Abstract
Archaeological bones are usually dated by radiocarbon measurement of extracted collagen. However, low collagen content, contamination from the burial environment, or museum conservation work, such as addition of glues, preservatives, and fumigants to “protect” archaeological materials, have previously led to inaccurate dates. These inaccuracies in turn frustrate the development of archaeological chronologies and, in the Paleolithic, blur the dating of such key events as the dispersal of anatomically modern humans. Here we describe a method to date hydroxyproline found in collagen (∼10% of collagen carbon) as a bone-specific biomarker that removes impurities, thereby improving dating accuracy and confidence. This method is applied to two important sites in Russia and allows us to report the earliest direct ages for the presence of anatomically modern humans on the Russian Plain. These dates contribute considerably to our understanding of the emergence of the Mid-Upper Paleolithic and the complex suite of burial behaviors that begin to appear during this period.
Keywords: HPLC, accelerator mass spectrometry, Kostenki, Sungir
Radiocarbon dating of bone collagen routinely focuses on the extraction of bulk proteins that are then purified before radiocarbon measurement. However, the extracted bulk gelatin can be heterogeneous and include, or be cross-linked to, potential contaminants from the depositional environment, such as humic and fulvic acids, rootlets, cellulose, sediments, and other plant and animal remains including amino acids from bacteria and microorganisms (1, 2). For some samples curated in museums, additional contaminating compounds, such as glues, consolidants, and fumigants, can affect accurate dating, if not removed. In archeology, reliable chronologies are critical if an accurate picture of the human past is to be reconstructed. One area of pressing need in this respect is dating the spread of early anatomically modern humans out of Africa and into Europe and Eurasia. Direct dating of hominin fossils, as a means to assess the nature and timing of major demographic dispersals, Neanderthal extinctions, and admixture across Eurasia is usually based on radiocarbon dating and to a lesser extent optically stimulated luminescence (OSL) measurements. The radiocarbon method can be problematic, however, due to the difficulties associated with geological and museum-derived contamination, which become increasingly important as the ∼50-ky dating limit of radiocarbon is approached. Evidence suggests that perhaps ∼70% or more of the bone dates from the Middle and Early Upper Paleolithic are liable to be underestimates of the true age (3). The significance of this statistic should not be underestimated; its effect on our understanding of archaeological chronology has profound implications. Although the application of more rigorous ultrafiltration protocols has improved this situation recently (3), if the contaminants in bone are of high molecular mass, then they will not be removed using this technique.
Standard sample preparation protocols for dating bones generally follow an acid–base–acid (ABA) treatment, involving a decalcification step to mobilize hydroxyapatite, followed by a dilute NaOH or KOH wash that removes some humic and fulvic acids, followed by reacidification. After washing, the extracted collagen is usually gelatinized (solubilized) at pH 3 at temperatures ranging from 58 °C to 100 °C and filtered. Some laboratories apply ultrafiltration to remove low molecular mass material, retaining >30,000-Da peptides for dating (4). More elaborate approaches to dating compound-specific fractions from bone have been explored since the 1960s, most having focused on hydroxyproline (Hyp) because collagen is almost unique in nature in containing large amounts of this amino acid (5–15). One factor that makes them difficult to evaluate is that the amount of carbon derived from the laboratory protocols themselves was not reported. These approaches have not been widely adopted.
We have developed a protocol on the basis of preparative HPLC separation of amino acids hydrolyzed from bone collagen. Mixed-mode HPLC extraction of Hyp could prove very useful in dating bone with too little surviving collagen to be datable by the bulk collagen method. Analysis of bones of this type using our technique shows that it is possible to extract sufficient Hyp from a large enough sample of bone and thereby produce a radiocarbon determination where previously this had been impossible. Further application of the method to low collagen bones, as well as to highly contaminated ones, may result in reliable archaeological chronologies for parts of the world that have previously been impossible to effectively date.
We have applied the technique to a set of important anatomically modern human bones from the Early and Mid-Upper Paleolithic of Russia. These are bones that previously have proved impossible to reliably date due, it is thought, to the effects of museum conservation or to site-based organic contaminants.
