Abstract
Autophagy is a self-degradative process in which cellular material is enclosed within autophagosomes and trafficked to lysosomes for degradation. Autophagosomal biogenesis is well described; however mechanisms controlling the growth and ultimate size of autophagosomes are unclear. Here we demonstrate that the Drosophila membrane protein Ema is required for the growth of autophagosomes. In an ema mutant, autophagosomes form in response to starvation and developmental cues, and these autophagosomes can mature into autolysosomes; however the autophagosomes are very small, and autophagy is impaired. In fat body cells, Ema localizes to the Golgi complex and is recruited to the membrane of autophagosomes in response to starvation. The Drosophila Golgi protein Lva also is recruited to the periphery of autophagosomes in response to starvation, and this recruitment requires ema. Therefore, we propose that Golgi is a membrane source for autophagosomal growth and that Ema facilitates this process. Clec16A, the human ortholog of Ema, is a candidate autoimmune susceptibility locus. Expression of Clec16A can rescue the autophagosome size defect in the ema mutant, suggesting that regulation of autophagosome morphogenesis may be a fundamental function of this gene family.
Autophagy is an essential cellular process of self-digestion in which cytosolic components are engulfed in double-membrane autophagosomes and digested by lysosomal acidic hydrolases (1). Autophagy occurs at a basal level at rest but can be induced during development or in response to cell stressors including starvation, growth factor deprivation, protein aggregation, and pathogen invasion. Autophagy serves a variety of physiological functions that are context dependent; for example, autophagy can promote cell survival in yeast and mammalian cells during nutrient limitation or induce growth arrest and a nonapoptotic form of cell death in human tumors and in the Drosophila salivary gland during pupariation (2). In addition to its physiological role, both normal and aberrant autophagy likely contributes to a variety of pathologic conditions including cancer, neurodegeneration, and infectious disease (3, 4).
Many autophagy-related genes (ATGs) were identified in yeast genetic studies and subsequently were shown to play conserved roles in the formation, maturation, and fusion of autophagosomes (1). However, molecular details of autophagosome biogenesis remain to be defined fully. In particular, it is unclear how the size of autophagosomes is regulated during autophagy. Autophagosomes are assembled at the single phagophore assembly site (PAS) in yeast but emerge as isolation membranes at multiple locations in higher eukaryotic cells (5) and may originate from endoplasmic reticulum (ER), mitochondria, and/or plasma membrane (6–9). In 1965, the Golgi complex was suggested as a possible membrane source for the double-membrane organelles observed in developing invertebrate fat body cells (10). The origin of autophagosomal membranes remains unclear, in part because so few integral membrane proteins localize to autophagosomes, making it difficult to trace their biogenesis. Nucleated autophagosomes (phagophores or isolation membranes) may expand by addition of membranes that could be de novo synthesized or delivered from a preexisting organelle via vesicular membrane traffic. The small ubiquitin-like molecule Atg8 that exhibits fusogenic activity in vitro is a candidate to mediate such membrane expansion in yeast (11–13). However, an analogous process in higher eukaryotes is less well understood, and its molecular mechanism remains to be defined.
We previously demonstrated that ema (endosomal maturation defective) promotes endosomal maturation and showed that it is a functional ortholog of human CLEC16A, a candidate susceptibility factor for autoimmune disorders (14). Using the Drosophila fat body, we investigated whether ema functions in autophagy. We hypothesized that ema might promote autophagosomal maturation because Ema is required for endosomal maturation. Unexpectedly, we find in the ema mutant that autophagosomes form and mature into autolysosomes; however, autophagic structures are dramatically smaller than in wild type because of a defect in their growth. In addition, autophagy is functionally impaired. Ema localizes to the Golgi complex and upon starvation is recruited to the autophagosomal membrane. Interestingly, autophagy also recruits the Golgi protein Lava lamp (Lva) to autophagosomes, and this process requires ema. Thus, we propose a model in which Ema mediates the recruitment of Golgi vesicles to autophagosomes and that these Golgi vesicles participate in a process of autophagosomal growth that is distinct from their initial formation and subsequent maturation.
Results
Ema Promotes the Formation of Large Autophagosomes.
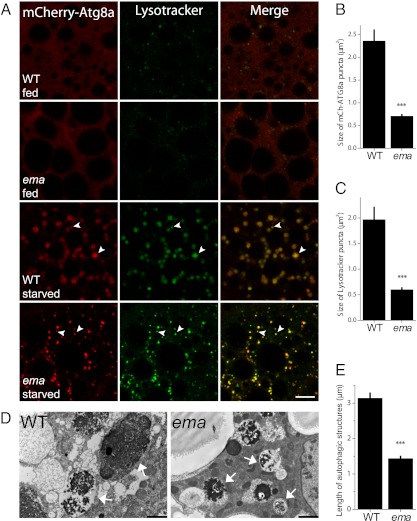
Drosophila fat body cells undergo extensive autophagy that is developmentally programmed and readily inducible under metabolic stresses (15). To test whether ema is required for autophagy, we expressed the fluorescent autophagosomal marker mCherry-Atg8a in fat body cells and labeled these cells live with the fluorescent pH-sensitive dye LysoTracker (DND-26) (16). In both wild-type and ema-mutant third-instar fat body cells under fed conditions, mCherry-Atg8a is distributed diffusely throughout the cell, and occasional LysoTracker-positive acidic puncta are observed (Fig. 1A and Fig. S1) (16). Upon starvation, numerous LysoTracker- and Atg8a-positive autophagosomes are generated in both genotypes (arrowheads in Fig. 1A and Fig. S1). However, in the mutant the autophagosomes are dramatically smaller, with an approximately threefold decrease in area; the size of mCherry-Atg8a puncta is 2.36 ± 0.24 μm2 in wild-type cells vs. 0.71 ± 0.03 μm2 in ema-mutant cells (P < 0.00001; t test), and the size of LysoTracker puncta is 1.97 ± 0.24 in wild-type cells vs. 0.60 ± 0.03 μm2 in ema-mutant cells (P < 0.0003; t test) (Fig. 1 B and C). The presence of acidic autophagosomes in the mutant indicates that autophagosomes can form and mature in the mutant, but their small size indicates that ema is required for their normal development into large autophagosomes.
Fig. 1.
Ema is required for formation of normal autophagosomes. (A) ema-mutant autophagosomes are smaller than wild type but can mature into acidic autolysosomes. Shown are representative single confocal sections of Drosophila fat body cells expressing the autophagosomal marker mCherry-Atg8a (red) and stained with LysoTracker DND-26 (green) from early third-instar larvae fed on yeast extract-enriched fly food or starved in EBSS buffer for 6 h. Arrowheads indicate LysotTracker-positive autophagosomes. Wild type = CG-Gal4/+,UAS-mCherry-Atg8a/+; ema = CG-Gal4/+,UAS-mCherry-Atg8a/+,ema1. (Scale bar, 10 μm.) (B and C) Quantification of the size of autophagic structures in A: mCherry-Atg8a autophagosomes (B) and LysoTracker-positive autolysosomes (C). (D and E) Ultrastructural analysis confirms the presence of small autophagosomes in the ema mutant. (D) Representative electron micrographs of starved Drosophila fat body cells. Autophagic structures (arrows) contain remnants of rough ER and mitochondria. (Scale bars, 2 μm.) (E) Quantification of the length of autophagosomes in D. n = 219 autophagic structures from eight sections from wild type; n = 326 from 12 sections from ema mutant. Data in B, C, and E represent mean ± SE; ***P < 0.001 (t test).
