Abstract
Nonenzymatic manganese was first shown to provide protection against superoxide toxicity in vivo in 1981, but the chemical mechanism responsible for this protection subsequently became controversial due to conflicting reports concerning the ability of Mn to catalyze superoxide disproportionation in vitro. In a recent communication, we reported that low concentrations of a simple Mn phosphate salt under physiologically relevant conditions will indeed catalyze superoxide disproportionation in vitro. We report now that two of the four Mn complexes that are expected to be most abundant in vivo, Mn phosphate and Mn carbonate, can catalyze superoxide disproportionation at physiologically relevant concentrations and pH, whereas Mn pyrophosphate and citrate complexes cannot. Additionally, the chemical mechanisms of these reactions have been studied in detail, and the rates of reactions of the catalytic removal of superoxide by Mn phosphate and carbonate have been modeled. Physiologically relevant concentrations of these compounds were found to be sufficient to mimic an effective concentration of enzymatic superoxide dismutase found in vivo. This mechanism provides a likely explanation as to how Mn combats superoxide stress in cellular systems.
Keywords: pulse radiolysis, oxidative stress
The role of manganese in cells has been of great interest recently, led both by the characterization of the extraordinarily radiation-resistant bacterium Deinococcus radiodurans, which seems to owe some of its remarkable survival in high-radiation fields to high levels of cellular Mn (1) and by the demonstrated ability of Mn ions to rescue mutant yeast cells that have had their superoxide dismutases (SOD) removed (2).
Nonenzymatic Mn protection against superoxide O2− toxicity in vivo was first described in 1981 by Archibald and Fridovich (3) for the lactic acid bacterium Lactobacillus plantarum, which accumulates extremely high Mn levels, has no measurable enzymatic SOD activity, and yet grows normally in air. Similarly, Neisseria gonorrhoeae, which lack the SodA and SodC enzymes that are known to be important in Neisseria meningitidis, survive the challenge of O2− produced by the immune system. Mn accumulation has been implicated as protecting the virulent form N. gonorrhoeae from O2−, and a highly active catalase protects it from H2O2 (4).
Dramatic demonstrations of the antioxidant effects of Mn were reported in studies of yeast or bacteria that had been engineered to lack SOD enzymes. The health of these SOD-deficient strains, which show many defects and grow poorly in air, were greatly improved by supplementation of the growth medium with Mn(II) ion returning all tested phenotypes to normal levels (2, 5–7). The same degree of rescue was observed even if MnSOD, which is localized in the mitochondrial matrix, was also removed (6). Moreover, recent work by McNaughton et al. (8) has provided direct evidence in whole yeast cells that the concentrations of Mn phosphate complexes correlate with the ability of Mn to protect against superoxide-induced stress.
Mn can also play an antioxidant role in higher organisms, such as the nematode Caenorhabditis elegans. Deleterious effects due to mutations in the electron transport chain that cause higher-than-normal fluxes of reactive oxygen species and effects due to heat-shock stress are alleviated by MnSO4 supplementation, which also leads to an increase in mean lifetime (9, 10).
Though the ability of Mn to protect against superoxide toxicity has now been convincingly documented in many different systems (1, 2, 4, 5, 7, 10), the in vivo chemical form of the Mn-based antioxidants and the chemical reactions responsible for their biological action remain unclear. Elucidating the exact chemical nature of the Mn-containing antioxidant molecules has proven to be particularly challenging due to the labile nature of the Mn(II) ion, which rapidly exchanges its ligands when moved from one environment to another, as occurs when it is isolated from cells. Another challenge has been the absence of a convenient direct assay for SOD activity, leading to reliance on indirect assays that may work well for SOD enzymes but are less effective for low molecular-weight metal ion complexes.
Earlier researchers explored possible mechanisms of the observed in vivo protective properties of Mn using indirect SOD assays (see below), which appeared to demonstrate that some simple Mn(II) complexes are efficient catalysts of superoxide disproportionation (11). Using those indirect assays, Mn(II) pyrophosphate, lactate, and higher concentrations of phosphate appeared to be particularly effective at superoxide removal (11). However, subsequent studies using more direct kinetic methods, such as pulse radiolysis, contradicted the earlier findings (12–14). Cabelli and Bielski (12, 13) and Barnese et al. (15) showed that O2− reacts rapidly with several Mn(II) compounds to form the intermediate species, MnOO+, but there was little evidence of catalytic behavior.
To understand better the in vivo reactivity of Mn ions with superoxide, we have estimated the relative concentrations of the potential low molecular-weight ligands for Mn ions present in cells and determined, using their relative binding affinities for Mn(II) and Mn(III) ions, that carbonate, citrate, phosphate, and pyrophosphate bind most of the cellular Mn ions not bound to proteins (16, 17). We then investigated experimentally the reactivity of these major Mn-containing species with O2− under two very different conditions that might occur in vivo: O2− generated at lower concentrations at a constant flux or generated as a single, high-concentration burst. We report here that manganous carbonate and manganous phosphate, under physiologically relevant conditions of concentration and pH, are each capable of catalytically removing superoxide from solution at rates that are competitive with those of the cellular SOD enzymes, regardless of whether the superoxide is generated at a slow constant flux or in a single high-concentration burst.
