Abstract
Astrocytes are the most abundant cell of the CNS and demonstrate contact inhibition in which a nonproliferative, nonmotile cellular state is achieved once stable intercellular contacts are formed between mature cells. Cellular injury disrupts these intercellular contacts, causing a loss of contact inhibition and the rapid initiation of healing. Dysregulation of the molecular pathways involved in this process is thought to lead to an aggressive cellular state associated with neoplasia. We investigated whether a comparable correlation exists between the response of astrocytes to injury and the malignant phenotype of astrocytomas. We discovered that the loss of contact inhibition plays a critical role in the initiation and regulation of reactive astrocytes in the healing of wounds. In particular, injury of the astrocytes interrupts and destabilizes the cadherin-catenin complexes at the cell membrane leading to nuclear translocation of β-catenin and characteristic changes associated with the activation of astrocytes. Similar signaling pathways are found to be active—but dysregulated—in astrocytomas. Inhibition of β-catenin signaling diminished both the response of astrocytes to injury and induction of the malignant phenotype of astrocytomas. The findings shed light on a unique mechanism associated with the pathogenesis of astrocytomas and provide a model for the loss of contact inhibition that may broadly apply to understanding the mechanisms of tissue repair and tumorigenesis in the brain.
Keywords: astrocyte activation, glioblastoma multiforme, wound healing, adherent junction
Astrocytes play a critical role in numerous activities that support the function of the CNS. They are responsible for tissue repair, the integrity of the blood brain barrier, metabolic support of neurons, fluid and electrolyte balance, and other essential activities (1, 2). Following injury to the nervous system, astrocytes respond through a repair process where they undergo significant proliferation and differentiation (3, 4). The activation of astrocytes is a tightly regulated process that is rapidly initiated and promptly terminated when repair is achieved (3, 5). The dynamic nature of this process indicates the presence of an unidentified sensor that initiates the activation of astrocytes.
Although the molecular mechanisms leading to the activation of astrocytes are not completely established, we hypothesized that dysregulation of these pathways could result in abnormal cellular proliferation and neoplastic transformation. Therefore, understanding the cellular changes that occur in response to injury could help identify critical signaling pathways involved in the pathogenesis of astrocytomas, a highly malignant tumor of humans. To determine the mechanisms underlying the activation of astrocytes, including the initiating events and regulatory mediators of this process, the molecular signaling changes after a scratch of adult mouse astrocytes were examined and correlated with the processes and signaling pathways that participate in the pathogenesis of human malignant gliomas.
Results
Spatial and Temporal Changes in Reactive Astrocytes.
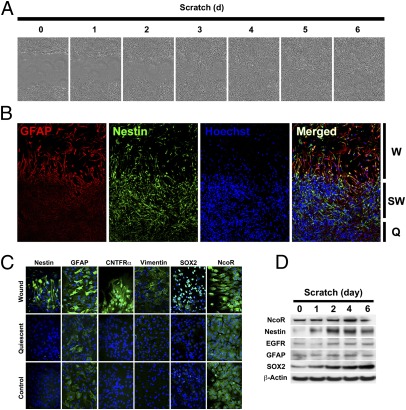
Current models of the activation of astrocytes include morphological, migratory, and molecular changes (5). Other key features of this process include growth and proliferative changes, differentiation of quiescent astrocytes, and an increase in the expression of stem cell biomarkers (6). An in vitro scratch assay for the healing of wounds in astrocytes was performed. Wounds were observed at defined time points to determine the morphological changes that occur during the activation of astrocytes (Fig. 1A). This assay demonstrated the migration of astrocytes into the wound within 1 h of injury and complete infiltration by 6 d postinjury. High-frequency serial images of astrocytes were captured following injury to observe these changes in greater detail. Cells in the region adjacent to injury developed either a migratory phenotype, with processes extending into the cavity of the wound, or a proliferative phenotype along the margins of the wound. Cells farthest from the margins remained quiescent (Movies S1, S2, and S3).
Fig. 1.
Characterization of changes in astrocytes following injury. (A) Scratch assay in cultured mouse astrocytes at various time points following injury demonstrating wound closure by 6 d postinjury. (Magnification: 100×.) (B) Immunofluorescence staining for GFAP (red) and Nestin (green) demonstrating differential spatial pattern of expression following injury to astrocytes. Cell nuclei are counterstained with Hoechst stain (blue). Q, quiescent region; SW, submarginal wound region; W, wound region. (Magnification: 200×.) (C) Immunofluorescence staining of the expression of biomarkers in different regions following the injury to astrocytes. (Magnification: 400×.) (D) Western blot analysis and time course of expression of biomarkers following injury.