To examine ultrastructural aspects of autophagy, we performed electron microscopic analysis of the fat body. Both wild-type and ema-mutant fat body cells contain numerous lipid droplets, glycogen deposits, membrane tubules, and cellular organelles including mitochondria and ER (Fig. 1D and Fig. S2A). Consistent with the low basal level of LysoTracker-positive acidic compartments (Fig. 1A), very few vesicular profiles that could be endosomes and lysosomes are detected in either wild-type or ema-mutant cells under fed conditions (Fig. S2A). On the other hand, in wild-type fat body cells starvation induces extensive vesicular and vacuolar autophagic structures, many of which contain remnants of mitochondria, ER, and glycogen particles (arrows in Fig. 1D). Autophagosomes are also present in the starved ema-mutant fat body cells, but they are much smaller than those in wild-type cells. To quantify this difference, we measured the length of autophagic structures along their longest axes. Histogram and cumulative probability plots indicate that autophagic structures rarely reach 5 μm in length in the ema mutant but often exceed 10 μm in wild type (Fig. S2 B and C). On average, autophagic structures are approximately two times shorter in the ema mutant than in wild type (Fig. 1E). Such a change in length suggests an approximately eightfold decrease in autophagosome volume. As autophagosomes and autolysosomes mature, they become progressively electron dense (17), and such electron-dense structures are apparent in both wild-type and ema-mutant fat body cells. Hence, this ultrastructural analysis confirms and extends the light microscopic analysis: In the absence of ema, autophagosomes form and mature but are abnormally small.
ema Acts Cell Autonomously for Autophagy.
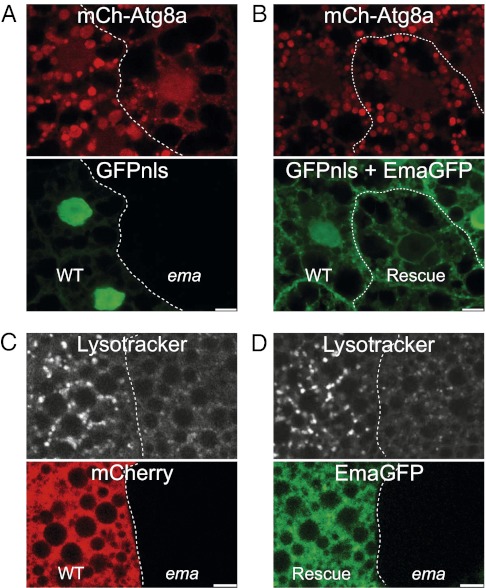
To investigate whether ema function is required cell autonomously, we performed mosaic analyses in the ema mutant. Postmitotic clones of cells in mosaic fat bodies of ema were generated via heat shock at 6 h after egg laying (18). All fat body cells express the autophagosomal marker mCherry-Atg8a, and only wild-type, but not ema-mutant, fat body cells express a nuclear GFP protein, GFPnls, as a clonal marker (Fig. 2A). Under fed conditions, neighboring wild-type and ema-mutant clones display diffuse mCherry-Atg8a (Fig. 2A). Under starvation conditions, autophagosomes in ema-mutant clones (GFPnls-negative) are approximately three times smaller than those in neighboring wild-type clones (GFPnls-positive); the mean size of mCherry-Atg8a autophagosomes is 4.4 ± 0.7 μm2 in wild-type clones vs. 1.5 ± 0.3 μm2 in ema-mutant clones (P < 0.006; paired t test) (Fig. 2A). To test whether the ema mutation causes this phenotype, we performed a rescue experiment by expressing a GFP-tagged Ema transgene (Ema-GFP). Expression of Ema-GFP in the wild-type fat body cell clone (GFPnls-positive) does not affect the formation of starvation-induced autophagosomes. However, its expression in the ema-mutant fat body cells (GFPnls-negative) rescues the mutant phenotype, allowing the formation of normally sized autophagosomes (the mean size of mCherry-Atg8a autophagosomes is 4.2 ± 0.6 μm2 in wild-type clones vs. 4.2 ± 0.7 μm2 in rescue clones; P > 0.9; paired t test) (Fig. 2B). We performed a parallel series of mosaic experiments using LysoTracker rather than mCherry-Atg8 to label autophagic structures and found very similar results. Autophagic lysosomes (autolysosomes) in ema-mutant fat body cell clones are smaller than those in wild type; the mean size of LysoTracker puncta is 2.8 ± 0.6 μm2 in wild-type clones vs. 0.8 ± 0.2 μm2 in ema-mutant clones (P < 0.007; paired t test) (Fig. 2C). The expression of the Ema transgene rescues autolysosome size in the mutant (mean size of LysoTracker puncta is 2.4 ± 0.2 μm2 in rescue clones vs. 0.5 ± 0.1 μm2 in ema-mutant clones; P < 0.0002; paired t test) (Fig. 2D). Thus, mosaic analyses and genetic rescue demonstrate that ema is cell autonomously required for the formation of normally sized autophagosomes and autolysosomes.
Fig. 2.
Ema acts cell autonomously for autophagy. (A and B) Cell-autonomous function of ema for normal autophagosome formation. (A) Representative single confocal section of starved mosaic fat body cell clones expressing the autophagosomal marker mCherry-Atg8a (red). Expression of nuclear GFP (GFPnls, green) marks wild-type clones. Genotype of the animal: hs-flp/+;CG-Gal4/+;UAS-mCherry-Atg8a, FRT82B,UAS-GFPnls/FRT82B,ema1. (B) Representative single confocal section of starved mosaic fat body cell clones expressing the autophagosomal marker mCherry-Atg8a (red) and Ema-GFP (green). Note expression of GFPnls (green) in the wild-type clone but not in the ema-mutant clone. Genotype of the animal: hs-flp/+;CG-Gal4/+;UAS-Ema-GFP/+;UAS-mCherry-Atg8a, FRT82B,UAS-GFPnls/FRT82B,ema1. (C and D) Cell-autonomous function of ema for normal autolysosome formation. (C) Representative live single confocal section of starved mosaic fat body cell clones stained with LysoTracker DND-26. mCherry expression marks wild-type clones (red). Genotype of the animal: hs-flp/+;r4-Gal4,FRT82B,UAS-mCherry/FRT82B,ema1. (D) Representative live single confocal sections of starved mosaic fat body cell clones stained with LysoTracker DND-99. Ema-GFP (green) marks rescue clone. Genotype of the animal: da-Gal4,UAS-Ema-GFP, ema1. (Scale bars, 10 μm.)
Autophagosome Fusion with Endosomes and Lysosomes Does Not Require ema.
Why might autophagosomes be small in the absence of ema? We previously demonstrated that ema is required for endosomal maturation, and thus the abnormal autophagosome development could be caused by a defect in the fusion of autophagosomes with endosomes and/or lysosomes. Defects in endolysosomal trafficking can cause ectopic accumulation of autophagosomes in fat body cells even under fed conditions as autophagosomes originating from constitutive basal autophagy fail to mature (19). However, unlike mutants in the endosomal and lysosomal pathway, ema-mutant fat body cells do not exhibit any ectopic autophagosomal structures (Fig. 1A). This observation indicates that lysosomes in the ema-mutant fat body cells can cope with basal autophagy. Endosomal maturation also is essential for autophagosomal maturation (20). However, upon starvation, mature autophagosomes form in the absence of ema. Mutant fat body cells have abundant LysoTracker-positive mCherry-Atg8a autophagosomes (arrowheads in Fig. 1A and Fig. S1), and there is no difference between mutant and wild-type cells in the proportion of LysoTracker-positive autophagosomes or in the time course of their formation (Fig. 1A and Fig. S1).