Results
What are suitable ligands for catalytic removal of superoxide by Mn? Mn phosphate complexes have been found as the major dialyzable compound from lysates of cells with Mn-related SOD activity (16, 17). Recent work provides evidence in whole cells directly showing a MnHPO4 complex; even more tantalizing is that its presence correlates with the ability of Mn to protect against superoxide-induced stress (8). Additionally, phosphate is a well-documented ligand in yeast and bacterial systems for Mn transport into cells and for Mn sequestering (18).
Citrate and carbonate share the characteristics of relatively strong binding affinity to Mn and relatively high concentrations in vivo. Carbonate is known to be a high-concentration component of human cells (∼10 mM), and citrate is a ligand that is widely present in vivo. Additionally, Stadtman et al. (19) have demonstrated that Mn carbonate is a catalyst of H2O2 disproportionation, and O2− is an intermediate in that reaction, which demonstrates that an interesting chemistry occurs between O2− and MnHCO3+. Finally, carboxylate and phosphate motifs are the most commonly available ligands for Mn in vivo, being found in amino acids and nucleotides.
Initially we sought to investigate Mn compounds using the well-established cytochrome c SOD assay in which xanthine oxidase and xanthine in air are used to produce O2− (20). In agreement with earlier work, we found that Mn concentration in a phosphate buffer inversely correlated with rate of cytochrome c reduction, which appeared to indicate that Mn was a SOD. Upon further investigation, however, we found that the ratio of reduced cytochrome c to oxidized cytochrome c was correlated with the amount of Mn used in the experiment regardless of whether we started with fully reduced cytochrome c, fully oxidized cytochrome c, or a mixture of the two (Fig. S1). These results indicated that cytochrome c could be oxidized by a species created during the experiment that was proportional to available Mn (not H2O2), a complication that made this an unsuitable assay for SOD activity of low molecular-weight complexes of Mn.
Rather than study the mechanism behind the anomalous oxidation of cytochrome c, we used a different system that would allow us to simulate the traditional SOD assay without its interferences. We chose 5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl) tetrazolium (MTS; structure in Fig. S2A) as the reporter ligand, which, unlike cytochrome c, is not reversibly oxidized. We generated O2− by 60Co γ-irradiation of oxygenated aqueous solutions containing 0.5 M ethanol, which produces a constant flux of O2− (21). Upon reaction with O2−, MTS forms the colored monoformazan (MF; ε480nm = 27,500 M−1⋅cm−1; Scheme 1 and Fig. S2) compound in a rapid disproportionation reaction (22). MF forms at a rate proportional to the rate of O2− formation, unless the O2− is scavenged by another reactant. Thus, the MF formation rate is inversely proportional to the concentration and rate of the competing reactant with superoxide.
Scheme 1.
Reduction of MTS by O2− to form MF. The reduction can be inhibited by SOD. MTS and MF structures in SI Text.
Experiments with Steady-State Conditions of Superoxide.
With Mn(II) citrate present, MTS was not immediately reduced by O2− because the O2− reacted instead with the Mn(II), leading to the initial flat portion in Fig. 1A. Once all of the Mn(II) citrate was consumed, i.e., after the O2− to Mn(II) ratio surpassed 1:1, MTS was then reduced by O2− and the formation of MF was observed. The 1:1 stoichiometry of [Mn(II) citrate]:[O2−] demonstrates that O2− was not catalytically removed (Fig. S2C for close-up of area near y = 0 in Fig. 1A).
Fig. 1.
MnHCO3+ catalyzes the disappearance of O2−, but Mn(II) citrate does not as measured by MTS reduction. O2− generated at 0.45 μM⋅s−1 by 60Co. [O2−] was determined by measuring MF formation. The arrows and symbols below the x axis show the point of stoichiometric equivalence between O2− and Mn(II). Solutions contained 0, 25, 50, or 100 μM Mn, 0.5 M ethanol, 150 μM MTS, and 50 mM citrate, pH 7 (A), or 50 mM carbonate, pH 8 (B).
In contrast, MnHCO3+ [Mn(II) carbonate is noted as MnHCO3+, but in solution it may appear as MnCO3; see Fig. S3 for speciation] was found to react catalytically with O2−. The rate of O2− reduction of MTS remained low, indicating that MnHCO3+quickly removed O2−. The negligibly small amount of MF formed well past the point of 1:1 of [Mn(II)]:[O2−] indicates that the reaction was catalytic.
The data in Fig. 2A provide evidence as to why Mn(II) citrate reacts stoichiometrically with O2−. When Mn(II) citrate was reacted with O2−, a species that was spectroscopically similar to Mn(III) appeared at λmax = 250 nm (open arrow), indicating oxidation of the Mn(II) citrate by O2−. Once all of the Mn(II) had been oxidized by a equal amount of O2−, the excess O2− then reacted with MTS to form the MF product at λmax = 490 nm (filled arrow). The absorbance at 250–350 nm was not due to reduced MTS, because reduced MTS has much less absorbance in this region than at 490 nm (Fig. S2D). The complete oxidation of Mn(II) by O2− is the basis for the stoichiometric reaction.