Immunofluorescence staining was performed to identify a spatial and temporal pattern of the expression of molecular markers associated with the activation of astrocytes. Following injury, the expression of both glial fibrillary acidic protein (GFAP) and Nestin increased at the margins of the wound. GFAP was strongly expressed in migratory cells in the cavity of the wound but cells expressing Nestin were localized at the margins of the wound and the immediately adjacent region (Fig. 1B). Expression of several markers associated with growth and proliferation of astrocytes [nuclear receptor corepressor (NcoR), ciliary neurotrophic factor receptor CNTFR)], the differentiation of astrocytes (GFAP, vimentin), and stem and progenitor cells [Nestin, sex-determining region Y-box 2 (SOX2)] also increased following injury (Fig. 1C). The expression of these markers followed a distinct spatial localization and pattern of separation at the site of injury. The expression of SOX2 was increased at the margins of the wound, but other potential stem cell biomarkers, including Nanog and Oct4, were not increased following injury to the astrocytes (Fig. S1). We then determined the time course of the expression of several markers in the astrocytes that were up-regulated in response to injury. Within a day after injury, several markers, including NcoR, epidermal growth factor receptor (EGFR), GFAP, and SOX2 had increased. The expression of Nestin increased transiently, peaking at 3 d postinjury, and the expression of SOX2 gradually increased without evidence of decline up to 6 d after injury (Fig. 1D and Fig. S2).
β-Catenin Is a Molecular Initiator of the Activation of Astrocytes.
Cellular changes occurred mainly at the margins of the wound after injury to the astrocytes. Cells remote from the site of injury remained dormant. These findings indicated that interruption of cell-cell contact might underlie the changes observed in reactive astrocytes following mechanical injury of these cells. It seemed plausible that elements of the cell membrane responsible for cell adhesion and communication, such as cadherin-catenin complexes, could play a role in sensing such injury. β-Catenin has been shown to be an important regulator of the migratory phenotype in epithelial cells following injury (7). As a transcription factor ubiquitously located on the surface of many different types of cells, β-catenin can be activated at the cell membrane, resulting in its nuclear translocation and the transcription of several genes favoring proliferation (8).
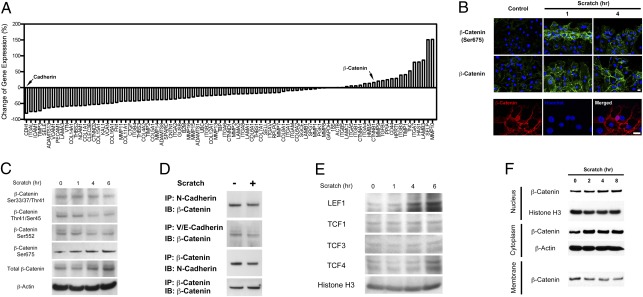
Gene-expression analysis of various molecules associated with cellular adhesion in injured astrocytes was performed using PCR array to determine if cellular constituents, including β-catenin, could sense mechanical injury. β-Catenin increased in injured astrocytes, but cadherin was significantly reduced (Fig. 2A). These findings suggested that β-catenin signaling plays a role in the activation of astrocytes following mechanical injury.
Fig. 2.
β-Catenin phosphoryation and nuclear translocation following the injury of astrocytes. (A) Quantitative PCR analysis quantifying the change in expression of various genes associated with cell adhesion following injury. Arrows indicate the expression of cahedrin and β-catenin. (B) Immunofluorescence staining for phospho-β-catenin at Ser-675 (Top) and total β-catenin (Middle), 1 and 4 h postinjury. Following the injury of astrocytes, β-catenin is present in the nuclei of cells (Bottom). (Magnification: Upper, 200×; Lower, 400×.) (C) Western blot analysis demonstrating increased expression of total β-catenin and phospho-β-catenin at the Ser-675 site and decreased expression of phospho-β-catenin at other residues following injury. (D) Immunoprecipitation (IP) of N-cadherin and VE-cadherin and immunoblotting (IB) for β-catenin demonstrating decreased association of both N-cadherin and VE-cadherin with β-catenin following injury. (E) Western blot analysis demonstrating increased expression of downstream targets of β-catenin, LEF1, and TCF4, following injury. (F) Western blot analysis demonstrating expression of β-catenin in various cellular fractions at various time points following injury.
Considering the rapid changes in proliferative and molecular patterns of expression associated with the activation of astrocytes, it would indicate that a molecular trigger of this process would also be highly dynamic in nature. Based on the finding that β-catenin levels were increased in astrocytes after injury, we hypothesized that during injury to the nervous system, the activation of astrocytes occurred because of a loss of contact inhibition mediated by membrane cadherin-catenin complexes that rapidly sensed mechanical injury. The expression of β-catenin before and after injury was measured to determine whether it was directly involved in the activation of astrocytes. The expression of β-catenin was increased at 1 and 4 h following injury. Specifically, there was up-regulation of β-catenin phosphorylated at serine 675 (Ser-675) (Fig. 2B). Western blot analysis of astrocytes after injury demonstrated an increase in both total and Ser675-phosphorylated β-catenin (Fig. 2C and Fig. S3).