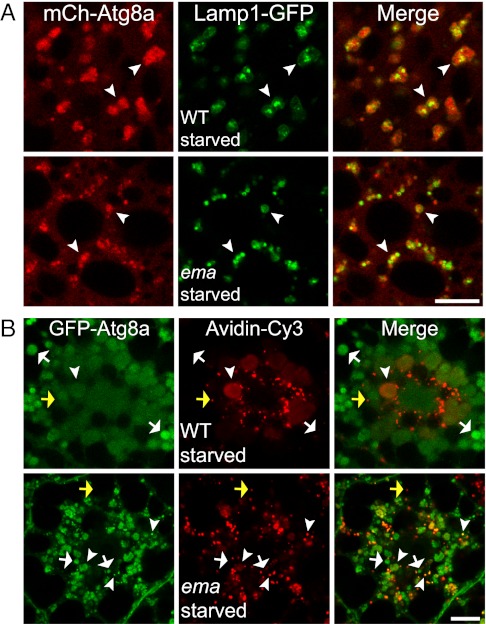
To test directly whether fusion between autophagosomes and lysosomes occurs in ema-mutant fat body cells, we expressed both the lysosomal protein Lamp1-GFP and the autophagosomal marker mCherry-Atg8a. Under fed conditions, small Lamp1-GFP speckles are distributed throughout the cell with concentrations at the perinuclear region in both wild-type and ema-mutant fat body cells (Fig. S3). In starved wild-type fat body cells, the large mCherry-Atg8a autophagosomes colocalize with Lamp1-GFP lysosomes (Fig. 3A). Similarly, the majority of Lamp1-GFP lysosomes colocalize with the small mCherry-Atg8a autophagosomes in starved ema-mutant fat body cells. Thus, autophagosomes in the ema mutant can fuse with lysosomes despite their small size. Because endosomes also fuse with autophagosomes and may contribute to their growth, we next tested fusion between endosomes and autophagosomes by labeling the active endosomal compartment with the endocytic tracer avidin-Cy3. In starved wild-type fat body cells, avidin-Cy3–labeled endosomal compartments are concentrated mainly at the perinuclear regions, whereas GFP-Atg8a autophagosomes are distributed throughout the cytosol (Fig. 3B). Notably, autophagosomes at the periphery do not contain the endocytic tracer avidin-cy3 (white arrows in Fig. 2B), indicating that endosomal fusion is not required for the formation of autophagosomes. In the starved ema-mutant fat body cell, colocalization between the autophagosomal marker GFP-Atg8a and the endosomal tracer avidin-Cy3 also is observed predominantly at the perinuclear region (arrowheads in Fig. 3B). Hence, fusion between autophagosomes and endosomes occurs in the ema mutant. In addition, endosomes that are not associated with autophagosomes are comparable in size in wild type and the ema mutant (yellow arrows in Fig. 3B). It also is noteworthy that, despite successful fusion between autophagosomes and endosomes, autophagosomes are small in the ema-mutant fat body cells (arrowheads in Fig. 3B). Interestingly, there is a trend toward a higher fraction of autophagosomes being labeled with the endocytic tracer in the ema mutant, but this trend is not statistically significant; the fraction of autophagosomes containing the endocytic tracer is 43 ± 7% in wild type vs. 61 ± 9% in ema mutant (P = 0.152; t test). Hence, ema is not required for autophagosomal fusion with endosomes. Taken together, these findings demonstrate that the small autophagosomes in the ema-mutant fat body cells do not result from a lack of fusion of autophagosomes with endosomes and/or lysosomes. Hence, in the ema-mutant autophagosomes can mature via fusion with endosomes and lysosomes and become acidified; however, other aspects of maturation could be abnormal in these small autophagosomes.
Fig. 3.
ema is dispensable for autophagosomal fusion with endosomes and lysosomes. (A) Autophagosomal fusion with lysosomes. Representative single confocal sections show part of a starved Drosophila fat body cell that expresses both the lysosomal marker Lamp1-GFP (green) and the autophagosomal marker mCherry-Atg8a (red). Arrowheads indicate autolysosomes (fusion products of autophagosome and lysosome) that contain both Lamp1-GFP and mCherry-Atg8a. Wild type = CG-Gal4/+,UAS-Lamp1-GFP/+,UAS-mCherry-Atg8a/+; Ema = CG-Gal4/+,UAS-Lamp1-GFP/+,UAS-mCherry-Atg8a/+, ema1. (B) Autophagosomal fusion with endosomes. Representative single confocal sections of fat body cells expressing the autophagosomal marker mCherry-Atg8a (green) and labeled with the endocytic tracer avidin-Cy3 (red). Arrowheads indicate an amphisome (fusion product of autophagosome and endosome) that contains both GFP-Atg8a and avidin-Cy3. White arrows indicate autophagosomes that have not fused with endosomes. Yellow arrows indicate an endosome that has not fused with autophagosomes. Wild type = da-Gal4/+,UAS-GFP-Atg8a/+; Ema = da-Gal4/+,UAS-GFP-Atg8a/+,ema1.
ema Impairs Autophagosomal Growth.
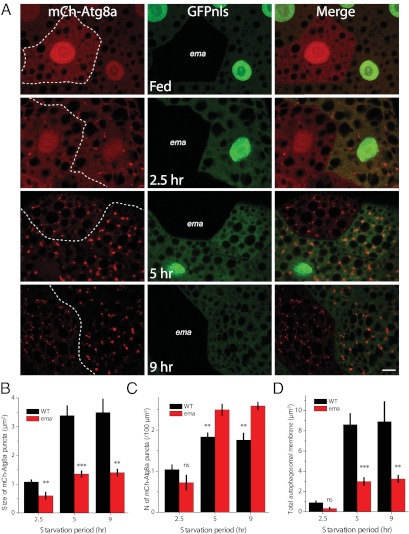
Because autophagosomal maturation and fusion with endosomes and lysosomes occurs in the ema-mutant fat body cells, we hypothesized that the small autophagosomes could arise from either improper growth or fragmentation of autophagosomes. To distinguish between these possibilities, we analyzed autophagosomes in the mosaic ema clones after starvation periods of different durations. To minimize any interference with developmentally programmed autophagy during the third-instar larval stage, we analyzed second-instar larvae. As previously reported, second-instar larvae do not display any trace of developmentally programmed autophagy under fed conditions (Fig. 4A). After 2.5 h of starvation, a similar number of mCherry-Atg8a autophagosomes emerge in wild-type and ema-mutant clones (Fig. 4 A and C). Notably, autophagosomes in wild-type clones already are twofold larger (1.1 μm2) than those in ema (0.6 μm2) (Fig. 4B), suggesting that the ema mutation affects an early stage of autophagy. Following 5 h of starvation, autophagosomes grow by an additional threefold in wild-type clones (to 3.4 μm2) but only by twofold in the ema mutant (to 1.4 μm2). At this point, there is an ∼2.5-fold difference in the size of autophagosomes in wild type and ema mutants. There is a small increase in the number of autophagosomes in ema-mutant fat body cells compared with wild-type cells (the mean number of autophagosomes per 100-μm2 cell area is 1.8 ± 0.1 in wild type vs. 2.5 ± 0.1 in ema mutant) (Fig. 4C). An additional 4 h of starvation does not affect either the size or the number of autophagosomes, suggesting that a maximal steady-state level of starvation-induced autophagy has been achieved. This small increase in the number of autophagosomes does not compensate for the decrease in size: The total autophagosomal membrane [total volume = (average volume) × (the number of autophagosomes)] is decreased significantly in the ema-mutant fat cells compared with wild-type cells at late starvation periods (Fig. 4D). This analysis of autophagosomal development indicates that the ema mutation mainly impairs autophagosome size and that this defect is apparent in the early stages of autophagy. These data are consistent with the hypothesis that ema is required for the growth of autophagosomes.