Fig. 2.
MnHCO3+ and MnHPO4 do not form an oxidized Mn final product when they react with O2− but Mn(II) citrate and MnP2O72− do. (A) Mn-citrate forms a Mn(III) product (open arrow), reduction of MTS (filled arrow) occurs only after Mn(III) oxidation. (B) MnHCO3+ did not form a Mn(III), the reduction of MTS (filled arrow). (C) MnP2O72− was similar to Mn-citrate (A). (D) MnHPO4 was similar to MnHCO3+ (B). Solutions were irradiated with 60Co to produce O2−. Solutions contained 50 μM MTS, 50 μM MnSO4, 0.5 M ethanol, 2 U/mL catalase, and 50 mM citrate (A), pyrophosphate (B), carbonate (C), or phosphate (D). All solutions were adjusted to pH 7 except carbonate (C), which was pH 8.7.
However, when MnHCO3+ was reacted with O2−, a small percentage of the O2− reduced the MTS, and no oxidized Mn species were observed (Fig. 2C). These measurements were made at time points significantly longer than the lifetime of MnOO+, thus there was no spectroscopic trace of the intermediate. These data indicate that MnHCO3+ catalytically cycles, because O2− was efficiently removed and no final stable Mn(III) product formed.
Similar experiments were performed for MnP2O72− and MnHPO4. Our previous work showed that MnP2O72− reacted stoichiometrically with O2− (15). As with Mn(II) citrate, we see here MnP2O72− becomes oxidized by one stoichiometric unit of O2−, as indicated by the growth of a peak at λmax = 260 nm (open arrow; Fig. 2B). MnHPO4 was shown previously to react catalytically with O2− (15), and in these experiments it acted similarly to MnHCO3+ (Fig. 2D). These data indicate that the mechanisms for MnP2O72− and MnHPO4 are similar to those for Mn(II) citrate and MnHCO3+, respectively. Comparison of the amount of MF formed in Fig. 2 C and D implies that O2− disappears more rapidly with MnHCO3+ than MnHPO4, an observation that was further investigated by fast kinetics.
Experiments with High Initial Concentrations of Superoxide.
Pulse radiolysis was used to generate bursts of O2−, and the changes in absorbance of any transients/final products were monitored between 225 and 375 nm as a function of time (21). This approach allowed for the direct determination of the rate constants and extinction coefficients of the intermediates. Superoxide formed within the first microsecond after the pulse (Fig. 3A, green triangles). The O2− disappeared in the presence of Mn(II) phosphate with the concomitant formation of intermediate 1 (Fig. 3A, purple squares). Under these conditions, this reaction occurs from 10−4 to 10−3 s. Intermediate 1 disappeared with the concomitant formation attributed to intermediate 2 (Fig. 3A, red circles).
Fig. 3.
Determination of rate constants and intermediate spectra by pulse radiolysis. (A) Spectra of different species in the reaction of O2− with MnHPO4. The absorption bands were measured immediately after the pulse (green triangles), 2 ms after the pulse (blue squares), and 20 ms after the pulse (red circles). The pulse radiolysis trace at 280 nm (B) and 230 nm (C) were made by piecing together a 20-ms and a 500-ms time-scale trace. The solution contained 50 mM phosphate, 50 μM MnSO4, 0.5 M ethanol, and ∼2.4 μM O2− (pH 7.0).
The changes in absorbance with time, as measured at both 280 and 230 nm, are shown in Fig. 3 B and C, respectively. Notice that at 230 nm (Fig. 3A) the extinction coefficient of the first intermediate is similar to that of O2−, so in Fig. 3C the formation of the first intermediate appears as a very small change in absorbance in the first 2 ms. At 280 nm, the extinction difference between O2− and the first intermediate is much greater, so the rate of formation of the first intermediate is much easier to measure accurately. In contrast, the absorbance at 280 nm on the 2- to 20-ms time scale does not change much, whereas the absorbance at 230 changes significantly in the same time scale. Using these data, the absorbance changes with time for both of these processes can be fit to using pseudo-first-order kinetics. On the 50- to 500-ms time scale, the absorbances at both 230 nm and 280 nm disappear by a process that is bimolecular.
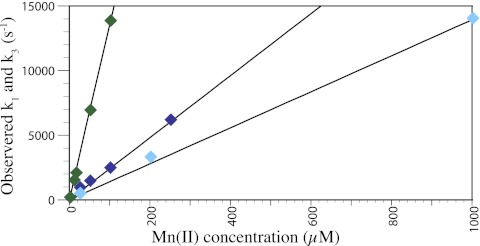
For Mn(II) successfully to sequester O2−, a complex must be formed that does not readily dissociate. Although Mn(II) sulfate and Mn2+(aq) react with O2− to readily form a MnOO+ intermediate, the rate of dissociation to Mn(II) and O2− was sufficiently large that free O2− was released back into the system at low concentrations of Mn(II) (12, 23). For a Mn(II) complex to act as a SOD, the forward rate must be large with respect to the back rate. Accordingly, the reaction of Mn(II) phosphate, citrate, and carbonate complexes with O2− show a lack of a large intercept when a straight line is fitted through the plot of observed rates vs. [Mn] (Fig. 4), which indicates that any back reaction is minimal relative to the forward reaction.