Increased Ser-675-phosphorylated β-catenin in response to injury suggests a mechanism where β-catenin dissociates from membrane complexes after phosphorylation, accumulates in the cytoplasm, and then translocates into the nucleus. Immunoprecipitation was performed to investigate this mechanism by measuring β-catenin binding to various cadherins at the membrane surface following injury. β-catenin was found to have decreased association with N-cadherin, an important mediator of cellular adhesion in the nervous system (9) (Fig. 2D and Fig. S4). Because these findings are suggestive of a process in which dissociation of β-catenin from the cell membrane occurs, we investigated whether translocation of β-catenin into the nucleus also occurs during the activation of astrocytes by measuring downstream targets within the lymphoid enhancer factor (LEF) and T-cell factor (TCF) families of transcription factors (10). Expression levels of LEF1 and TCF4 were up-regulated 6 h following injury, suggesting an increase in the nuclear effects of β-catenin (Fig. 2E and Figs. S5 and S6).
Expression of β-catenin in membrane-bound, cytoplasmic, and nuclear cell fractions following injury was measured to determine how the expression of β-catenin increases in response to injury. Cytoplasmic and nuclear expression of β-catenin increased following injury, but membrane-bound β-catenin is decreased shortly after injury (Fig. 2F and Fig. S7). The findings indicate that injury of astrocytes causes destabilization and phosphorylation of β-catenin at the cell membrane, translocation to the nucleus, and the initiation of activation of astrocytes.
Inactivation of β-Catenin Diminishes the Reactive Astrocyte Phenotype.
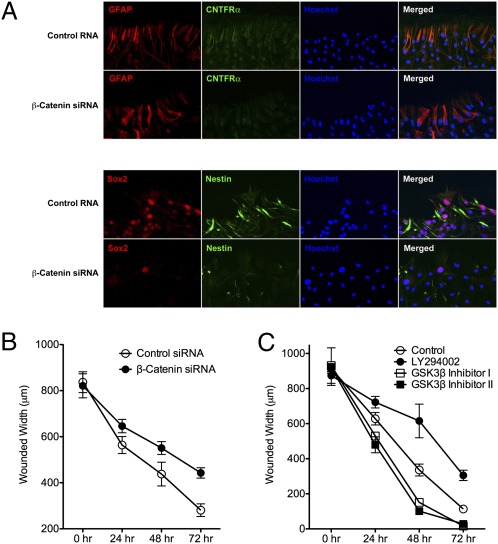
siRNA knockdown of β-catenin was performed and the expression of various markers previously found to be associated with this process was measured to further elucidate the role of β-catenin in the activation astrocytes (Figs. S8 and S9). Following injury, knockdown of β-catenin resulted in minimally decreased expression of GFAP, but significantly decreased expression of CNTFR at the margin of the wound. Additionally, expression of the stem cell markers Nestin and SOX2 was nearly abolished by the knockdown of β-catenin (Fig. 3A). The findings indicate an important role of β-catenin in the proliferative capacity of cells and the expression of stem-cell markers during the activation of astrocytes.
Fig. 3.
Effects of knockdown of β-catenin on the activation of astrocytes. (A) Immunofluorescence staining for GFAP (red) and CNTFR (green) demonstrating minimal change in expression before and after siRNA knockdown of β-catenin (Upper). Immunofluorescence staining for SOX2 and Nestin (Lower), indicating a decrease in expression of these two biomarkers following knockdown of β-catenin. (Magnification: 200×.) (B) Measurement of the width of the wound before (○) and after (●) knockdown of β-catenin demonstrating delayed wound closure following inhibition of β-catenin. (C) Measurement of the wound before (○) and after (●) LY294002, demonstrating delayed wound closure following treatment with PI3K agonist. Use of two GSK3β inhibitors (□ and ■) result in faster wound closure, presumably because of their effects on β-catenin signaling.
The ability of astrocytes to repair after injury before and after the knockdown of β-catenin was measured to determine whether astrocyte repair of injury is affected by the loss of β-catenin. The studies revealed that inhibition of β-catenin had a detrimental effect on the repair of astrocytes (Fig. 3B). Additionally, the use of an inhibitor of glycogen synthase kinase-3β (GSK3β), a kinase that increases the degradation of β-catenin, resulted in faster healing of the wound (Fig. 3C). These results suggest that β-catenin is a critical mediator of the activation of astrocytes and that a reduction of its function results in a diminished response to injury by astrocytes.