Fig. 4.
Ema promotes autophagosomal growth. Small autophagosomes in the ema mutant result from defective autophagosomal growth. (A) Representative single confocal sections of mosaic fat body cell clones expressing the autophagosomal marker mCherry-Atg8a (red) at the indicated starvation periods. Expression of GFPnls (green) marks wild-type clones. Genotype of the animal: hs-flp/+;CG-Gal4/+;UAS-mCherry-Atg8a, FRT82B,UAS-GFPnls/FRT82B,ema1. (Scale bar, 10 μm.) (B and C) Quantification of the size (B) and number (C) of autophagosomes. (D) Total membrane (spherical volume; 4/3Πr3) of autophagosomes in A (n = 7 animals at 2.5 h, 8 animals at 5 h, and 5 animals at 9 h). Data represent mean ± SE (**P < 0.01; ***P < 0.001, ANOVA). ns, not significant.
Ema Protein Localizes to Autophagosomes.
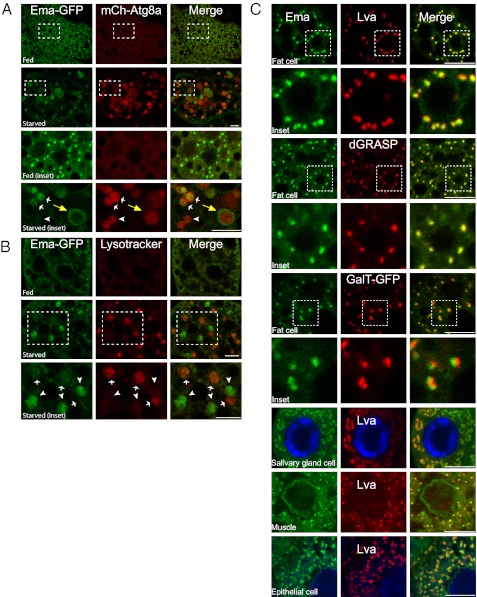
To explore how ema could promote autophagosomal growth, we first tested whether Ema protein localizes to autophagic structures. Ema-GFP, which rescues all known ema phenotypes (Fig. 2 B and D) (14), labels small punctate structures that distribute throughout the cytosol in fat body cells under fed conditions (Fig. 5A). When autophagy is induced by starvation, Ema-GFP becomes localized to a subset of the mCherry-Atg8a autophagosomes (white and yellow arrows in Fig. 5A, Inset) and is absent from others (arrowhead in Fig. 5A, Inset). Once autophagosomes form completely by the fusion of both tips of the isolation membranes, Atg8 remains on their inner membrane surface but dissociates from the outer membrane surface (21). Interestingly, Ema-GFP often is present at the periphery of autophagosomes and does not overlap with mCherry-Atg8a fluorescence (yellow arrow in Fig. 5A, Inset), suggesting that Ema localizes to the outer membrane of autophagosomes. Autophagic substrates are sequestered into the lumen of autophagosomes; hence, the membrane localization of Ema-GFP on autophagosomes likely reflects a functional role for Ema rather than its recruitment as a substrate for degradation.
Fig. 5.
Ema localizes to the Golgi complex and moves to autophagosomes under starvation conditions. (A and B) Localization of Ema protein to autophagosomes during autophagy. (A) Representative single confocal sections of fed and starved fat body cells expressing Ema-GFP (green) and mCherry-Atg8a (red). Insets show autophagosomes with (white arrows) or without (arrowhead) Ema-GFP. Note that Ema-GFP localizes at the perimeter of autophagosomes (yellow arrows). (B) Representative single confocal sections of fed and starved fat body cells expressing Ema-GFP (green) and stained with LysoTracker DND-99 (red). Insets show the lack of colocalization of Ema-GFP (arrowheads) and LysoTracker structures (arrows). (C) Ema protein localizes to the Golgi complex. Representative single confocal sections of fat body cells, salivary gland cells, muscles, and epithelial cells expressing Ema protein tagged with either GFP (green) or mCherry(red) and labeled for the Golgi proteins Lva, dGRASP-GFP, or GalT-GFP. (Scale bars, 10 μm.)
mCherry-Atg8a marks both immature autophagosomes and mature autolysosomes. The finding that Ema-GFP localizes to a subset, but not to all, mCherry-Atg8a structures (Fig. 5A, Inset) suggests that Ema localizes preferentially to either autophagosomes or autolysosomes. To investigate this issue, we performed LysoTracker staining on the fat body cells expressing Ema-GFP. In a fed fat body cell, LysoTracker staining is diffuse, and small Ema-GFP structures distribute throughout the cytosol (Fig. 5B). Upon starvation, we rarely detect overlapping of LysoTracker-positive autolysosomes (arrows in Fig. 5B) and large Ema-GFP autophagic structures (arrowheads in Fig. 5B). Therefore, the large Ema-GFP structures are largely immature autophagosomes rather than mature autolysosomes. Similar results were obtained with mCherry-tagged Ema that is resistant to low pH (Fig. S4E); thus the loss of Ema from autolysosomes is not caused by quenching of GFP but rather reflects the absence of Ema protein. Such restricted localization of Ema to early autophagosomes is consistent with the early defect in autophagosome growth observed in the ema mutant and suggests that Ema may function in an early step of autophagy.
Steady-State Localization of Ema to the Golgi Complex.
Identifying the membrane sources for autophagosome formation is challenging because of the scarcity of transmembrane proteins that both localize to autophagosomes and are required for autophagy. We previously showed that Ema is likely a transmembrane protein: Ema contains a stretch of 23 hydrophobic amino acids predicted to form a transmembrane domain; Ema is strongly enriched in the membrane fraction; Ema is not extracted from the membrane with high salt; and Ema is extracted from the membrane with detergents (14). Therefore, the localization of Ema protein in the autophagosomal membrane could provide a crucial clue as to the membrane source for autophagosome growth. To define the identity of the membrane compartment in which Ema localizes before it is recruited to autophagosomes, we performed colocalization experiments with a battery of organelle markers ranging from ER to lysosomes. Our previous study demonstrated that Ema colocalizes with endosomal markers in the highly endocytic garland cells (14); in fat body cells, however, only a small fraction of Ema-GFP localizes to the endocytic compartments. Ema-GFP puncta are spread throughout these huge cells, whereas the endocytic compartments labeled by the endocytic tracer avidin-cy3, Rab5, Rab7, or Lamp1-GFP are enriched around the nucleus. Near the nucleus, there is some colocalization of Ema-GFP with these endosomal markers (Fig. S4). Although Ema can localize with endocytic structures in fat body cells, these endocytic structures do not account for the vast majority of Ema-GFP puncta. Instead, the Ema-GFP structures throughout the fed fat body cell colocalize mainly with Lva, a Drosophila peripheral Golgi protein (Fig. 5C) (22, 23). The Golgi markers dGRASP-GFP and GalT-GFP (24, 25) also colocalize with Ema-GFP (Fig. 5C). Although these results demonstrate that Ema localizes to the Golgi complex in the fat body cell, the incomplete overlap between Ema and those Golgi puncta suggests that Ema protein is restricted to a specific membrane domain within the Golgi complex (Fig. 5C, Insets). Ema also localizes to the Golgi in other types of Drosophila cells including salivary gland cells, muscle cells, and epithelial cells (Fig. 5C); however we still failed to detect Ema in the Golgi of the endocytic garland cells (14). It is possible that the steady-state distribution of Ema protein shifts when subcellular membrane compartments either are extensively specialized, as in the highly endocytic garland cells, or are experiencing dramatic remodeling, as during autophagy. In this latter scenario, the localization of Ema in fat body cells suggests that the Golgi may be a source for the autophagosomal membrane.