Fig. 4.
The forward reaction (pseudo-first-order) of Mn(II) phosphate (blue, k3), MnHCO3+ (green, k3), and Mn(II) citrate (cyan, k1) with O2− is fast, whereas the back reaction is slower, as determined by pulse radiolysis. Rates depend on initial [O2−] (1–10 μM). Solutions contained 0.5 M ethanol and 50 mM phosphate, carbonate, or citrate. All solutions were adjusted to pH 7, except carbonate, which was pH 8.7.
Fast kinetic experiments show that the reaction of Mn(II) citrate with O2− occurs with a mechanism that is analogous to the reaction of MnP2O72− with O2− (Scheme 2). At pH 7, the Mn(II) citrate reaction yielded an intermediate with a calculated rate constant for k1 = 1.3 × 107 M−1⋅s−1 and k−1 < 10 s−1. That intermediate then decayed to a final Mn(III) species with a rate of k2 = 2.0 × 103 s−1 (Fig. S4).
Scheme 2.
Proposed noncatalytic mechanism of O2− reaction with MnP2O72− or Mn(II) citrate. L, citrate/pyrophosphate.
As noted above, MnHCO3+and MnHPO4 also react with a sizable forward rate and a small back rate (MnHPO4 k3 = 2.4 × 107 M−1s−1 and k−3 = 10 s−1; MnHCO3+ k3 = 1.4 × 108 M−1⋅s−1 and k−3 = 7 s−1) (Scheme 3). The successive reactions of MnOO+ in phosphate or carbonate are more complicated then when citrate is the ligand, and each system is therefore discussed separately.
Scheme 3.
Proposed catalytic mechanism of MnHPO4 and MnHCO3+ disproportionation of O2. L, phosphate/carbonate. Anionn− is additional bound L.
Reaction Kinetics of Mn(II) Phosphate with Superoxide.
We found that the reaction mechanism of MnHPO4 with O2− was dependent upon multiple factors. The rate at which Mn(II) reacts with O2−, k3, was only dependent upon phosphate at phosphate concentrations <50 mM (Fig. S4). This effect is most likely due to the need for a minimum amount of phosphate to form the MnHPO4 complex (8).
The observed rates for k4, the reaction whereby a second intermediate is formed, showed a dependence on phosphate concentration (Fig. 5A). The rates for k4 increased in a linear manner with increasing phosphate concentration; i.e., phosphate is fit as a first-order reactant. Phosphate concentration below 10 mM was neglected due to the small percentage of MnHPO4 formed at that low phosphate concentration. We calculated the rate constant for k4 to be 3.2 × 103 M−1s−1 and k−4 to be 130 s−1 ± 60 s−1.
Fig. 5.
Kinetics determined by pulse radiolysis. (A and B) Dependence of k4 and k5 on [HPO42−]. (C and D) Dependence of k4 and k5 on [HCO3−]. Rates were fitted on initial [O2−] (1–10 μM). All solutions contained 0.5 M ethanol and 100 μM MnSO4, and phosphate solutions were pH 7 and carbonate pH 8.3.
The MnHPO4 complex catalyzes superoxide removal through the disproportionation of the MnOO+ complex. We studied the effects of phosphate concentration, Mn(II) concentration, and pH on k5, the second-order rate of disappearance of the second transient. In Fig. 5B, we demonstrate that at higher concentrations of phosphate there was significantly slower second-order decay. Because k5 is bimolecular, the measured rate is more affected by concentration than a first-order reaction, and the amount of the first intermediate present in the K4 equilibrium strongly affects the observed rate of k5. Thus, the effect of phosphate on k5 is likely due to shifting the equilibrium (K4) toward a greater percentage of the second intermediate and greatly slowing k5.
At higher pH, k5 decreases with an apparent equilibrium point at ∼pH 7.0 (Fig. S4). Speciation calculations showed that near pH 7 the dominant species is MnHPO4 (Fig. S3), which may indicate that the first intermediate is more likely HPO4MnOO− than PO4MnOO2−. Initial Mn(II) concentration did not alter k5 (Fig. S4) as predicted in Eq. 6 (from the rate law for Scheme 3; see SI Text, S1 for derivation, k−3 neglected), because Mn(II) concentration was always higher than O2−. The calculated rate with only MnOO+ dependence for k5 = 8.9 × 106 M−1⋅s−1.
Reaction Kinetics of Mn(II) Carbonate with Superoxide.
