured for the Y807C mutant LDLR was at least two orders of magnitude lower (Fig. 4B).
These data are consistent with site-directed mutagenesis showing a role for aromatic residues at Y0 in promoting the spontaneous reverse-turn conformation of NPxY peptides (25) and in LDL internalization (32). The hydrophobic nature of the ARH pocket for Y0, combined with the potential π-π stacking interaction between Y0 and Q+1 of the LDLR tail in the ARH-bound state (Fig. 3D) may in part explain the requirement for aromatic residues at this position.
In contrast to the numerous missense mutations identified on the LDLR gene in FH patients, most mutations identified thus far in ARH patients are null, resulting in frame-shifted or premature truncation products of nonfunctional ARH. Recently, the mutation (T56M) of ARH has been associated with hypercholesterolemia (33). Thus far, this is the only point mutation identified on the PTB domain of ARH, but no effect on its binding to LDLR could be detected in a pull-down assay (34). We studied the binding of the LDLR peptide by the corresponding rARH mutant (T55M) in comparison with WT rARH by SPR. T55M rARH showed ≈10-fold lower affinity (KD ≈38 μM) for LDLR (Fig. 4C) and a significantly reduced stability, which resulted in poor protein yields.
The location of T55 away from the LDLR binding site suggests that the T55M mutation affects the tail binding allosterically. Fig. 4D shows that T55 is located on the β1b strand of ARH and forms an H-bond with R72 located on the α1 helix. In fact, R72 serves as a bidentate ligand that forms an additional H-bond with the carbonyl oxygen of L56, thereby facilitating the proper orientation of the α1 helix with respect to the second β-sheet of ARH. Interestingly, the connecting loop between the β1b strand and the α1 helix forms part of the pocket for Y0. On this basis, a plausible model can be imagined according to which the loss of the attachment of R72 to the β1b strand due to the T55M mutation disturbs the proper orientation of the α1 helix. This, in turn, perturbs the structural integrity of the pocket for Y0 (Fig. 4D), leading to the observed reduction in association rate.
The putative in vivo loss of ARH function due to the T56M mutation may arise from the loss of stability of T55M rARH or from its decreased affinity for LDLR, or both may contribute additively. Future investigations of the effect on LDL internalization are required to support this hypothesis and to establish this mutation as a primary cause for hypercholesterolemia.
ARH Interacts with Specific LDLR Residues Outside the FxNPxY Motif.
In the crystal, ARH clearly interacts with residues flanking the canonical F-5xNPxY0 internalization motif of LDLR (i.e., positions −7 and +1) (notice that the interaction with K+2 is mediated via its main-chain carbonyl and not the side-chain). To determine the contribution of these newly identified interactions to the binding energy, we performed steady-state binding experiments on ARH and three LDLR mutated peptides (I-7>A, I-7>R, and Q+1>A) in comparison with the WT biotinylated tail (Biotin-PEG8-SI-7NFDNPVY0Q+1KT). If the G1 helical groove has a hydrophobic role as seen in the structure (Fig. 3A), then reducing the hydrophobicity of the LDLR at position −7 by the I-7>A mutation should decrease the binding affinity, and an even stronger effect is expected for the I-7 > R mutation. Similarly, the Q+1>A mutation was designed to learn about the involvement of the side-chain of Q+1, which is seen to interact with the ARH pocket for Y0 and likely with Y0 itself (Fig. 3D).
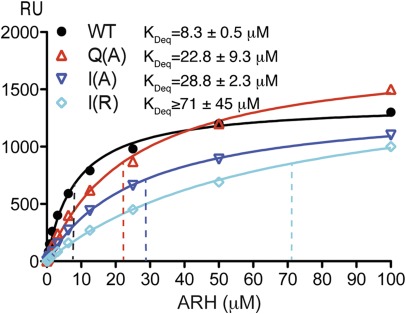
We noticed that the binding affinity of ARH for the WT LDLR, as derived from the steady-state experiment, was slightly lower than that obtained by the kinetic experiment (Fig. 4). However, using the identical experimental setup and protein batch, the measured affinity of ARH for the I-7>A mutated peptide was approximately threefold lower than for WT LDLR peptide, whereas the affinity for I-7>R mutated peptide was almost 10-fold lower (Fig. 5). These significant differences are consistent with a decrease in the hydrophobicity of I-7>A mutated peptide and likely with increased repulsion introduced by the I-7>R mutation, thereby strongly suggesting a hydrophobic role for the G1 helical groove of ARH. The affinity for the Q+1>A peptide was also significantly lower and similarly highlights the involvement of the +1 position of LDLR in binding ARH.