Results and Discussion
Radiocarbon dating of Paleolithic bones has frequently resulted in severe underestimates of the real age, but direct dating of Neanderthal and modern human fossil remains is crucial to understanding the mechanics of the extinction of the former and the initial wide dispersal of the latter. Paleogenetic studies have shown that humans sharing haplogroup U characteristics dispersed into Europe (U5) and North Africa (U6 and M1), but dating of this diaspora is not certain (16). The Kostenki 14 (Markina Gora) human skeleton excavated near Voronezh, Russia (Fig. S1), is one of only three fossil human remains with a “complete” published mtDNA sequence (17) and it shows the five diagnostic substitutions defining haplogroup U2, present also in modern populations in Europe. Although the specimen is suspected of being Paleolithic in age, direct radiocarbon dates are much younger (∼3.7–13.6 kaBP) (18, 19). One indication of a problem with the collagen from the bone is that the C:N ratio is higher than expected (Table 1: values outside 2.9–3.5 are considered problematic). Pure biochemically characterized collagen has a carbon to nitrogen ratio of 3.21 (20). Values higher than this indicate exogenous carbon (Table 1). We extracted bone powder from the right tibia of the skeleton and attempted a new direct date using an ultrafiltration protocol but this again resulted in high C:Ns (3.8) and the date was not attempted. We then took 40 mg of the contaminated collagen and used the HPLC protocol described above to separate the Hyp fraction. The C:N ratio of the separated Hyp was 5.1, close to the theoretical value of 5.0. The resulting 1.2 mg graphite, produced for dating by accelerator mass spectrometry (AMS), yielded an age of 33,250 ± 500 yBP (Table 1 and Table S1). This date is significantly older than all previous determinations.
Table 1.
C:N atomic ratios and radiocarbon ages from Kostenki 14 (Markina Gora) skeleton (a tibia)
| Fraction/treatment | Laboratory code | C:N | 14C age, BP, ±1 σ error | Source |
| Gelatin | OxA-7126 | 3.7 | 4,750 ± 40 | (18) |
| Gelatin | GrA-9303 | NP | 3,730 ± 40 | (18) |
| Gelatin | SR-7366/UCIAMS-61666 | NP | 13,610 ± 40 | (19) |
| Ultrafiltered collagen | NA | 4.1 | Not dated | (17) |
| HPLC-separated Hyp fraction | OxA-X-2395-15 | 5.1 | 33,250 ± 500 | This paper |
Note the theoretical C:N ratio of Hyp is 5.0. NA, not available; NP, not published.
There is independent evidence for the age of the burial, based on the excavated sequence at Kostenki. The burial lies under cultural layer III, but no signs of a burial pit were observed from the level of this cultural layer. A. N. Rogachev (21, 22), the excavator, rejected any possibility for the burial to be attributed to cultural layer III, which is dated to ∼28.3–31.7 kaBP (19). The pit containing the body had cut through the volcanic ash horizon at the site, the Campanian Ignibrite (CI), which was clearly visible in the walls but absent from the burial fill (22, 23). The most probable context for the burial is thought to be with the “cultural layer in volcanic ash” of Aurignacian attribution (between cultural layers III and IVa). This level was unknown in 1954. The stratigraphic context and direct radiocarbon dates of material from the same cultural level therefore suggest that the age of the human must be at least 30 kaBP. Its maximum age is probably ∼35 kaBP, because of the presence of the CI tephra within and below cultural layer IVa [the CI is dated to ∼39.3 ka calibrated (cal) BP, which, based on the IntCal09 calibration curve, would be equivalent to ∼35 kaBP] (24). The radiocarbon date therefore fits perfectly into this chrono-stratigraphic schema.
At Sungir, another key Russian Upper Paleolithic site, discovered in 1955, several spectacular burials were excavated (25). The remains of eight individuals were found, buried and ornamented with ivory spears, bracelets, brooches, numerous ivory beads, and perforated fox teeth, attesting to the technical sophistication of its inhabitants. The cultural assemblage and the red ochre covering the skeletons imply strongly that the burials are related to the wider Mid-Upper Paleolithic cultures of European modern humans (26). Sungir is the northernmost of these Upper Paleolithic sites and has a Streletskian artifact assemblage, which comprises triangular bifacial points with concave bases and therefore suggests the site is a transitional cultural phase related to the previous Early Upper Paleolithic (EUP). An Aurignacian component in the lithic assemblage supports this EUP affiliation, albeit of a more recent manifestation. Direct radiocarbon dating of three of the skeletons was attempted previously in Oxford, Arizona, and Kiel (26–28), but the results were highly inconsistent, both between the laboratories and between the different individuals dated. The results ranged between 19,160 and 27,210 yBP and this wide variability has led to problems in placing the burials into their proper context. The relatively high C:N ratio of some of the samples (Table 2) again indicates that carbonaceous conservation material may have been applied to the bone, which the various chemistry pretreatment methods performed on the sample were unable to remove. It should be noted, however, that C:N ratios are not greatly sensitive to small amounts of exogenous carbon contamination so could mask potential problems. Dobrovolskaya et al. (28) provided two new determinations recently from Sungir 1 and 3, which they suggested were sufficient to establish the geological age of the skeletons at 26–27 kaBP because the results overlapped at 2 SDs (Table 2).