Ema Promotes Recruitment of Golgi Elements to Autophagosomes.
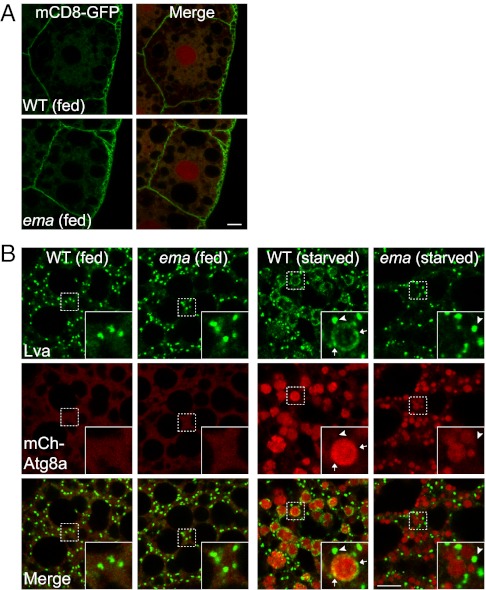
The Golgi localization of Ema protein suggests its possible role in Golgi-mediated membrane trafficking. We first investigated general membrane trafficking through the Golgi complex by assessing the localization of the plasma membrane protein mCD8-GFP, whose trafficking is critically regulated by the Golgi complex. In both wild-type and ema-mutant fat body cells, mCD8-GFP is properly localized at the cell surface (Fig. 6A), indicating that Ema is dispensable for general membrane trafficking through the Golgi complex. However, the inducible localization of Ema to autophagosomes (Fig. 5 A and B) suggests that Ema could mediate a specific form of membrane trafficking from the Golgi compartment to autophagosomes. To test this hypothesis, we investigated whether the Drosophila Golgi protein Lva localizes to autophagosomes in wild-type and/or ema-mutant fat body cells. In wild-type fat body cells under fed conditions, small Lva-containing Golgi complexes distribute throughout the cytosol (Fig. 6B). Upon autophagy induction, Lva is localized to two distinct classes of structures. One type (arrowheads in Fig. 6B, Inset) is similar in size and shape to the Golgi structures observed in fed cells, whereas the other appears smaller and vesicular (arrows in Fig. 6B, Inset). Interestingly, these smaller vesicles localize along the perimeter of mCherry-Atg8a autophagosomes, demonstrating a starvation-induced recruitment of Lva-containing vesicles to autophagosomes. The scarcity of Lva fluorescence in the lumen of autophagosomes suggests that recruitment of these Golgi elements to autophagosomes does not simply reflect autophagic degradation of the Golgi complex.
Fig. 6.
Defective Golgi-to-autophagosome traffic in the ema fat body cell. (A) ema does not affect general Golgi membrane trafficking. Shown are representative single confocal sections of fat body cells expressing the plasma membrane protein mCD8-GFP. Note the proper localization of mCD8-GFP at the cell surface in both wild-type and ema-mutant cells. mCherry-Atg8a (red) was used as reference for the cell nuclei. (B) Recruitment of the Golgi protein Lva to autophagosomes requires ema. Shown are representative single confocal sections of fat body cells expressing mCherry-Atg8a (red) and labeled for Lva protein (green). Insets show large and small Lva structures (indicated by arrowheads and arrows, respectively) separated from or associated with mCherry-Atg8a autophagosomes of starved cells. (Scale bars, 10 μm.)
The localization of Lva on autophagosomes shows that protein traffics between the Golgi complex and autophagosomes. We next asked whether ema is required for this localization of Lva. As in wild-type cells, numerous small Lva structures are distributed throughout the ema-mutant fat body cell under fed conditions, indicating that ema does affect the steady-state distribution or membrane trafficking of the Golgi complex. In contrast to the wild type, however, none of the autophagosomes in the starved ema-mutant fat body cell are associated with Lva structures (arrowheads in Fig. 6B). Hence, ema is required for the recruitment of these Lva-positive Golgi elements to autophagosomes. However, Ema is not required for trafficking of all Golgi components to the autophagosome. The Golgi membrane protein GFP-GalT is recruited to autophagosomes under starvation conditions in both wild-type and ema-mutant fat cells (Fig. S5). However, GFP-GalT appears to localize to the lumen of the autophagosomes and so could be an autophagic substrate rather than a membrane source for growth. The recruitment of the membrane Golgi protein Ema and peripheral Golgi protein Lva to the limiting membrane of autophagosomes is consistent with the model that Ema promotes autophagosome growth by facilitating the recruitment of Golgi membrane components to the developing autophagosome.
Ema Is Required for Autophagic Degradation of p62 and Mitochondria.
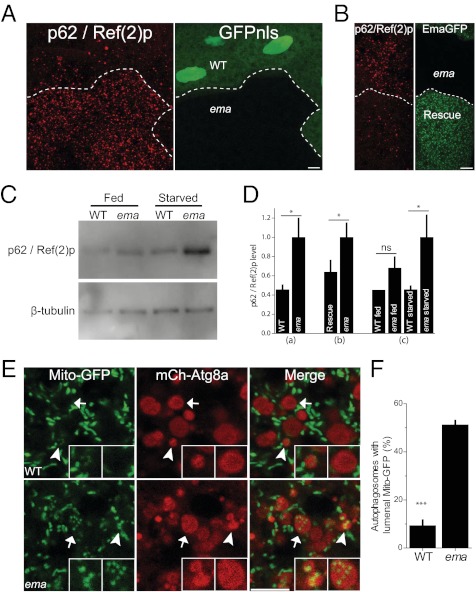
Ema is required for autophagosomal growth; is it also required for their function? To assess autophagy, we measured p62 turnover and mitophagy. The p62/SQSTM1 protein directly binds to ubiquitin via its ubiquitin-binding domain and recruits ubiquitinated proteins to autophagosomes for their degradation. Impaired p62 turnover occurs in many Atg mutants and is observed in human disease conditions such as cancers and neurodegeneration (26, 27). The Drosophila p62 homolog is Ref(2)p (28). To test whether ema is required for the autophagic turnover of Ref(2)p (hereafter called “p62”), we first examined the p62 protein in mosaic ema-mutant fat body cell clones by immunocytochemistry. p62 immunoreactive puncta distribute sparsely in the starved wild-type fat body cell clones (GFPnls-positive), although they accumulate in the neighboring ema-mutant clones (GFPnls-negative) (2.5 fold increase in the ema-mutant cells; P < 0.02; paired t test) (Fig. 7 A and D). Transgenic expression of Ema-GFP in ema-mutant cells reduces this accumulation of p62 by ∼40% ( P < 0.04; paired t test) (Fig. 7 B and D). We also examined the p62 protein level by immunoblot (Fig. 7C). A similar amount of p62 protein is detected in whole-cell lysates of wild type and ema mutants under fed conditions (P > 0.6; ANOVA) (Fig. 7D). Under starvation conditions, p62 levels are unchanged in wild type but increase significantly (by two fold) in the ema mutant (P < 0.04; ANOVA) (Fig. 7D). Taken together, these results demonstrate that ema is required for autophagic p62 turnover upon starvation.
Fig. 7.