The MnHCO3+ system was also studied by pulse radiolysis, and the reaction was found to be mechanistically similar to that of MnHPO4 (Scheme 1), but with significantly different reaction rates. The experimental values for k3 were relatively independent of carbonate concentration (Fig. S4), but the value of k3 did fluctuate with changing pH (Fig. S4). Speciation calculations demonstrated that near pH 8, where the complex was studied, MnCO3 and MnHCO3+ are the dominant species (Fig. S3). That the reaction rate decreases with higher pH may indicate that MnHCO3+ is a better reactant than MnCO3. Also, loss of carbonate as carbon dioxide becomes a factor with lower pH.
At pH 8.3 (MnHCO3), k4 increases with increasing [HCO3−], analogous to the MnHPO4 mechanism (Fig. 5C). Treating HCO3− as a first-order reactant, k4 and k−4 were calculated to be 3.7 × 103 M−1⋅s−1 and 250 s−1 ± 30 s−1, respectively. The rate of reaction 4 decreased at higher pH. This inhibition may be due to ionic effects from the increase of HCO3− or to the higher stability of HOOMnHCO3+ compared with HOOMnCO3.
The calculated rate for MnHCO3+ k5 (1.5 × 106 M−1⋅s−1) was six-fold slower than that for MnHPO4 (Fig. 5D). As was the case with phosphate, increased carbonate had a deleterious effect on the second-order rate of decay of the MnO2+ intermediate. There was also a similar trend with increasing pH. The protons either encouraged the formation of the MnHCO3+ species over the MnCO3 species and/or led to an available proton to drive the formation of hydrogen peroxide from O2−.
Spectra of the Mn Intermediates 1 and 2.
Using pulse radiolysis, intermediates generated from the reactions of O2− with Mn(II) citrate, MnHPO4, and MnHCO3+ were found to have similar spectra (Fig. 6A). The λmax was found near 275 nm with extinction coefficients between 4,000 and 5,000 M−1⋅cm−1. The spectra are unlike the spectrum for O2− (λmax = 245 nm and ε245 = 2,250 M−1⋅cm−1) (24).
Fig. 6.
Spectra of MnOO+ intermediates are similar. (A) First intermediates and O2− (black line) spectra. (B–D) Second intermediates spectra. Pulse radiolysis was used to determine the extinction coefficient for each species at the indicated wavelengths for points in B–D. The dashed lines are Mn(III) samples made from Mn(III) acetate and 0.5 M carbonate or phosphate (C and D) or from 60Co irradiation (B). Pulse radiolysis: Mn(II) citrate (circles), MnHCO3+ (squares), and MnHPO4 (triangles), were made by adding 50 μM MnSO4 (MnHPO4 and MnHCO3+) or 200 μM MnSO4 [Mn(II) citrate] to 50 mM of the corresponding anion. All solutions contained 0.5 M ethanol and were pH 7 except MnHCO3+, which was pH 7.7.
The spectra of the intermediates formed by reaction 4 (phosphate and carbonate) and the product formed by reaction 2 (citrate) were found to be similar to those of the respective Mn(III) complexes. Pulse radiolysis was used to generate the spectra shown in Fig. 6 B–D (points), and the Mn(III) complexes of the aforementioned ligands were made by various procedures and the spectra measured (lines). With MnP2O72−, this second intermediate was shown previously to be Mn(III) pyrophosphate (15). The shapes of the spectra are consistent with those of the Mn(III) ion, with a characteristic absorbance peak near 450 nm (25), indicating the species are likely in the Mn(III) oxidation state. Based on the lack of kinetic dependence of H2O2, intermediate 2 appears to be a Mn(III)-peroxo.
Kinetic Modeling of Rate Constants.
Using Kintecus computer modeling software, we demonstrated that a physiological concentration of Mn(II) could substitute for physiological concentration of enzymatic SOD. Using rate constants determined in this work for Mn and those from Rotilio et al. (26) for yeast CuZnSOD (2 × 109 M−1⋅s−1), we show that with a mere 20- to 100-fold more metal ion (Mn:Cu), Mn(II) removes O2− at rates similar to CuZnSOD.
Burst conditions using an initial concentration of 25 μM O2− (other concentrations of O2− showed a similar pattern) were modeled in the CuZnSOD and in the two catalytic inorganic Mn systems. To compare the different systems, the amount of O2− removed by the competing uncatalyzed disproportionation reaction (5.4 × 106 M−1⋅s−1 at pH 7) was used as a standard, and concentrations of MnHPO4 or MnHCO3+ were selected so that the amount of O2− lost by self-disproportionation was equal to the amount lost self-disproportionation with 1 μM CuZnSOD (Fig. 7A). We calculated that 1 μM of CuZnSOD is necessary for normal growth (SI Text, S5). Our calculations are similar to those done by Anjem et al. (27), except that they neglect to take into account that organisms can survive with much less SOD than is normally expressed (28). A concentration of 91 μM of MnHPO4 or 25 μM MnHCO3+ was necessary to mimic 1 μM of CuZnSOD. The small amount of enzymatic SOD removed the O2− by quick catalytic cycling (Fig. 7B). At the chosen concentrations, Mn would quickly remove stoichiometric amounts of O2− to form the Mn intermediates. However, after the O2− is removed from the system, >99% of the Mn(II) ions would regenerate in <1.5 s.
Fig. 7.