Dysregulation of Reactive Astrocyte Signaling Mediated by β-Catenin Plays a Role in the Pathogenesis of Astrocytomas.
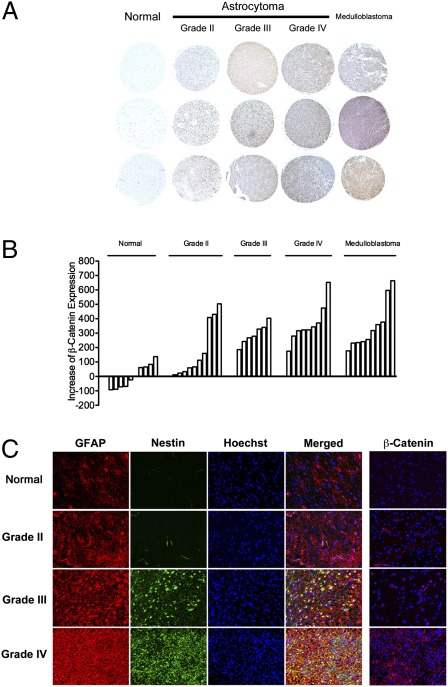
Previous studies have implicated β-catenin signaling and its nuclear translocation in the malignant phenotype of astrocytomas and demonstrated Wnt and the overexpression of β-catenin in these tumors (11–13). The finding that β-catenin signaling and nuclear translocation plays an important role in the activation and proliferation of astrocytes in response to injury led us to examine whether this signaling pathway might be common to these two processes (repair and tumorigenesis). To measure and confirm the expression of β-catenin in astrocytomas, an immunohistochemical and Western blot analysis was conducted on multiple astrocytoma samples of varying grade. This analysis demonstrated increased expression of β-catenin in primary brain tumors compared with normal brain tissue, and showed an increasing trend of expression of β-catenin with increased histological grade of astrocytomas (Fig. 4 A and B and Fig. S10).
Fig. 4.
Changes associated with reactive astrocytes in astrocytomas of varying grade. (A) Tissue microarray analysis of β-catenin expression in astrocytomas of varying grade demonstrating a positive correlation of expression of β-catenin with the grade of the astrocytoma. (Magnification: 40×.) (B) Semiquantitative analysis of expression of β-catenin in samples of normal brain and tumors (individual bars) also demonstrating a positive correlation between expression of β-catenin and the grade of the astrocytoma. (C) Immunofluorescence staining for GFAP (red) and Nestin (green) demonstrating an increase in expression of these reactive astrocyte biomarkers in astrocytomas of increasing grade. In all astrocytomas, the pattern of expression of these markers appears heterogeneous and dysregulated. Far right column: Immunofluorescence staining for β-catenin (red) demonstrating increased β-catenin in astrocytomas of increasing grade. (Magnification: 200×.)
Immunofluorescence analysis was carried out on normal brain tissue and astrocytomas of different grades to determine the pattern of expression of markers previously found in this study to be up-regulated as a consequence of the activation of astrocytes. This analysis revealed that the expression of GFAP and Nestin are present in all grades of astrocytoma, but are more robustly expressed in high-grade (grade III and IV) compared with low-grade astrocytomas (grade II). There appears to be a random intermingling and heterogeneous dispersement of GFAP- and Nestin-expressing cells within astrocytomas in contrast to the organized pattern of distribution in normal reactive astrocytes. Thus, it appears that there is dysregulation of the localization of these two phenotypic cell types within astrocytomas. We further confirmed by immunofluorescence staining that β-catenin is increased in high-grade astrocytomas compared with low-grade tumors and normal brain (Fig. 4C). The finding that the markers associated with the activation of astrocytes are also expressed in astrocytomas, albeit in a dysregulated manner, indicates that these processes are phenotypically related and that their pathogenic mechanisms may be linked.
Down-Regulation of β-Catenin Inhibits Growth of Primary Glioma Cells.
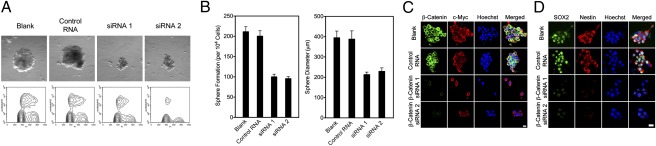
The common expression of stem cell biomarkers, such as Nestin, and astrocyte differentiation markers, such as GFAP, during the activation of astrocytes and in astrocytomas suggests a shared pathway through which both biological phenomena occur. To investigate whether β-catenin signaling underlies this potential functional association, β-catenin was knocked down with specific siRNA in tumor sphere-forming primary glioma cultures and various properties associated with their proliferation and viability were examined (14). Following the knockdown of β-catenin, tumor sphere size was considerably decreased with the use of two different siRNA constructs for β-catenin.