Fig. 5.
Steady-state SPR binding analysis of the interaction of ARH with residues flanking the FxNPxY motif of LDLR. The PTB domain of rARH was injected at various concentrations (indicated in μM) over a CAP Biacore sensor chip coated with either WT LDLR or mutated LDLR peptide. Average equilibrium constant KDeq ± SEM values calculated from two experiments are shown. Representative sensorgrams can be seen in Fig. S5. All mutants show significantly reduced affinity for ARH. For the I-7(R) mutation, saturation of binding has not been reached at 100 μM ARH, thus the calculated KDeq value likely represents an underestimation of the true value.
Taken together, ARH uses its β5 strand as a nonselective platform for backbone H-bonds with the LDLR tail, whereas its α2 helix and the pocket for F/Y0 are used as selectivity modules for interaction with side-chains of receptor tails. The tail side-chains at positions −7, −5, −3, −2, 0, and +1 (Ι−7xF-5xN-3P-2xY0Q+1) fit into four discrete structural determinants; G1, G2, G3, and the pocket for F/Y0 (Fig. 3).
Discussion
The comprehensive work by Brown and Goldstein and their colleagues over the past several decades established that the clearance of plasma cholesterol-LDL occurs primarily through endocytosis via the LDLR, and that mutations disrupting either the binding of LDL by LDLR or the internalization of the complex results in FH. Investigations in the last decade show that the hepatic internalization of LDL, which accounts for uptake of more than 70% of the total LDL, is dependent on the interaction between the endocytic adaptor protein ARH and the LDLR. This interaction is mediated by the PTB domain of ARH and the cytoplasmic tail of the LDLR receptor, and the destabilization of this interface leads to hypercholesterolemia and compromised cardiovascular health as seen in FH and ARH patients.
PTB domains are structural modules for interaction with mostly linear protein segments bound in a shallow surface groove (Fig. 1). Despite their similar fold, they are made by substantially different sequences, which result in significantly distinct surface properties for interactions with many different biological partners. The PTB domain of ARH seems to have an exclusive role in cholesterol homeostasis via interaction with the LDLR tail. Herein we describe this molecular interface structurally. The structure, reported at a remarkably high resolution of 1.37 Å, reveals an extended molecular complementarity between LDLR and ARH, which is distinct from that seen in the related PTB structures of the Dab proteins. These unique features, including a longer pattern of recognition, a double β-turn conformation, a selectivity helix with three hydrophobic helical grooves, and a hydrophobic rather than polar pocket for F/Y0, render ARH more specific for LDLR compared with other PTB proteins.
We demonstrate that residues located outside the canonical FxNPxY0 motif contribute significantly to the binding energy between ARH and the LDLR tail. The interactions with the LDLR side-chains at positions −7, −5, −3, −2, −1, 0, and +1 define the ARH binding determinants for protein interactions (Fig. 3). The imperative positions for binding are occupied by the conserved residues N-3, P-2, and [Y/F]0. These residues allow receptor tails to assume a “Hook”-like structure, which seems to be a precondition for binding ARH and probably for other PTB domains. The LDLR side-chains in the other positions also contribute significantly, but additively, to the binding energy (Fig. 5) as they are accommodated by ARH structural determinants of varying degrees of promiscuity. On the basis of these unique determinants, the 3D specificity of the PTB domain of ARH for proteins can now be formulated as a one-dimensional pattern, Φ−7xΦ−5xNPψ[F/Y]0Ω+1, which can be used to identify new ARH binding partners (Table S1).