Ema is required for autophagy of p62 and mitochondria. (A–D) Ema promotes p62 turnover. (A) Representative confocal images of starved fat body cell clones labeled for p62 protein (red). GFPnls (green) marks wild-type clones. Genotype of the animal: hs-flp/+;r4-Gal4,FRT82B,UAS-mCherry/FRT82B,ema1. n = 10 pairs of mosaic clones. *P < 0.05 (paired t test). (B) Representative confocal images of starved fat body cell clones labeled for p62 protein (red). Ema-GFP (green) marks rescue clones. Genotype of the animal: da-Gal4,UAS-Ema-GFP, ema1. n = 7 pairs of mosaic clones. (C) Immunoblot of p62 protein in whole-animal lysates. The p62 level is normalized to β-tubulin. n = 5 independent Western blot experiments. (D) Quantifications of p62 level in A, B, and C (mean ± SE). *P < 0.05, (paired t test). ns, not significant. (E and F) Ema promotes mitophagy. (E) Representative single confocal sections of starved fat body cells expressing the mitochondria marker Mito-GFP (green) and autophagosomal marker mCherry-Atg8a (red). Arrowheads indicate small autophagosomes; arrows indicate luminal Mito-GFP puncta in the large autophagosomes. Left Inset depicts small autophagosomes in both wild-type and mutant fat body cells containing Mito-GFP puncta (note very strong luminal Mito-GFP fluorescence in the ema mutant). Right Inset depicts luminal Mito-GFP puncta in the large autophagosomes in the mutant but not in the wild type. (F) Quantification of the fraction (%) of autophagosomes with luminal Mito-GFP puncta in E (mean ± SE). ***P < 0.001 (t test). (Scale bars, 10 μm.)
To test whether ema is required for autophagic degradation of mitochondria, we expressed both the autophagosomal protein mCherry-Atg8a and the mitochondrial protein Mito-GFP in Drosophila fat body cells. In the starved wild-type fat body cells, Mito-GFP–labeled mitochondria display extensive tubular morphologies and rarely colocalize with autophagosomes (Fig. 7E). In the starved ema-mutant fat body cells, Mito-GFP structures are much shorter than those in wild type, and there is excessive accumulation of Mito-GFP fragments in autophagosomes. In the ema-mutant cells, ∼50% of autophagosomes contain Mito-GFP fragments, whereas only ∼10% of autophagosomes contain such fragments in wild-type cells (P < 0.0001; t test) (Fig. 7F), consistent with defective mitophagy. The presence of Mito-GFP inside the mutant autophagosomes suggests that defects in autophagosomal growth occur after the engulfing process and/or the completion of autophagosome formation. However, the small size of the autophagosomes does not explain the functional deficits in autophagosomes. Although ema-mutant autophagosomes can fuse with endosomes and lysosomes (Fig. 3), the defect in mitophagy may reflect some abnormality in these endosomes or lysosomes that may contribute to the abnormal autophagic degradation (14). In conclusion, the accumulation of p62 and the failure to degrade Mito-GFP demonstrate that ema impairs not only the size of autophagosomes but also the clearance of autophagic substrates.
Discussion
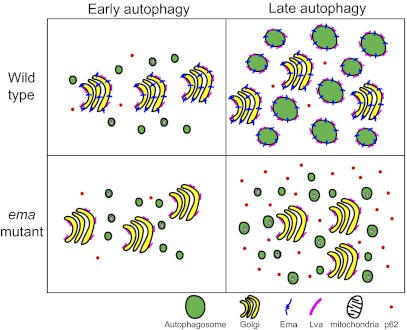
Here we demonstrate that Ema is required for autophagosomal growth and efficient autophagy and propose that Ema functions to promote membrane traffic from the Golgi complex to autophagosomes.
Ema Participates in the Regulation of Autophagosome Size.
In this study we were able to investigate the morphology of autophagosomes in detail because autophagosomes in Drosophila fat body cells are much larger than those in yeast and mammalian cell lines, often reaching 10 μm in diameter rather than the ∼1-μm diameter of autophagosomes present in other systems. Although how the invertebrate fat body cells form such large autophagosomes remains unclear, their presence likely is related to the need for efficient self-digestion, because the fat body is degraded during metamorphosis. Although detected most easily in Drosophila, variation in the size of autophagosomes and related structures is observed in other systems. For example, the cytoplasm-to-vacuole targeting (Cvt) vesicle (∼150 nm) is smaller than an autophagosome (∼500–900 nm) in yeast, even though the Cvt pathway and autophagy share many components. Context-dependent regulation of autophagosome size also occurs in mouse tissues and cultured cell lines (29).
The size of autophagosomes likely is determined at two distinct autophagic steps. During the early stage of autophagy, autophagosomes can expand by the addition of membrane to the forming isolation membranes. The ubiquitin-like conjugation system for Atg8 and the lipid phosphatidylethanolamine is important in this early growth phase. Mutation of the mouse Atg7, which exerts an E1-like activity for Atg8, results in small autophagosomes (30). This membrane expansion may be mediated by the membrane-tethering and hemifusion activities of the ubiquitin-like Atg protein Atg8 (11). Indeed, there is an ∼30% decrease in the size of autophagic bodies in the most severe hypomorphic Atg8 yeast mutants, whereas atg8-null mutants (∆atg8) completely lack autophagic bodies (13). Therefore, Atg8 is essential for both autophagosome formation and growth. On the other hand, our temporal analysis of autophagosomal development demonstrates that in wild-type cells autophagosome size continues to increase as autophagy proceeds (Fig. 3 A and B), consistent with an additional growth process following autophagosome completion. At the late stage of autophagy, autophagosomes may grow by fusion with endosomes and lysosomes. However, impaired lysosomal fusion in Drosophila and mammalian cells results in the accumulation of autophagosomes but has little effect on their size (19, 20). In addition, the presence of amphisomes (the fusion product of endosomes and autophagosomes) and autolysosomes in the ema mutant (Figs. 1 and 3) indicates that late events in autophagy such as endosomal/lysosomal fusion are not sufficient for proper autophagosomal growth. Therefore, additional membrane apart from that of the endocytic pathway may be added to autophagosomes after completion, and ema likely is required for this process.
Membrane Traffic from the Golgi Complex to Autophagosome.
Nearly 50 years ago Locke and Collins performed ultrastructural analysis of the fat body from the butterfly Calpodes ethlius undergoing metamorphosis/starvation and proposed that the Golgi complex contributes to the membrane of the autophagosome-like compartments they observed (10). We now support the hypothesis that the Golgi complex is a membrane source for autophagosomes by demonstrating that the Golgi proteins Ema and Lva are recruited to the limiting membrane of autophagosomes upon autophagic induction in the Drosophila fat body (Figs. 5 and 6B). Several other studies have proposed an interaction between the Golgi complex and autophagosomes. Two essential Atg proteins, mammalian Atg9 and Beclin 1/Atg6, localize to the Golgi complex (31, 32). Two recent studies demonstrated that severe defects in Golgi membrane trafficking disrupt the expansion of the phagophore, suggesting that the Golgi complex contributes to an early stage of autophagy (33, 34). In addition, a specific set of yeast secretory components are required for autophagy including the biogenesis of autophagic structures (35–38). Because the ema mutation does not grossly affect Golgi trafficking but does impair autophagy (Fig. 6A), our data are consistent with the model that a specialized form of Golgi trafficking promotes autophagy in Drosophila fat body cells.