Cell-cycle analysis was carried out on the primary glioma cell lines to further examine their proliferative capacity. The cell demonstrated decreased S-phase population after knockdown of β-catenin, suggesting cell-cycle arrest and diminished proliferation as a result of the inhibition of this pathway (Fig. 5A). Additionally, using a sphere-formation assay, tumor sphere formation was noticeably reduced following the knockdown of β-catenin and spheres that were able to form in culture were of decreased diameter (Fig. 5B). Immunofluorescence analysis of various markers known to be involved in the activation of astrocytes and the pathogenesis of astrocytomas confirmed that expression of β-catenin was strongly inhibited with siRNA transfection, and that c-myc, a downstream target of β-catenin, was also reduced (Fig. 5C and Fig. S11).
Fig. 5.
Effect of knockdown of β-catenin on the malignant phenotype of primary glioma cell lines. (A) Formation of tumor spheres (Upper) and analysis of the cell cycle (Lower) of primary glioma cells before and after knockdown of β-catenin with two different siRNA constructs. (Magnification: 40×.) (B) (Left) Assay of the formation of spheres in tumors. (Right) Diameter of the spheres before and after knockdown of β-catenin with two different siRNA constructs. (C) Immunofluorescence staining of primary glioma cells for β-catenin (green) and c-Myc (red) before and after siRNA knockdown of β-catenin. (Scale bar: 10 μm.) (D) Immunofluorescence staining of primary glioma cells for SOX2 (green) and Nestin (red). (Scale bar: 10 μm.)
The expression of putative glioma stem cell biomarkers was measured before and after knockdown of β-catenin to determine whether β-catenin signaling plays a role in conferring or maintaining the characteristics of glioma cells. The expression of Nestin and SOX2 were dramatically decreased in primary glioma cell lines following transfection with either of the two siRNA constructs for β-catenin (Fig. 5D). These findings demonstrate that β-catenin signaling plays a significant role in the proliferative capacity, viability, and maintenance of primary glioma cells. Thus, β-catenin signaling may be a critical cellular pathway involved in the activation of astrocytes and its dysregulation may contribute to the pathogenesis of astrocytomas.
Discussion
Destabilization of the cell membrane plays a critical role in the initial sensing of mechanical injury and in the activation of astrocytes. Astrocytes respond in a defined way following injury that includes changes in migration, proliferation, and cell differentiation that contribute to the repair of injured tissue in the CNS. These changes occur rapidly. Migration and proliferation of astrocytes in the region of injury are apparent within 15 min following the in vitro scratch assay (Fig. S1). Similarly, the response of astrocytes to injury is briskly terminated once repair is complete, implying that the activation of astrocytes is a highly regulated and dynamic process.
Many cells within the human body demonstrate contact inhibition of growth, in which cell migration and proliferation (major components of the process of activation of astrocytes) cease and a passive, nonmotile cellular state is established following the formation of stable contacts between adjacent cells (15). Cell-to-cell adhesion is mediated by membrane catenin-cadherin complexes that are ubiquitous and is responsible for the contact inhibition of growth in differentiated mature cells. Destabilization of membrane complexes has been implicated in the migratory phenotype of epithelial cells during development and tissue remodeling, as well as in neoplastic states where the loss of contact inhibition contributes to the malignant phenotype of many cancers (7, 16, 17).
The rapid dynamic changes that occur in vitro following the injury of astrocytes could be attributable to a loss of contact inhibition, resulting in a state of proliferation and motility. Similar changes were observed in a stab-wound model of mouse brain cortex in vivo, although the dynamic process could not be recorded (Fig. S12). Our observations suggest that injury of astrocytes results in a loss of contact inhibition at the margins of the wound as a result of decreased interaction between cadherins, catenins, and other membrane complex elements at the cell surface (Fig. 2). The results indicate that deconjugation of β-catenin from cell-membrane adhesion complexes likely serves as a molecular sensor of astrocyte injury and that its translocation to the nucleus is critical to the changes associated with the activation of astrocytes (Fig. 3).