Analysis of sequences for which experimental evidence for binding ARH has been reported along with the data presented here suggests that neither of the variable positions (Greek characters) has a dominant role on binding, but they have an incremental contribution to the overall binding affinity. The overall significance of binding is determined by the integration of all of the individual complementarities at each structural determinant. In this manner, binding of less favorable side-chains at a certain ARH structural determinant is compensated for by favorable side-chains bound at the other determinants. This implies that a longer list than that in Table S1 can be obtained using the above formula in a less conservative search. Indeed, a search with more promiscuity at the variable positions is necessary to pick up the cytoplasmic sequences of megalin, the renal potassium channel ROMK, and cubam, all of which are known to interact with ARH (30, 31).
The structural data and tools presented here are fundamental for the understanding of the intimate interaction between ARH and LDLR for the hepatic internalization of LDL, but they are also useful resources for identifying new binding partners for ARH, which may associate it with novel cellular functions and/or help discover additional regulators for LDL internalization.
Materials and Methods
The PTB domain (residues 43–174) of rARH was overexpressed in Esherichia coli, BL21 DE3 strain (Invitrogen), and purified initially by Ni+2 affinity. Thrombin-digested products were further purified on a HiTrap SP column at pH 6.5, followed by size exclusion chromatography on a Superdex-200 column (GE Healthcare). Expression of rARH yielded 5–10 mg/L of 99% pure protein, whereas the PTB domain of rARH yielded 20–30 mg/L, and crystallized only in the presence of the LDLR peptide. Before crystallization the protein was mixed with 5- to 10-fold molar excess of the synthetic LDLR peptide (NSINFDNPVYQKTT) and concentrated to ≈4.7 mg/mL.
Crystallization and Structure Determination.
Crystals grew by the sitting-drop vapor diffusion method by mixing the protein at a 1:1 ratio with 0.1 M Hepes, 30% (wt/vol) PEG 6000 at pH 7.0, as a reservoir, at 4 °C. X-ray data were collected at 100K on beamline 11-1 at the Stanford Synchrotron Radiation Lightsource. The data were indexed and integrated using MOSFLM (35) and merged and scaled using SCALA (36, 37) in space group P212121 (Table 1). The structure was solved by Molecular Replacement using PHASER (38) searching with the PTB structure of Dab1, including its bound peptide [Protein Data Bank (PDB) ID 1NTV]. The MR search identified one PTB domain in the asymmetric unit, yielding an initial R-factor of 59%. Manual model editing using COOT (39) followed by several cycles of rigid-body refinement using REFMAC5.5 (40) at 1.5-Å resolution resulted in an R = 56% and Free-R = 59%. Using phases from this model the ARH chain and the LDLR peptide were completely retraced using ARP/wARP (41) at 1.5-Å resolution, yielding R = 22.5% and Free-R = 27.3%.
After several cycles of restrained refinement with anisotropic temperature factors (46.9–1.37-Å resolution) and manual model editing the R and Free-R factors converged to 15.2% and 20.6%, respectively (Table 1). Structural illustrations were made using PyMol (42). The coordinates and structure factors have been deposited in the PDB (ID 3SO6).
SPR Binding Studies.
Here, a longer rARH PTB construct (residues 29–197) was used. All measurements were done at 25 °C on a Biacore 3000 (GE Healthcare) using, as a running buffer, 20 mM Hepes·HCl (pH 7.4), 500 mM NaCl, and 0.005% Polyoxyethylene 20 sorbitan monolaurate (Tween 20) at 30 μL/min, and the four-channels sensor chip CAP (GE Healthcare). Conjugated streptavidin capture reagent was immobilized to ≈3,000–4,000 response units (RU) onto all four channels. The biotinylated synthetic LDLR peptide [Biotin-PEG8-SINFDNPVYQKT (CPC Scientific)] was captured to ≈20–50 RU, whereas ≈200 RU of the J.D. mutant peptide (Biotin-PEG8-SINFDNPVCQKT) was required for proper detection of ARH binding. For the steady-state analysis of the LDLR mutant peptides, ≈100 RU of WT peptide and ≈200 RU of mutant peptides (Biotin-PEG8-SINFDNPVYAKT, Biotin-PEG8-SANFDNPVYQKT, Biotin-PEG8-SRNFDNPVYQKT) were immobilized on the chip. Two channels were captured with peptides, and two remained peptide free. The free streptavidin sites on all four channels were then blocked to a 1,000–1,500 RU with an unrelated biotinylated protein (mouse CD1d). Specific ARH binding was measured from the difference in RU between peptide-coated channels and peptide-free channels. Binding affinities were calculated from rate constants derived from fitting the data to a 1:1 Langmuir binding model for a series of protein concentrations (J.D. mutant) or steady-state analysis (Q+1 and I-7 mutants) in BIAevaluation v4.1. Average values from two independent experiments are reported.