Table 2.
C:N atomic ratios and radiocarbon ages from Sungir
| Laboratory code | C:N | 14C age BP, ±1σ error | Source | |
| Sungir 1 | AA-36473 (vertebra fragment) | NP | 19,160 ± 270 | (27) |
| OxA-9036* | NP | 22,930 ± 200 | (26) | |
| KIA-27006† | 3.1 | 27,050 ± 210 | (28) | |
| Sungir 2 | AA-36474 (right side ribs) | NP | 27,210 ± 710 | (27) |
| AA-36475 (left side ribs) | NP | 26,200 ± 640 | (27) | |
| OxA-9037* | 3.5 | 23,830 ± 220 | (26) | |
| OxA-15753† | 3.3 | 25,020 ± 120 | NP | |
| OxX-2395-6 Hyp fraction | 5.0 | 30,100 ± 550 | This paper | |
| Sungir 3 | AA-36476 (rib fragments) | NP | 26,190 ± 640 | (27) |
| OxA-9038* | 3.4 | 24,100 ± 240 | (26) | |
| OxA-15751† | 3.2 | 25,430 ± 160 | NP | |
| OxA-15754† | 3.2 | 24,830 ± 110 | NP | |
| KIA- 27007† | 3.5 | 26,000 ± 410 | (28) | |
| OxX-2395-7 Hyp fraction | 5.0 | 30,000 ± 550 | This paper | |
| Sungir mammoth bone | OxA-9039* | 3.5 | 27,460 ± 310 | (26) |
| OxA-15752† | 3.1 | 29,640 ± 180 | NP | |
| OxA-15755† | 3.2 | 29,450 ± 180 | NP | |
| OxX-2395-8 Hyp fraction | 5.1 | 30,100 ± 400 | This paper |
Note that the theoretical C:N ratio of Hyp is 5.0. NP, not published.
*Samples pretreated with a gelatinization method.
†Samples ultrafiltered before AMS dating.
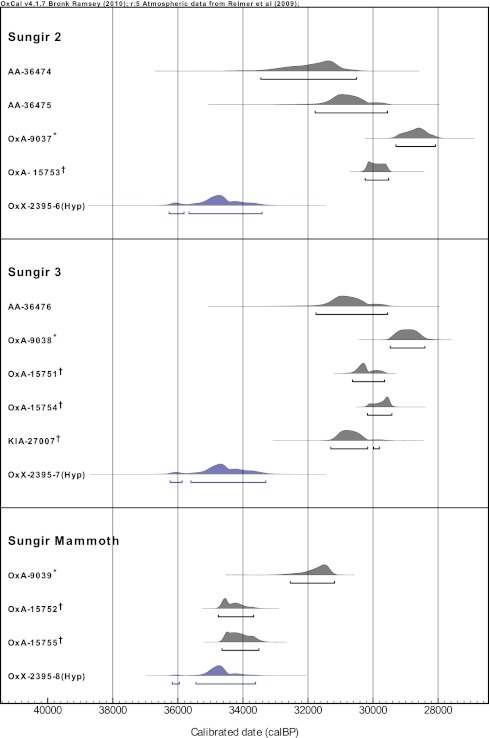
Hyp was separated from two samples of 30 mg collagen that had previously been extracted and dated. The samples came from the Sungir 2 and 3 individuals, both buried in the same grave. Unfortunately, there was not enough material remaining to redate Sungir 1 as well. In addition, Hyp was also extracted from a 30-mg collagen sample of a mammoth bone that came from the same occupation area of the site. The Sungir 2 and 3 Hyp fractions both yielded graphites of ∼0.7 mg. The Sungir mammoth yielded a graphite of 0.9 mg (Table S2). The new dates, shown in Table 2, are in close agreement and therefore consistent with a single event for the burials (Table S1). The fact that the two human dates are internally consistent provides some support for their accuracy because they are known to be contemporaneous burials and were interred together. Fig. 1 shows the calibrated ages for the Sungir samples. The new date for the Sungir burials, 30.1 ± 0.3 kaBP (between 34.1 and 35.2 ka cal BP) is appreciably older than previously assumed. Before this work, the earliest direct date for a Mid-Upper Paleolithic individual was ∼29,000 yBP for the “Red Lady” of Paviland in the British Isles (29). These dates may suggest an earlier onset in the beginnings of Mid-Upper Paleolithic technocomplexes and complex ritual burial behaviors in Eastern Europe compared with Western Europe, but more dating is required.