β-Catenin signaling occurs through two pathways, one involving cadherin-catenin complexes at the cell membrane and the other occurring through Wnt/Frizzled signaling (18, 19). During embryological development, Wnt plays an important role in the patterning of cells in the nervous system and the regulation of development (20). Binding of Wnt to receptors of the Frizzled family leads to downstream evasion by β-catenin from degradation in the cytoplasm (21). The accumulation of β-catenin in the cytoplasm results in its entry into the nucleus and the transcription of various genes, including the LEF1/TCF family of transcription factors (22). In contrast to Wnt-dominated β-catenin signaling in embryogenesis, membrane cadherin-catenin complexes are responsible for both cell adhesion and contact inhibition of growth between astrocytes in adulthood. When cell-to-cell interactions are compromised by injury, or when activation of cell membrane-associated kinases, such as src or EGFR occurs, phosphorylation of β-catenin is thought to disrupt its interaction with constituents of the membrane complex, such as cadherins, α-catenin, and p120. As a result, increased cytoplasmic and nuclear β-catenin increases gene transcription and proliferation (23).
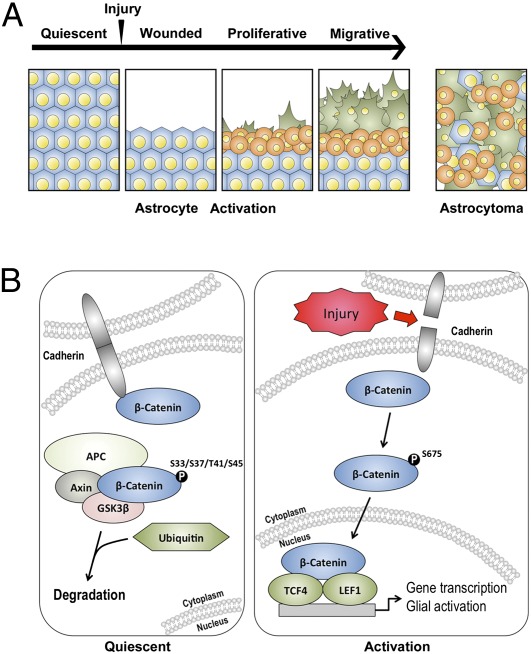
Cell-to-cell adhesion and normal tissue architecture are compromised after mechanical injury to quiescent astrocytes. Based on the current observations, it appears that translocation of β-catenin to the nucleus plays a critical role in propagating the changes associated with the activation of astrocytes (Fig. 6). The induction of this molecular cascade following injury to the astrocytes produces three populations of astrocytes, each of which contributes to the changes in proliferation, migration, and differentiation associated with the activation of astrocytes. One group is comprised of reactive migratory cells characterized by inwardly growing pseudopodia and processes into the cavity of the wound. These cells are distinguished by changes involving their differentiation and activation, particularly their increased expression of GFAP, a well-recognized marker of reactive astrocytes (Fig. 1B). A separate group of astrocytes consists of reactive proliferating cells with stem cell-like properties that are characterized by their localization to the margins and immediate submarginal regions of the wound. Their expression of Nestin and SOX2 biomarkers are thought to identify neural stem and progenitor cells within the nervous system (24, 25). A third group of astrocytes is the quiescent cell population located farthest from the wound, with no identifiable change in response to injury. Once repair is complete, reactive astrocytes revert to this quiescent state in which GFAP, Nestin, and other expression markers associated with activation of astrocytes are no longer detectable.
Fig. 6.
Schematic depiction of the sequence of events following wounding of astrocytes and the molecular mediators of the activation of astrocytes. (A) Timeline indicating changes in astrocytes following injury (arrowhead). After quiescent astrocytes (blue cells) are injured, they react by (i) proliferating (round, orange cells) and (ii) differentiating and migrating into the space created by injury (spiculated, green cells). These reactive changes follow a distinct and reproducible pattern in vitro, in which proliferating cells are initially found at the margins of the wound and motile, differentiated astrocytes migrate from the margins into the cavity of the wound following injury. (Right) In astrocytomas, similar reactive changes are found, but a dysregulation of the spatial and temporal pattern of these changes occurs. (B) (Left) Cadherin–cadherin interactions between adjacent cells in quiescent astrocytes confer cell-cell adhesion. In this state, β-catenin is bound to these complexes on the cytosolic surface of the cell membrane. β-Catenin in the cytoplasm is phosphorylated at specific residues by kinases, such as GSK3β, and subsequently degraded by ubiquitin-mediated proteasomal degradation. (Right) Cadherin–cadherin interactions are interrupted and dissociation of β-catenin from membrane complexes occurs in astrocytes after mechanical injury. Phosphorylation of β-catenin at the Ser-675 site mediates its evasion of cytoplasmic degradation machinery and translocation from the cell membrane to the nucleus, where it promotes cellular proliferation through activation of genes such as TCF4 and LEF1.