Computer modeling of the catalytic superoxide dismutase activity of Mn(II) phosphate and carbonate. CuZnSOD (yellow squares) was modeled at 1 μM in all cases. The rate of autodismutation of superoxide at pH 7 was used as a competing reaction to determine noncatalytic reaction of superoxide (purple diamonds). (A and B) Superoxide was modeled as a 25-μM superoxide burst, MnHPO4 (blue circles) or MnHCO3 (green triangles) removed an amount of superoxide equal to that removed by 1 μM CuZnSOD. (C and D) Superoxide was modeled as a slow constant flux of 6.0 μM⋅s−1. [Mn] with 5 mM HPO42− or 5 mM HCO3− were chosen to have a steady-state [O2−] identical to that of 1 μM CuZnSOD.
Under physiological conditions, a relatively constant O2− generation rate is expected. We estimated this rate at 6.0 μM/s (SI Text, S5). As with burst conditions, the enzymatic system cycled to remove the O2−, but under these conditions the Mn systems were also cycling. The rate law derived in SI Text, S2 (which neglects k−3), gives similar solutions as the computer modeling (which takes all rates into account).
The steady-state [O2−] under conditions where O2− is only removed by uncatalyzed disproportionation is 750 nM (Fig. 7D). With 1 μM CuZnSOD, the steady-state [O2−] was 3.0 nM. Mn concentrations were selected so that the steady-state concentration of O2− was also 3.0 nM; 165 μM of Mn in 5 mM phosphate or 36 μM Mn with 5 mM carbonate was necessary. The estimated value for Mn phosphate is similar to the 115 μM MnHPO4 measured in yeast that was necessary to combat O2− stress (8), which reinforces the concept that this relatively low concentration of Mn in vivo is biologically significant.
Why is MnHCO3+ better than MnHPO4 at catalytically removing O2− even though the rate-limiting constant for k5 is much slower for carbonate system? The equilibrium constant for K3 is larger with MnHCO3+ than MnHPO4; therefore, the MnHCO3+ system is able to sequester the O2− fivefold faster than MnHPO4. As a result, a higher concentration of Mn carbonate intermediate is formed than Mn phosphate intermediate from the same amount O2−.
Discussion
Our interest in these systems was to shed light on the observed Mn rescue of yeast and the radiation resistance that seems to be a function in part of Mn accumulation in bacterial cells. We have been able to provide a mechanism that is consistent with these phenomena and that invokes biologically relevant ligands at biologically relevant concentrations. In the process we are able to corroborate an earlier suggestion that Mn can serve as a SOD (3, 16, 29). We conclude that Archibald and Fridovich (11) were entirely correct in their conclusion that Mn(II) could catalytically remove O2−. However, their claim that Mn at high phosphate concentrations and MnP2O72− could catalytically remove O2− were not correct because reduced cytochrome c, their marker of O2− concentrations, reacted with Mn(III) (Fig. S1).
The subsequent pulse radiolysis studies missed the catalysis by MnHPO4 because the experimental conditions used in that study required the use of phosphate concentrations >250 mM due to the presence of high concentrations of the competing ligand formate, which was present in the solutions to scavenge primary radicals (13). These high concentrations of phosphate would make the intermediates appear to be stable final products on a >10-s time scale.
What gives rise to the difference between the Mn(II) phosphate and carbonate systems, which are catalytic, and the Mn(II) pyrophosphate and citrate systems, which are not? Most likely the Mn(II) citrate and pyrophosphate systems dissociate H2O2 (during reaction 2), but the carbonate and phosphate systems do not. Even though all of the intermediates (reactions 2 and 4) appear spectroscopically similar to Mn(III), excess hydrogen peroxide has little effect on the observed rates of the reactions. That is, free H2O2 does not alter observed k4 and hence is not a reactant in k−4. Additionally, speciation results (Fig. S3) show that, near pH 7, the Mn(II) citrate and MnP2O72− complexes lack a dissociable proton, whereas the MnHPO4 and MnHCO3+ do have one. The role of carbonate and phosphate may be to stabilize the superoxo/peroxo intermediate through hydrogen bonding so that H2O2 does not dissociate to form a Mn(III) final product.
The second-order decay has been modeled as two MnOO+ intermediates interacting. This mechanism is the most likely, based on consideration of the alternatives. Another mechanism that would fit a second-order decay stems from the reaction of O2− with MnOO+, similar to the fast reaction of O2− with HO2. However, in pulse radiolysis experiments, the weak back reaction of MnOO+ in carbonate or phosphate would make the concentration of O2− low. Additionally, one would expect that at higher Mn concentrations (less free O2−) this reaction would be inhibited, but we saw little dependence of Mn(II) concentration on k5 (Fig. S4). A more likely alternative mechanism would be that the first intermediate formed reacts with the second intermediate formed. The anion concentration necessary to produce a 50/50 mixture of the first and second intermediates where the mixed reaction would be the fastest is ∼50 mM (SI Text, S4). Our experiments do not show this trend, but experiments <50 mM may be distorted by incomplete complex formation.