Fig. 1.
Calibrated radiocarbon dates (radiocarbon likelihoods) for Sungir 2, Sungir 3, and the Sungir mammoth bones, produced using OxCal 4.1 and the INTCAL09 calibration curve (30, 31). * denotes samples pretreated with a gelatinization method; † denotes samples ultrafiltered before AMS dating. Bulk calibrated dates range between ∼28 ky cal BP and 35 ky cal BP, but the three Hyp fraction dates all fall between 33.3 ky cal BP and 36.3 ky cal BP and can be combined with >95% probability to a single calibrated date range that falls between 34.1 and 35.2 ky cal BP. Interestingly, for the mammoth bone, the ultrafiltered bulk dates (OxA-15752 and OxA-15755) are very similar to the Hyp date, implying probably that this bone was not preserved and so the contaminant that made it appear more modern was effectively removed by the ultrafiltration. Sungir 2 and 3 probably had some preservation material that was not fully removed by the ultrafiltration, and only the Hyp method was able to date them accurately as implied by the fact that the three Hyp dates were the same.
The results demonstrate the potential problems that exist with direct dating of human remains using less rigorous pretreatment chemistries. Often these bones are those that are conserved in museums and collections and potentially contaminated with more modern carbon. The few human fossils dating to the Middle to Upper Paleolithic make it imperative that when direct dating is undertaken, reliable measurements can be ensured. In the case of Sungir and Kostenki the direct radiocarbon dates previously obtained are erroneous and should henceforth be set to one side by prehistorians. Our methodology provides a chemically characterizable, compound-specific molecule that eliminates the contamination that other methods cannot. Although in the majority of cases an ultrafiltration preparation is quite sufficient to decontaminate bones before AMS dating, our work shows that in some cases, where there is contamination and the samples are precious, the single amino acid method is the most optimal technique that can be applied.
Methods
The collagen is extracted by crushing ∼0.5 g cleaned bone (or more, depending on its collagen yield). It is then demineralized and gelatinized. The resulting collagen is hydrolyzed and finally separated into individual amino acids, using a mixed-mode HPLC separation method incorporating weak cation exchange combined with reversed-phase components combined in the same stationary phase. The amino acid retention times were identified using standards, and spiking natural samples confirmed these retention times in archaeological samples. Detection by UV absorbance (32–34) and LC/MS analysis of selected amino acid fractions was used to confirm identification of peaks by mass. It is crucial that laboratory-derived carbonaceous material be kept as close to zero as possible. Amino acids do not require derivatization and no organic solvents are used, to avoid adding carbon to the eluate (35). We have verified, to a precision of ±30 y, that independently isolated Hyp fractions from known-age bone give the correct 14C age, indistinguishable statistically from the bulk collagen age (Fig. S2). Independently isolated Hyp fractions from a 14C-free bone were all >41 kaBP (Fig. S3 and Table S1). The procedure background was calculated to be 3.3 ± 1.4 μg carbon, of which 1.5 ± 0.3 μg was modern and 1.8 ± 1.1 μg was 14C-free. These results demonstrate that the isolation of hydroxyproline does not add a significant background carbon to the radiocarbon measurement (SI Methods, Figs. S2–S4, and Tables S1, S3 and S4). The Hyp isolation procedural background has been quantified. This is an important requirement for validating the method before it can be applied to archaeological samples of unknown age. The Hyp method is dramatically different from most protocols used for dating bone and teeth today, as the date is produced from a specific, endogenous single molecule. In addition to the traditional pretreatment method, a preparative HPLC system with UV detection and an evaporator capable of removing several hundred milliliters of water-based eluent are required. The additional time required is mostly for sample hydrolysis (24 h) and separation and evaporation of the mobile phases (38 h). Different approaches have now shortened this significantly in our laboratory. After isolation of Hyp the graphitization procedure is the same as for bulk samples. The benefit of the compound-specific approach is the ability to effectively remove exogenous contamination from a wide range of samples, providing the potential for it to play a significant role in the expansion of more accurate archaeological chronologies.