Given the critical role of β-catenin signaling in the activation of astrocytes, this molecular pathway could also be implicated in the pathogenesis of astrocytomas. Overexpression of β-catenin in gliomas is well recognized and present observations confirm a positive correlation between the expression of β-catenin and the histological grade of astrocytomas (26) (Fig. 4). Our observations that specific downstream targets of β-catenin signaling, LEF1 and TCF4, are up-regulated in astrocytes following injury, and that β-catenin accumulates in the nucleus during the activation of astrocytes as well as in astrocytomas (13), suggest a common pathway between injury repair and tumorigenesis. The expression of neural stem cell markers Nestin and SOX2 in certain reactive astrocytes and in primary glioma cultures also suggests that similar molecular mechanisms contribute to the activation of astrocytes and pathogenesis of astrocytomas (27, 28). Treatment of primary glioma cell lines with β-catenin siRNA resulted in a markedly decreased potential for tumor sphere formation and the proliferative capacity of these cells (Fig. 5 A and B). The expression of putative glioma stem cell markers was strongly inhibited after the knockdown of β-catenin (Fig. 5 C and D). These findings indicated that the malignant phenotype of astrocytomas is at least partially regulated by β-catenin signaling and that the molecular mechanisms underlying the activation of astrocytes are closely related to the mechanisms of pathogenesis and the progression of growth of astrocytomas. Patients with Turcot syndrome highlight the involvement of β-catenin signaling in tumorigenesis within the CNS, where mutations of the APC gene, an important regulator of β-catenin signaling, cause the formation of brain tumors (29).
Following the injury of astrocytes, distinct populations of reactive astrocytes express either GFAP or Nestin and localize to the margins of the wound with a consistent and replicable pattern (Fig. 1B). Expression of both GFAP and Nestin were also shown to correlate positively with increasing grade of astrocytomas. Cells expressing these markers minimally colocalized with each other and were dispersed in a heterogeneous manner in gliomas of increasing grade (Fig. 4C). The correlation between histological grade of tumors and the increasingly “reactive” nature of higher-grade astrocytomas provides evidence that dysregulation of the mechanisms involved in the normal activation of astrocytes could contribute to the pathogenesis of astrocytomas. Specifically, dysregulation of normal β-catenin signaling pathways involved in the activation of astrocytes could result in the functional loss of contact inhibition and an invasive, migratory phenotype observed in neoplastic states (30).
The time course of the activation of astrocytes have been characterized both morphologically and molecularly (Fig. 1 A and D). The dysregulation of this signaling pathway in astrocytomas appears to produce a desynchronized biological growth pattern in which the normal patterns of reactive astrocytes are lost and properties, such as proliferation and migration, occur in a chaotic, unregulated manner where GFAP (−), GFAP (+), and Nestin and Sox2 (+) cells are found throughout the chaotic 3D milieu of an astrocytoma. The potential loss of the dynamic regulation of the activation of astrocytes seen in normal injury compared with the dysregulated state of astrocytomas suggests a potential explanation for the dysregulated microenvironment of these tumors that appears to accelerate as tumors increase in grade. Although the association between CNS injury and tumor predisposition requires further investigation, genetic insult to the critical pathways involved in the activation of astrocytes demonstrated in this study could create a dysregulated state that results in the formation of brain tumors.
Materials and Methods
Tissue Culture.
Primary human and mouse astrocyte tissue cultures were purchased and maintained according to the manufacturer’s protocol (Sciencell Research Laboratories). An in vitro scratch assay was used for the injury of astrocytes, as previously described (31–33). Monolayer cells were scraped with a sterile pipette tip. Cells were then incubated in fresh growth medium. Cellular morphology and healing of the wound were subsequently measured and recorded.
Primary glioma cell cultures were maintained under conditions described previously (34, 35). Cultures were dissociated into a single-cell suspension using TrypLE Express (Invitrogen) for transfection and assayed for the formation of clones. Cells were resuspended at 50,000 cells/mL in DMEM/F12 supplemented with 50 ng/mL EGF, 20 ng/mL bFGF, and B27 (Invitrogen).
Glioma Tumor Samples and Tissue Dissection.
Tissue samples were collected at the Cleveland Clinic Foundation. All tissue samples and clinical information were obtained as part of an Institutional Review Board-approved study for molecular analysis on brain tumor in Cleveland Clinic Foundation. Frozen tissue samples included grade II astrocytomas (n = 7), grade III astrocytomas (n = 7), grade IV astrocytomas (n = 2), and normal brain (n = 1). Tissue dissection was performed as previously described (36).
Paraffin-embedded brain tumor and normal tissue array (US Biomax) were used to for immunohistochemistry staining. Normal brain tissue (n = 10), grade II glioma (n = 10), grade III glioma (n = 7), grade IV glioma (n = 9), and medullablastoma (n = 10) specimens were stained for β-catenins. Protein expression was measured by ImageJ software.