We calculated that the conditions in yeast could allow Mn to serve as a defense from superoxide in vivo. However, for this inorganic Mn SOD system to be active, an organism would require concentrations of anion high enough to ensure proper binding but low enough to inhibit the k4 reaction. This need for tightly regulated anion concentrations makes this system unwieldy in vivo, and is a possible explanation why enzymatic SOD evolved to be nearly ubiquitous in aerobic organisms.
Currently, MnHPO4+ seems to be the best prospect as the in vivo source of SOD activity at high Mn concentrations. Although Mn carbonate remains possible, in vivo levels of carbonate can fluctuate depending on growth conditions, whereas phosphate is more tightly regulated because there are multiple enzymes dealing with import and there is a storage system. Mn carbonate also can act as a catalase (19), yet in vivo Mn has been shown not to be a catalase (27), possibly indicating that Mn is not bound to carbonate in vivo. Additionally, Daly et al. (17) have implicated Mn phosphate as the likely species protecting proteins from oxidation from radiation in D. radiodurans. Finally, increased in vivo Mn phosphate has been found to correlate with the ability of mutant yeast to survive superoxide stress (8).
Materials and Methods
To measure the ability of Mn(II) complexes to act as a SOD at low constant superoxide fluxes, O2− was generated by 60Co irradiation of oxygenated 0.5 M ethanol solutions and detected by its reaction with MTS (Molecular Probes). The experiment was detailed previously (15). Briefly, 60Co irradiation of samples containing 0.5 M ethanol was used to generate a flux of O2−. γ-Irradiation from the 60Co source reacts with water, O2, and ethanol to create superoxide at a rate of 0.45 μM/s of superoxide. MTS reacts with O2− to ultimately yield 1/2 monoformazan (ε490 nm 27,500 M−1⋅cm−1) at a rate of 2 × 105 M−1⋅s−1 at pH 7 (22). In all cases, the concentration of MTS was kept sufficiently low so that the eaq− formed in the radiolysis of water reacted preferentially with O2. Solutions buffered with different anions (phosphate, citrate, pyrophosphate, and carbonate) were oxygenated for 10 min, and then 0, 25, 50, or 100 μM Mn(II) was added. The solution was then irradiated for the time required to generate the specified amount of O2−. Between 5 and 10 min after the irradiation, the absorbance was read at 490 nm or the entire spectrum was recorded on a Cary UV-VIS-NIR spectrophotometer. Each time point represents the average of three separate determinations.
Pulse radiolysis experiments were carried out using a 2-MeV Van de Graff generator at Brookhaven National Laboratory. Solutions were made to the desired pH by titrating with either strong base (NaOH) or strong acid (H2SO4). All solutions contained 0.5 M ethanol and were bubbled with O2 so that radicals formed by radiolysis would convert to O2− (21). (Carbonate solutions were oxygenated before adding concentrated carbonate buffer to inhibit loss of CO2.) After the radiolytic pulse, O2− and intermediates were monitored spectrophotometrically over time (2 μs to 10 s) using a deuterium lamp (235–400 nm) and a path length of 2 cm. Because the experimental setup allows only one wavelength to be monitored at a time, spectra were determined by performing a separate experiment for each wavelength. PR software, a program designed to record and analyze the pulse radiolysis data, was used to fit data and solve for the observed rate constants and the extinction coefficients. Measurements of rate constants and spectra are within 10% error except where noted.
Solutions of Mn(III) with different ligands were made from Mn(III) acetate as follows. Both Mn(III) phosphate and carbonate were made by a modification of published protocols. Five to 10 mg of Mn(III) acetate were mixed into 5 mL of 500 mM buffer (pH 7). The slurry was syringe filtered using a 0.22-μm filter into a quartz cuvette, and the spectrum was immediately determined by a Hewlett Packard model 8452 diode array spectrophotometer. The spectrum was measured shortly after the solution was made because of the tendency for these Mn(III) complexes to disproportionate to Mn(IV) and Mn(II). The concentration of Mn(III) was calculated from extinction coefficients of the same Mn(III) species formed by pulse radiolysis (12, 13).
Mn(III) citrate was formed by oxidation of Mn(II) citrate. Oxidation was carried out using superoxide formed by 60Co γ-irradiation of oxygenated 0.5 M ethanol solution. The spectrum of the Mn(III) species was then determined on a Cary UV-Vis-NIR spectrophotometer. The spectrum had the same λmax as previously reported (30).
Kinetic modeling was done using Kintecus software (v3.8). Chemical equilibria were calculated using CHEAQS (http://home.tiscali.nl/cheaqs/) software and binding constants from Table S1. The equilibria were calculated taking ionic effects into account.
Supplementary Material
Acknowledgments
We thank Prof. James J. Morgan of the California Institute of Technology for helpful discussions. This work was supported by National Institutes of Health Grant DK46828 (to J.S.V.). Radiolysis studies were carried out at the Accelerator Center for Energy Research at Brookhaven National Laboratory under Contract DE-AC02-98CH10886 with the US Department of Energy and supported by its Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203051109/-/DCSupplemental.