Supplementary Material
Acknowledgments
We thank Alexandra Buzhilova (Institute and Museum of Anthropology, Lomonosov Moscow State University) for comments on the new dates for Sungir and also thank Christopher Bronk Ramsey, Angela Bowles, Barbara Emery, Jane Davies, Peter Ditchfield, Fiona Brock, and Martin Humm (Oxford Radiocarbon Accelerator Unit, Research Laboratory for Archaeology, University of Oxford) for their assistance. We are grateful to V. Khartanovich and V. Moiseyev (Kunstkamera Museum of Anthropology and Ethnography, Russian Academy of Sciences, St. Petersburg) for permission and help in sampling the Kostenki XIV skeletal remains. Thanks are also due to T. Balueva (Institute of Ethnology and Anthropology, Moscow) for helping with the sampling of the Sungir remains. The radiocarbon dates were financed by an Oxford Radiocarbon Accelerator Dating Service (ORADS) grant through the Natural Environment Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116328109/-/DCSupplemental.
References
- 1.Collins MJ, et al. The survival of organic matter in bone: A review. Archaeometry. 2002;44:383–394. [Google Scholar]
- 2.Hedges REM, van Klinken GJ. A review on current approaches in the pretreatment of bone for radiocarbon dating by AMS. Radiocarbon. 1992;34:279–291. [Google Scholar]
- 3.Higham T. European Middle and Upper Palaeolithic radiocarbon dates are often older than they look: Problems with previous dates and some remedies. Antiquity. 2011;85:235–249. [Google Scholar]
- 4.Bronk Ramsey C, Higham TFG, Bowles A, Hedges R. Improvements to the pretreatment of bone at Oxford. Radiocarbon. 2004;46(1):155–163. [Google Scholar]
- 5.Gillespie R, Hedges REM, Humm MJ. Routine AMS dating of bone and shell proteins. Radiocarbon. 1986;28:451–456. [Google Scholar]
- 6.Gillespie R, Hedges REM, Wand JO. Radiocarbon dating of bone by accelerator mass spectrometry. J Archaeol Sci. 1984;11(2):165–170. [Google Scholar]
- 7.Ho TY, Marcus LF, Berger R. Radiocarbon dating of petroleum-impregnated bone from tar pits at Rancho La Brea, California. Science. 1969;164:1051–1052. doi: 10.1126/science.164.3883.1051. [DOI] [PubMed] [Google Scholar]
- 8.Stafford TW, Jr, Brendel K, Duhamel RC. Radiocarbon, 13C and 15N analysis of fossil bone: Removal of humates with XAD-2 resin. Geochim Cosmochim Acta. 1988;52(9):2257–2267. [Google Scholar]
- 9.Stafford TW, Jr, Duhamel RC, Haynes CV, Jr, Brendel K. Isolation of proline and hydroxyproline from fossil bone. Life Sci. 1982;31:931–938. doi: 10.1016/0024-3205(82)90551-3. [DOI] [PubMed] [Google Scholar]
- 10.Stafford TW, Jr, Hare PE, Currie L, Jull AJT, Donahue DJ. Accelerator radiocarbon dating at the molecular level. J Archaeol Sci. 1991;18(1):35–72. [Google Scholar]
- 11.van Klinken GJ, Bowels AD, Hedges REM. Radiocarbon dating of peptides isolated from contaminated fossil bone collagen by collagenase digestion and reverse-phase chromatography. Geochim Cosmochim Acta. 1994;58:2453–2551. [Google Scholar]
- 12.van Klinken GJ, Hedges REM. Experiments on collagen-humic interactions: Speed of humic uptake, and effects of diverse chemical treatments. J Archaeol Sci. 1995;22:263–270. [Google Scholar]
- 13.van Klinken GJ, Mook WG. Preparative high performance liquid chromatographic separation of individual amino acids derived from fossil bone collagen. Radiocarbon. 1990;32(2):155–164. [Google Scholar]
- 14.Hare PE, Gil-Av E. Separation of D and L amino acids by liquid chromatography: Use of chiral eluants. Science. 1979;204:1226–1228. doi: 10.1126/science.36662. [DOI] [PubMed] [Google Scholar]
- 15.Abelson PH, Hoering TC. Carbon isotope fractionation in formation of amino acids by photosynthetic organisms. Proc Natl Acad Sci USA. 1961;47:623–632. doi: 10.1073/pnas.47.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soares P, et al. Correcting for purifying selection: An improved human mitochondrial molecular clock. Am J Hum Genet. 2009;84:740–759. doi: 10.1016/j.ajhg.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause JA, et al. A complete mtDNA genome of an early modern human from Kostenki, Russia. Curr Biol. 2010;20:231–236. doi: 10.1016/j.cub.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 18.Sinitsyn A. 2004. Les sépultures de Kostienki: Chronologie, attribution culturelle, rite funéraire. La Spiritualité [Les sépultures de Kostienki: Chronology, cultural attribution, funeral rites: the spirituality]. Actes du Colloque de la Commission 8 de l'UISPP (Paléolithique supérieur) (Liège, 2003), ed Otte M (Études et Recherches Archéologiques de l'Université de Liège 106, Liège, Belgium), pp. 237–244.