How might material traffic from the Golgi complex to autophagosomes? Lva is a peripheral Golgi protein and can interact with dynein, so its movement does not necessarily involve membrane traffic (22). However, Ema is a membrane protein (14) and so most likely is transported via membrane trafficking. Based on electron microscopic studies in insect fat bodies, Locke and Collins (10) proposed that a vesicular membrane carrier moves from the Golgi complex to autophagosomes. The small Lva-positive structures that we observe in starved fat body cells could represent such a Golgi-derived vesicular carrier being trafficked to autophagosomes (Fig. 6). To promote such trafficking, Ema could mediate fusion of expanding autophagosomes with those Golgi-derived vesicles. Because Ema interacts with the class C Vps/HOPS tethering complex proteins (14), Ema could function in a manner equivalent to a tethering protein during such fusion. Recent studies demonstrated that SNARE proteins are essential for autophagosome biogenesis (39, 40). A potential collaboration among Ema, tethering complexes and SNAREs could occur before the completion of autophagosome formation in which Golgi-derived vesicles are added to the expanding isolation membrane or could mediate fusion between completed autophagosomes and Golgi vesicles. In addition, Ema also could function after it reaches the autophagosome. For example, Ema could mediate homotypic fusion of autophagosomes, potentially as a part of the tethering complex. In the future, live imaging and in vitro reconstitution experiments may address the molecular actions of Ema and its potential partners for membrane fusion during autophagosomal growth.
Evolutionary Role of Ema for Autophagy.
We describe a dramatic change in autophagosome size in the ema mutant and provide support for the model that Ema promotes Golgi trafficking in Drosophila fat body cells. The absence of an ema ortholog in yeast suggests that ema could add such functionality to the Golgi complex and thus promote the versatility of autophagic processes and function in higher multicellular eukaryotes. The ema phenotype may be so easy to visualize in the Drosophila fat body because in this cell enormous amounts of membrane are necessary to support extensive autophagy. However, Ema function in autophagosomal growth is not limited in the fat body cells; small autophagosomes also are present in the somatic muscles of the ema mutant, suggesting a more general role for Ema (Fig. S6). Indeed, it is plausible that ema could serve a similar role in the concurrent formation and growth of multiple autophagosomes in higher eukaryotes but is dispensable in yeast, where autophagosomes are formed at a single PAS site.
Many independent genomewide association studies have demonstrated that the human ema ortholog CLEC16A is a candidate susceptibility factor for human autoimmune disorders including type 1 diabetes and multiple sclerosis (41–44). ema and human CLEC16A are functionally conserved, in that human Clec16A rescues the lethality and membrane-trafficking defects in the ema-mutant fly (14) and restores normal autophagosomal growth when expressed in the ema-mutant fat body cells (Fig. S7). Interestingly, autophagy is implicated in MHC II antigen loading and in shaping the T-cell repertoire, and defective autophagy can lead to autoimmunity (45–47). Therefore, it is attractive to speculate that human CLEC16A may be an autoimmunity susceptibility gene because it promotes autophagy in the mammalian immune system.
Materials and Methods
Fly Stocks and Culture.
Flies were raised at 25 °C on standard cornmeal/molasses/agar medium. To induce autophagy, second- or third-instar larvae were placed on KimWipes (Kimtech Science) in Petri dishes soaked with Earle’s Balanced Salt Solution (EBSS) (Sigma) or PBS with 1% (wt/vol) glucose for 6 h, unless otherwise indicated. ema1 (emaf07675) and UAS-Ema-GFP were described previously (14). Thomas Neufeld (University of Minnesota, Minneapolis, MN) kindly provided the following autophagy flies: hsflp; r4-mCherry-Atg8a Act > CD2 > GAL4 UAS-GFPnls (48), hsFLP; Cg-GAL4; UAS-mCherry-Atg8a FRT82B UAS-GFPnls (49), hsFLP; r4-Gal4 FRT82B UAS-mCherry (49), CG-Gal4, UAS-GFP-Atg8a (48), and CG-Gal4, UAS-mCherry-Atg8a (48). Helmut Kramer (UT Southwestern Medical Center, Dallas, TX) kindly provided the UAS-Lamp1-GFP fly (50). The following flies were obtained from the Bloomington Drosophila Stock Center at Indiana University: da-Gal4 (Jean-Maurice Dura, CNRS UPR 1142, Montpellier, France), CG-Gal4 (Chuck Dearolf, Massachusetts General Hospital, Boston, MA), UAS-dGRASP-GFP (Henry Chang, West Lafayette, IN), UAS-mCD8-GFP (Tzumin Lee and Liqun Luo, Stanford University, Stanford, CA), UAS-Rab5-YFP and UAS-Rab7-YFP (Jun Zhang and Matthew P. Scott, Stanford University, Stanford, CA), UAS-GalT-GFP (Rikhy and Lippincott-Schwartz NIH, Bethesda, MD), and UAS-Mito-GFP (Bill Saxton and Aaron Pilling, Indiana University, Bloomington, IN).
Confocal Microscopy.
For the analysis of expressed fluorescent protein-tagged molecules, larvae were dissected in ice-cold PBS or EBSS, fixed in 2% (vol/vol) paraformaldehyde (PFA) in PBS for 30 min at room temperature, and mounted in VECTASHIELD (Vector Laboratories).
For immunocytochemistry, larvae were dissected in ice-cold PBS, fixed in 2% (vol/vol) PFA in PBS for 30 min, and processed in PBS containing 0.1% (vol/vol) Triton X-100. The following materials were used: anti-Lva at 1:1,000 (23), anti-Ref(2)p at 1:1,000 (51), NeuroTrace 640/660 deep-red fluorescent Nissl at 1:500 (Invitrogen), and Alexa 488-/Cy3-/Cy5-conjugated secondary antibodies at 1:1,000 (Jackson ImmunoResearch Laboratories, Inc.). After staining, specimens were equilibrated in 70% (vol/vol) glycerol in PBS and mounted with VECTASHIELD.
Western Blot.
The Western blot procedure was described previously (14). Briefly, larvae with guts removed were lysed in an SDS sample buffer, and the lysates were subjected to SDS/PAGE and immunoblotting according to standard procedures. The following antibodies and materials were used: anti-Ref(2)p at 1:1,000 (51), anti–β-tubulin (E7; Developmental Studies Hybridoma Bank), and HRP-conjugated anti-rabbit or HRP-conjugated anti-mouse IgG at 1:10,000 (Jackson ImmunoResearch Inc).
Avidin-Cy3 Uptake and LysoTracker Staining,
For avidin-Cy3 tracing of endocytic compartments (14), fat bodies from third-instar larvae starved for 4 h were incubated in EBSS containing 10 μg/mL of Extravidin-Cy3 (Sigma) for an additional 2 h, fixed in 2% (vol/vol) PFA in PBS for 30 min, and processed for confocal microscopy. For labeling with LysoTracker DND-99 (Invitrogen), specimens were dissected in ice-cold PBS, fixed in 2% (vol/vol) PFA in PBS for 3 min, rinsed with ice-cold PBS, incubated in 1 μM DND-99 in ice-cold PBS for 10 s, washed in PBS, and mounted in PBS. For labeling with LysoTracker DND-26 (Invitrogen), specimens were dissected in ice-cold EBSS, incubated in 1 μM DND-26 for 1 min, washed in ice-cold EBSS, and mounted in ice-cold EBSS. Imaging was performed only on freshly mounted specimens.
Imaging Analysis.
Confocal images were acquired with a confocal microscope (model C1; Nikon) and accompanying EZ-C1 software using argon (excitation at 488 nm) and HeNe (excitation at 543 and 633 nm) lasers and a 60× Plan-Apochromat NA 1.4 objective (Nikon) at room temperature. Samples for each experiment were processed using the same confocal gain setting, unless otherwise specified in figure legends. All quantifications were performed while blinded to genotype. ImageJ (National Institutes of Health) was used for all quantitative manipulation and analysis of images. All figure images were processed with Photoshop CS3 and Illustrator CS3 software (Adobe).