References
- 1.Daly MJ, et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 2.Chang EC, Kosman DJ. Intracellular Mn (II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J Biol Chem. 1989;264:12172–12178. [PubMed] [Google Scholar]
- 3.Archibald FS, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–1186. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 5.Al-Maghrebi M, Fridovich I, Benov L. Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch Biochem Biophys. 2002;402:104–109. doi: 10.1016/S0003-9861(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 6.Reddi AR, et al. The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic Biol Med. 2009;46:154–162. doi: 10.1016/j.freeradbiomed.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez RJ, et al. Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking CuZnSOD. J Biol Inorg Chem. 2005;10:913–923. doi: 10.1007/s00775-005-0044-y. [DOI] [PubMed] [Google Scholar]
- 8.McNaughton RL, et al. Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc Natl Acad Sci USA. 2010;107:15335–15339. doi: 10.1073/pnas.1009648107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YT, et al. Manganous ion supplementation accelerates wild type development, enhances stress resistance, and rescues the life span of a short-lived Caenorhabditis elegans mutant. Free Radic Biol Med. 2006;40:1185–1193. doi: 10.1016/j.freeradbiomed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Hessom JD, Srinivasan C. Using elevated temperature as an assay to determine the importance of various SODs and the life extending properties of various manganese salts in C. elegans. FASEB J. 2007;21:A1040–A1040. [Google Scholar]
- 11.Archibald FS, Fridovich I. The scavenging of superoxide radical by manganous complexes: In vitro. Arch Biochem Biophys. 1982;214:452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 12.Cabelli DE, Bielski BHJ. Pulse-radiolysis study of the kinetics and mechanisms of the reactions between manganese(II) complexes and Ho2/O2- eadicals. 1. Sulfate, formate, and pyrophosphate complexes. J Phys Chem-Us. 1984;88:3111–3115. [Google Scholar]
- 13.Cabelli DE, Bielski BHJ. Pulse-radiolysis study of the kinetics and mechanisms of the reactions between manganese(II) complexes and Ho2/O2- radicals. 2. The phosphate complex and an overview. J Phys Chem-Us. 1984;88:6291–6294. [Google Scholar]
- 14.Weiss RH, et al. Evaluation of activity of putative superoxide dismutase mimics. Direct analysis by stopped-flow kinetics. J Biol Chem. 1993;268:23049–23054. [PubMed] [Google Scholar]
- 15.Barnese K, Gralla EB, Cabelli DE, Valentine JS. Manganous phosphate acts as a superoxide dismutase. J Am Chem Soc. 2008;130:4604–4606. doi: 10.1021/ja710162n. [DOI] [PubMed] [Google Scholar]
- 16.Archibald FS, Fridovich I. Investigations of the state of the manganese in Lactobacillus plantarum. Arch Biochem Biophys. 1982;215:589–596. doi: 10.1016/0003-9861(82)90120-5. [DOI] [PubMed] [Google Scholar]
- 17.Daly MJ, et al. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS ONE. 2010;5:e12570. doi: 10.1371/journal.pone.0012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddi AR, Jensen LT, Culotta VC. Manganese homeostasis in Saccharomyces cerevisiae. Chem Rev. 2009;109:4722–4732. doi: 10.1021/cr900031u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stadtman ER, Berlett BS, Chock PB. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc Natl Acad Sci USA. 1990;87:384–388. doi: 10.1073/pnas.87.1.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 21.Bielski BHJ, Gebicki JM. Generation of superoxide radicals by photolysis of oxygenated ethanol solutions. J Am Chem Soc. 1982;104:796–798. [Google Scholar]
- 22.Sutherland MW, Learmonth BA. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radic Res. 1997;27:283–289. doi: 10.3109/10715769709065766. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen F, Holcman J, Sehested K. Manganese(II)-superoxide complex in aqueous solution. J Phys Chem A. 1997;101:1324–1328. [Google Scholar]
- 24.Bielski BHJ, Cabelli DE, Arudi RL, Ross AB. Reactivity of Ho2/O-2 radicals in aqueous-solution. J Phys Chem Ref Data. 1985;14:1041–1100. [Google Scholar]
- 25.Fackler JP, Chawla ID. Spectra of manganese(III) complexes. 1. Aquomanganese(III) ion, hydroxide, fluoride, and chloride complexes. Inorg Chem. 1964;3:1130–1134. [Google Scholar]
- 26.Rotilio G, Bray RC, Fielden EM. A pulse radiolysis study of superoxide dismutase. Biochim Biophys Acta. 1972;268:605–609. doi: 10.1016/0005-2744(72)90359-2. [DOI] [PubMed] [Google Scholar]
- 27.Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt PJ, et al. Multiple protein domains contribute to the action of the copper chaperone for superoxide dismutase. J Biol Chem. 1999;274:23719–23725. doi: 10.1074/jbc.274.34.23719. [DOI] [PubMed] [Google Scholar]
- 29.Archibald FS, Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981;146:928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klewicki JK, Morgan JJ. Kinetic behavior of Mn(III) complexes of pyrophosphate, EDTA, and citrate. Environ Sci Technol. 1998;32:2916–2922. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.