- 19.Hoffecker JF. The early upper Paleolithic of eastern Europe reconsidered. Evol Anthropol. 2011;20:24–39. doi: 10.1002/evan.20284. [DOI] [PubMed] [Google Scholar]
- 20.Ambrose SH. Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci. 1990;17:431–451. [Google Scholar]
- 21.Rogachev AN. Palaeolithic burial at the site Kostenki XIV (Markina gora) Sov Etnogr. 1955;1:29–38. [Google Scholar]
- 22.Rogachev AN. Multilayer sites of Kostenki-Borshchevo area on Don and the problem of cultural evolution on Russian plain in the Palaeolithic epoch. Mater Stud Archaeol USSR. 1957;59:9–134. [Google Scholar]
- 23.Sinitsyn AA. Kostenki 14 (Markina gora): Data, problems, and perspectives. Préhistoire Européenne. 1996;9:273–313. [Google Scholar]
- 24.De Vivo B, et al. New constraints on the pyroclastic eruptive history of the Campanian volcanic Plain (Italy) Mineral Petrol. 2001;73(1-3):47–65. [Google Scholar]
- 25.Bader ON, Bader NO. Homo Sungirensis. Upper Palaeolithic Man: Ecological and Evolutionary Aspects of the Investigation. Moscow: Nauchny Mir; 2000. [Google Scholar]
- 26.Pettitt PB, Bader NO. Direct AMS radiocarbon dates for the Sungir mid Upper Palaeolithic burials. Antiquity. 2000;74:269–270. [Google Scholar]
- 27.Kuzmin YV, Burr GS, Jull AJT, Sulerzhitsky LD. AMS 14C age of the Upper Palaeolithic skeletons from Sungir site, Central Russian Plain. Nucl Instrum Methods Phys Res B. 2004;223–224:731–734. [Google Scholar]
- 28.Dobrovolskaya M, Richards MP, Trinkaus E. Direct radiocarbon dates for the Mid Upper Paleolithic (eastern Gravettian) burials from Sunghir, Russia. Bull Mem Soc Anthropol Paris. 2011 10.1007/s13219-011-0044-4. [Google Scholar]
- 29.Jacobi RM, Higham TFG. The “Red Lady” ages gracefully: New ultrafiltration AMS determinations from Paviland. J Hum Evol. 2008;55:898–907. doi: 10.1016/j.jhevol.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Bronk Ramsey C. Bayesian analysis of radiocarbon dates. Radiocarbon. 2009;51:337–360. [Google Scholar]
- 31.Reimer PJ, et al. IntCal09 and Marine09 radiocarbon age calibration curves, 0-50,000 years cal BP. Radiocarbon. 2009;51:1111–1150. [Google Scholar]
- 32.Tripp JA, McCullagh JSO, Hedges REM. Preparative separation of underivatized amino acids for compound-specific stable isotope analysis and radiocarbon dating of hydrolyzed bone collagen. J Sep Sci. 2006;29(1):41–48. doi: 10.1002/jssc.200500247. [DOI] [PubMed] [Google Scholar]
- 33.McCullagh JSO, Juchelka D, Hedges REM. Analysis of amino acid 13C abundance from human and faunal bone collagen using liquid chromatography/isotope ratio mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2761–2768. doi: 10.1002/rcm.2651. [DOI] [PubMed] [Google Scholar]
- 34.McCullagh JSO, Marom A, Hedges REM. Radiocarbon dating of individual amino acids from archaeological bone collagen. Radiocarbon. 2010;52:620–634. [Google Scholar]
- 35.McCullagh JSO. Mixed-mode chromatography/isotope ratio mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:483–494. doi: 10.1002/rcm.4322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.