Transmission Electron Microscopy.
For ultrastructural analysis of the Drosophila fat body cells, samples were fixed in 2% (vol/vol) PFA/2.5% (vol/vol) glutaraldehyde (Polysciences Inc) in 100 mM phosphate buffer, pH 7.2, overnight at 4 °C. Samples were washed in phosphate buffer and postfixed in 0.5% (wt/vol) osmium tetroxide (Polysciences Inc)/0.08% (wt/vol) potassium ferricyanide (Electron Microscopy Sciences)/100 mM phosphate buffer for 1 h and subsequently in 1% (vol/vol) tannic acid (Electron Microscopy Sciences)/100 mM phosphate buffer for 1 h. Samples then were rinsed extensively in distilled H2O before en bloc staining with 1% (vol/vol) aqueous uranyl acetate (Ted Pella Inc) for 1 h. Following several rinses in distilled H2O, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections of 95 nm were cut with a Leica Ultracut UCT ultramicrotome (Leica Microsystems Inc), stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA). Electron micrographs were also taken on a transmission electron microscope (H-7500; Hitachi). For the analysis of the size of autophagic structures, one image per section was taken randomly from wild-type (n = 8) and mutant (n = 12) cells at a magnification of 10,000×. The length along the longest axis of autophagic structures was measured using ImageJ software.
Statistics.
Results are represented as mean ± SEM. Statistical tests were performed with Origin 8.0 software (Origin Laboratory). Groups of means were compared using one-way ANOVA, and comparisons between two means were performed using Student’s t-test or paired t test.
Supplementary Material
Acknowledgments
We thank Thomas P. Neufeld (University of Minnesota) for the many fly lines; Ophelia Papoulas (University of Texas at Austin) for anti-Lva antibody; Didier Contamine (Université Versailles Saint Quentin) for anti-Ref(2)p antibody; Helmut Kramer (University of Texas Southwestern Medical Center) for the UAS-Lamp1-GFP fly; the Bloomington Drosophila Stock Center (Indiana University) for flies; the Developmental Studies Hybridoma Bank for antibodies; Dr. Wandy Beatty (Washington University School of Medicine) for electron microscopy preparations; Herbert W. Virgin and Seungmin Hwang for critical advice and discussions; and members of our laboratory for support and helpful discussions. This work was supported by grants from the Juvenile Diabetes Research Foundation and by National Institutes of Health Grant DA 020812 (to A.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 6800 (volume 109, number 18).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120320109/-/DCSupplemental.
References
- 1.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–1148. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K, Ohsumi Y. Current knowledge of the pre-autophagosomal structure (PAS) FEBS Lett. 2010;584:1280–1286. doi: 10.1016/j.febslet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Hailey DW, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Axe EL, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747–757. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol. 2010;12:831–835. doi: 10.1038/ncb0910-831. [DOI] [PubMed] [Google Scholar]
- 10.Locke M, Collins JV. the structure and formation of protein granules in the fat body of an insect. J Cell Biol. 1965;26:857–884. doi: 10.1083/jcb.26.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Subramani S, Farré J-C. A ubiquitin-like protein involved in membrane fusion. Cell. 2007;130:18–20. doi: 10.1016/j.cell.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19:3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Wairkar YP, Daniels RW, DiAntonio A. The novel endosomal membrane protein Ema interacts with the class C Vps-HOPS complex to promote endosomal maturation. J Cell Biol. 2010;188:717–734. doi: 10.1083/jcb.200911126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neufeld TP. Genetic manipulation and monitoring of autophagy in Drosophila. Methods Enzymol. 2008;451:653–667. doi: 10.1016/S0076-6879(08)03236-9. [DOI] [PubMed] [Google Scholar]
- 16.Juhász G, et al. The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol. 2008;181:655–666. doi: 10.1083/jcb.200712051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Manfruelli P, Reichhart J-M, Steward R, Hoffmann JA, Lemaitre B. A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J. 1999;18:3380–3391. doi: 10.1093/emboj/18.12.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rusten TE, et al. ESCRTs and Fab1 regulate distinct steps of autophagy. Curr Biol. 2007;17:1817–1825. doi: 10.1016/j.cub.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Liang C, et al. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol. 2008;10:776–787. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papoulas O, Hays TS, Sisson JC. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol. 2005;7:612–618. doi: 10.1038/ncb1264. [DOI] [PubMed] [Google Scholar]
- 23.Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondylis V, Spoorendonk KM, Rabouille C. dGRASP localization and function in the early exocytic pathway in Drosophila S2 cells. Mol Biol Cell. 2005;16:4061–4072. doi: 10.1091/mbc.E04-10-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoh AK, O’Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- 26.Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584:1374–1378. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nezis IP, et al. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol. 2008;180:1065–1071. doi: 10.1083/jcb.200711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komatsu M, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young ARJ, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- 32.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng J, Nair U, Yasumura-Yorimitsu K, Klionsky DJ. Post-Golgi Sec proteins are required for autophagy in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:2257–2269. doi: 10.1091/mbc.E09-11-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Vaart A, Griffith J, Reggiori F. Exit from the Golgi is required for the expansion of the autophagosomal phagophore in yeast Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:2270–2284. doi: 10.1091/mbc.E09-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yen W-L, et al. The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J Cell Biol. 2010;188:101–114. doi: 10.1083/jcb.200904075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishihara N, et al. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol Biol Cell. 2001;12:3690–3702. doi: 10.1091/mbc.12.11.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reggiori F, et al. Early stages of the secretory pathway, but not endosomes, are required for Cvt vesicle and autophagosome assembly in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:2189–2204. doi: 10.1091/mbc.E03-07-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meiling-Wesse K, et al. Trs85 (Gsg1), a component of the TRAPP complexes, is required for the organization of the preautophagosomal structure during selective autophagy via the Cvt pathway. J Biol Chem. 2005;280:33669–33678. doi: 10.1074/jbc.M501701200. [DOI] [PubMed] [Google Scholar]
- 39.Nair U, et al. SNARE proteins are required for macroautophagy. Cell. 2011;146:290–302. doi: 10.1016/j.cell.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreau K, Ravikumar B, Renna M, Puri C, Rubinsztein DC. Autophagosome precursor maturation requires homotypic fusion. Cell. 2011;146:303–317. doi: 10.1016/j.cell.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todd JA, et al. Genetics of Type 1 Diabetes in Finland Wellcome Trust Case Control Consortium Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakonarson H, et al. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591–594. doi: 10.1038/nature06010. [DOI] [PubMed] [Google Scholar]
- 43.Hafler DA, et al. International Multiple Sclerosis Genetics Consortium Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 44.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 46.Paludan C, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 47.Schmid D, Pypaert M, Münz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arsham AM, Neufeld TP. A genetic screen in Drosophila reveals novel cytoprotective functions of the autophagy-lysosome pathway. PLoS ONE. 2009;4:e6068. doi: 10.1371/journal.pone.0006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang Y-Y, Neufeld TP. An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol Biol Cell. 2009;20:2004–2014. doi: 10.1091/mbc.E08-12-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulipparacharuvil S, et al. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J Cell Sci. 2005;118:3663–3673. doi: 10.1242/jcs.02502. [DOI] [PubMed] [Google Scholar]
- 51.Wyers F, Petitjean AM, Dru P, Gay P, Contamine D. Localization of domains within the Drosophila Ref(2)P protein involved in the intracellular control of sigma rhabdovirus multiplication. J Virol. 1995;69:4463–4470. doi: 10.1128/jvi.69.7.4463-4470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]