Supplementary Material
Acknowledgments
We thank Drs. Gordon Gill, Israel Silman, Lynn Hedrick, and Klaus Ley for critical reading of the manuscript; Dr. Steven F. Dowdy for providing the synthetic peptide for the LDLR tail; Dr. Christian Klammt for technical advice with the light scattering; and the Stanford Synchrotron Radiation Lightsource staff for their support during X-ray data collection.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors reported in this paper have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3SO6).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114128109/-/DCSupplemental.
References
- 1.Lloyd-Jones D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JL, Hobbs HH, Brown MS. Familial Hypercholesterolemia. In: Valle D, editor. The Metabolic and Molecular Bases of Inherited Diseases. 8th Ed. Vol II. New York: McGraw-Hill; 2001. pp. 2863–2913. [Google Scholar]
- 4.Brown MS, Goldstein JL. Analysis of a mutant strain of human fibroblasts with a defect in the internalization of receptor-bound low density lipoprotein. Cell. 1976;9:663–674. doi: 10.1016/0092-8674(76)90130-6. [DOI] [PubMed] [Google Scholar]
- 5.Lehrman MA, Goldstein JL, Brown MS, Russell DW, Schneider WJ. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985;41:735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- 6.Davis CG, et al. The J.D. mutation in familial hypercholesterolemia: Amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986;45:15–24. doi: 10.1016/0092-8674(86)90533-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 8.Khachadurian AK, Uthman SM. Experiences with the homozygous cases of familial hypercholesterolemia. A report of 52 patients. Nutr Metab. 1973;15:132–140. doi: 10.1159/000175431. [DOI] [PubMed] [Google Scholar]
- 9.Garcia CK, et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science. 2001;292:1394–1398. doi: 10.1126/science.1060458. [DOI] [PubMed] [Google Scholar]
- 10.Mishra SK, Watkins SC, Traub LM. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc Natl Acad Sci USA. 2002;99:16099–16104. doi: 10.1073/pnas.252630799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He G, et al. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J Biol Chem. 2002;277:44044–44049. doi: 10.1074/jbc.M208539200. [DOI] [PubMed] [Google Scholar]
- 12.Mishra SK, et al. Functional dissection of an AP-2 beta2 appendage-binding sequence within the autosomal recessive hypercholesterolemia protein. J Biol Chem. 2005;280:19270–19280. doi: 10.1074/jbc.M501029200. [DOI] [PubMed] [Google Scholar]
- 13.Maurer ME, Cooper JA. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J Cell Sci. 2006;119:4235–4246. doi: 10.1242/jcs.03217. [DOI] [PubMed] [Google Scholar]
- 14.Keyel PA, et al. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol Biol Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones C, et al. Normal sorting but defective endocytosis of the low density lipoprotein receptor in mice with autosomal recessive hypercholesterolemia. J Biol Chem. 2003;278:29024–29030. doi: 10.1074/jbc.M304855200. [DOI] [PubMed] [Google Scholar]
- 16.Stolt PC, et al. Origins of peptide selectivity and phosphoinositide binding revealed by structures of disabled-1 PTB domain complexes. Structure. 2003;11:569–579. doi: 10.1016/s0969-2126(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 17.Yun M, et al. Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J Biol Chem. 2003;278:36572–36581. doi: 10.1074/jbc.M304384200. [DOI] [PubMed] [Google Scholar]
- 18.Blacklow SC, Kim PS. Protein folding and calcium binding defects arising from familial hypercholesterolemia mutations of the LDL receptor. Nat Struct Biol. 1996;3:758–762. doi: 10.1038/nsb0996-758. [DOI] [PubMed] [Google Scholar]