Supplementary Material
Acknowledgments
We thank Dr. Dragan Maric for the assistance in flowcytometry assay and data analysis. This research was supported by the intramural research program in the National Institute of Neurological Disorders and Stroke at the National Institutes of Health; and by the Melvin Burkhardt chair in neurosurgical oncology and the Karen Colina Wilson research endowment within the Brain Tumor and Neuro-Oncology Center at the Cleveland Clinic Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118754109/-/DCSupplemental.
References
- 1.Barres BA. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 4.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 5.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Cheng X-P, Li J-W, Yao Q, Ju G. De-differentiation response of cultured astrocytes to injury induced by scratch or conditioned culture medium of scratch-insulted astrocytes. Cell Mol Neurobiol. 2009;29:455–473. doi: 10.1007/s10571-008-9337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson KJ, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- 10.Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–537. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pu P, et al. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther. 2009;16:351–361. doi: 10.1038/cgt.2008.78. [DOI] [PubMed] [Google Scholar]
- 12.Sareddy GR, Challa S, Panigrahi M, Babu PP. Wnt/beta-catenin/Tcf signaling pathway activation in malignant progression of rat gliomas induced by transplacental N-ethyl-N-nitrosourea exposure. Neurochem Res. 2009;34:1278–1288. doi: 10.1007/s11064-008-9906-3. [DOI] [PubMed] [Google Scholar]
- 13.Sareddy GR, Panigrahi M, Challa S, Mahadevan A, Babu PP. Activation of Wnt/beta-catenin/Tcf signaling pathway in human astrocytomas. Neurochem Int. 2009;55:307–317. doi: 10.1016/j.neuint.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Beier D, et al. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 15.Holley RW, Kiernan JA. “Contact inhibition” of cell division in 3T3 cells. Proc Natl Acad Sci USA. 1968;60:300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basan M, Idema T, Lenz M, Joanny J-F, Risler T. A reaction-diffusion model of the cadherin-catenin system: A possible mechanism for contact inhibition and implications for tumorigenesis. Biophys J. 2010;98:2770–2779. doi: 10.1016/j.bpj.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack RE, Green H, Todaro GJ. Growth control in cultured cells: Selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci USA. 1968;60:126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 19.Gavert N, Ben-Ze’ev A. Beta-catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 20.Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis. 2010;38:148–153. doi: 10.1016/j.nbd.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daugherty RL, Gottardi CJ. Phospho-regulation of Beta-catenin adhesion and signaling functions. Physiology (Bethesda) 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 23.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Rietze RL, et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 26.Utsuki S, et al. Relationship between the expression of E-, N-cadherins and beta-catenin and tumor grade in astrocytomas. J Neurooncol. 2002;57:187–192. doi: 10.1023/a:1015720220602. [DOI] [PubMed] [Google Scholar]
- 27.Gangemi RMR, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 28.Gilbertson RJ, Rich JN. Making a tumour’s bed: Glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 29.Chen TC. Hereditary neurological tumor syndromes: Clues to glioma oncogenesis? Neurosurg Focus. 1998;4:e1. [PubMed] [Google Scholar]
- 30.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 31.Ishiuchi S, et al. In vitro neuronal and glial production and differentiation of human central neurocytoma cells. J Neurosci Res. 1998;51:526–535. doi: 10.1002/(SICI)1097-4547(19980215)51:4<526::AID-JNR12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Liang C-C, Park AY, Guan J-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 33.Wu BY, Yu AC. Quercetin inhibits c-fos, heat shock protein, and glial fibrillary acidic protein expression in injured astrocytes. J Neurosci Res. 2000;62:730–736. doi: 10.1002/1097-4547(20001201)62:5<730::AID-JNR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Bao S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 35.Park DM, et al. N-CoR pathway targeting induces glioblastoma derived cancer stem cell differentiation. Cell Cycle. 2007;6:467–470. doi: 10.4161/cc.6.4.3856. [DOI] [PubMed] [Google Scholar]
- 36.Furuta M, et al. Protein patterns and proteins that identify subtypes of glioblastoma multiforme. Oncogene. 2004;23:6806–6814. doi: 10.1038/sj.onc.1207770. [DOI] [PubMed] [Google Scholar]
- 37.Yang C, et al. Missense mutations in the NF2 gene result in the quantitative loss of merlin protein and minimally affect protein intrinsic function. Proc Natl Acad Sci USA. 2011;108:4980–4985. doi: 10.1073/pnas.1102198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J, et al. Decreased glucocerebrosidase activity in Gaucher disease parallels quantitative enzyme loss due to abnormal interaction with TCP1 and c-Cbl. Proc Natl Acad Sci USA. 2010;107:21665–21670. doi: 10.1073/pnas.1014376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.