- 19.Daly NL, Scanlon MJ, Djordjevic JT, Kroon PA, Smith R. Three-dimensional structure of a cysteine-rich repeat from the low-density lipoprotein receptor. Proc Natl Acad Sci USA. 1995;92:6334–6338. doi: 10.1073/pnas.92.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon H, et al. Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol. 2001;8:499–504. doi: 10.1038/88556. [DOI] [PubMed] [Google Scholar]
- 21.Fisher C, Beglova N, Blacklow SC. Structure of an LDLR-RAP complex reveals a general mode for ligand recognition by lipoprotein receptors. Mol Cell. 2006;22:277–283. doi: 10.1016/j.molcel.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Fass D, Blacklow S, Kim PS, Berger JM. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature. 1997;388:691–693. doi: 10.1038/41798. [DOI] [PubMed] [Google Scholar]
- 23.Rudenko G, et al. Structure of the LDL receptor extracellular domain at endosomal pH. Science. 2002;298:2353–2358. doi: 10.1126/science.1078124. [DOI] [PubMed] [Google Scholar]
- 24.Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J. Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci USA. 2008;105:1820–1825. doi: 10.1073/pnas.0712064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal A, Gierasch LM. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991;67:1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson EG, Thornton JM. A revised set of potentials for beta-turn formation in proteins. Protein Sci. 1994;3:2207–2216. doi: 10.1002/pro.5560031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou MM, et al. Structural basis for IL-4 receptor phosphopeptide recognition by the IRS-1 PTB domain. Nat Struct Biol. 1996;3:388–393. doi: 10.1038/nsb0496-388. [DOI] [PubMed] [Google Scholar]
- 28.Zhou MM, et al. Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature. 1995;378:584–592. doi: 10.1038/378584a0. [DOI] [PubMed] [Google Scholar]
- 29.Uhlik MT, et al. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol. 2005;345:1–20. doi: 10.1016/j.jmb.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 30.Fang L, Garuti R, Kim BY, Wade JB, Welling PA. The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest. 2009;119:3278–3289. doi: 10.1172/JCI37950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen GA, Chakraborty S, Steinhauser AL, Traub LM, Madsen M. AMN directs endocytosis of the intrinsic factor-vitamin B(12) receptor cubam by engaging ARH or Dab2. Traffic. 2010;11:706–720. doi: 10.1111/j.1600-0854.2010.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis CG, van Driel IR, Russell DW, Brown MS, Goldstein JL. The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J Biol Chem. 1987;262:4075–4082. [PubMed] [Google Scholar]
- 33.Harada K, et al. A novel Thr56Met mutation of the autosomal recessive hypercholesterolemia gene associated with hypercholesterolemia. J Atheroscler Thromb. 2010;17:131–140. doi: 10.5551/jat.2873. [DOI] [PubMed] [Google Scholar]
- 34.Canizales-Quinteros S, et al. A novel ARH splice site mutation in a Mexican kindred with autosomal recessive hypercholesterolemia. Hum Genet. 2005;116:114–120. doi: 10.1007/s00439-004-1192-9. [DOI] [PubMed] [Google Scholar]
- 35.Leslie AGW. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography. 1992;26 [Google Scholar]
- 36.Evans PR. Scala. Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography. 1997;33:22–24. [Google Scholar]
- 37.CCP4 Collaborative Computational Project, Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 38.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 41.Morris RJ, Perrakis A, Lamzin VS. ARP/wARP and automatic interpretation of protein electron density maps. Methods Enzymol. 2003;374:229–244. doi: 10.1016/S0076-6879(03)74011-7. [DOI] [PubMed] [Google Scholar]
- 42.DeLano WL. The PyMOL Molecular Graphics System. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.