Abstract
What are the origins of humans’ capacity to represent social relations? We approached this question by studying human infants’ understanding of social dominance as a stable relation. We presented infants with interactions between animated agents in conflict situations. Studies 1 and 2 targeted expectations of stability of social dominance. They revealed that 15-mo-olds (and, to a lesser extent, 12-mo-olds) expect an asymmetric relationship between two agents to remain stable from one conflict to another. To do so, infants need to infer that one of the agents (the dominant) will consistently prevail when her goals conflict with those of the other (the subordinate). Study 3 and 4 targeted the format of infants’ representation of social dominance. In these studies, we found that 12- and 15-mo-olds did not extend their expectations of dominance to unobserved relationships, even when they could have been established by transitive inference. These results suggest that infants' expectation of stability originates from their representation of social dominance as a relationship between two agents rather than as an individual property. Infants’ demonstrated understanding of social dominance reflects the cognitive underpinning of humans’ capacity to represent social relations, which may be evolutionarily ancient, and may be shared with nonhuman species.
Keywords: cognitive development, naïve sociology, human evolution, social cognition, relational reasoning
Social relations have two key properties that distinguish them from other social entities. First, unlike social interactions, relations are stable across relatively long time periods and variable situations (1). Second, unlike individual dispositions, such as traits, relations apply over at least two individuals. We report four studies that investigated these two key properties in human infants’ representation of social dominance. Studies 1 and 2 focused on infants’ expectation of social dominance’s stability across time and situations. Studies 3 and 4 focused on whether infants represent social dominance as an individual property or as a relation.
In line with other approaches in biology (2), cognitive science (3), and social sciences (4), we define dominance as the tendency to prevail when one’s goals conflict with those of another agent. The goals of two agents “conflict” when the fulfillment of the goal of one of the agents would prevent the other agent from fulfilling her goal. For example, if individuals A and B both aim to acquire a resource, but only one of them can get it, then their goals conflict. Note also that the notion of “general tendency to prevail” does not specify the source of this capacity, and may map to notions, like “power” (4–6) or “social status” (7) in the literature. Dominance relations are characteristic to many animal species that live in social groups. In some species of nonhuman primates and in humans, individuals not only track who is dominant or subordinate to them, but also recognize the dominance relations of others. This capacity may appear early in human’s ontogeny and phylogeny (3, 8, 9).
Assumptions of stability are likely to play a crucial role in the monitoring of others’ dominance relations. They allow for (i) extracting dominance relations by observing social interactions, and (ii) applying dominance relations across situations. (i) Dominance relationships can be established on the basis of various cues (e.g., size, posture, physical aggression, age, and dominance and submission displays in nonhuman primates). These cues have some diagnostic value because they are causally related, in some way or another, to the capacity to prevail when two or more agents’ goals conflict. Another way to infer dominance relations is simply to look for its direct manifestations, by monitoring who prevails when two agents have conflicting goals. In these cases, the observation of a given interaction is used to infer a stable social relation. This type of process is likely to be important to assess the validity of cues of dominance. For example, in nonhuman primates, cues such as size or age are sometimes misleading, because young and small individuals may achieve a high dominance position through their social alliances (2, 8). Tracking who prevails when agents have conflicting goals may provide more reliable information about dominance relations than agents’ size or age in the long term. (ii) To use social relationships efficiently, one also has to have expectations about their effect on social interactions. Forming these expectations for novel situations entails the assumption of stability through time, and consistency across situations, of dominance relationships.
Studies 1 and 2 tested whether human infants (i) track which individuals prevail against others in the face of conflicting goals, and (ii) use this knowledge as a basis for inferences about the outcomes of future conflicts. We hypothesized that infants’ understanding of goal-directed actions might be sufficient to identify situations in which agents’ goals conflict: Infants can predict actions on the basis of goals and have some understanding of constraints limiting agents’ actions (10). They also have some sensitivity to agents’ failures in achieving their goals (11). These abilities might allow infants to track who prevails when the goals of two agents conflict.
Studies 3 and 4 investigated the format of infants’ representation of social dominance. The format of a representation specifies how the information it represents is rendered, meaning what aspects of the information are made explicit in the representation. It determines what stands for what (e.g., specifying a set of symbols and a set of rules for combining these symbols). These studies thus focused on what may be described as the algorithmic level (12) or syntactic aspects (13) of dominance processing.
There are two likely candidates for the representational format of social dominance. It may be treated as a (i) stable individual property organized on an ordered scale, similar to the ones one may use to measure height, for example. In this view, a dominance level would be attached to each individual, and the dominance relation between two agents can be directly read off this scale by comparing their ranks. Alternatively, dominance may be represented as a (ii) relationship, organized into pairs of agents with a directed, asymmetric relation between them. In this view, representations of dominance would apply over at least two individuals, and to the nature of the relation that unites them.
In all studies, infants watched computer animations of interacting geometrical figures to make sure that no familiarity with the agents or with their behavior could be used to assess dominance relationships. Rather, infants had to rely on the agents’ relative success and failure in goal-directed actions during a conflict situation. Moreover, we used a test in which two agents had conflicting goals (both wanted the same object), but did not engage in an actual physical conflict or in a direct competition for that object. Rather, one agent took the object, and the other one “let” her obtain it. As a result, our test did not simply display a situation in which one individual outcompeted the other. It is rather that the dominant prevailed and the subordinate deferred without the two of them engaging in genuine competition (see e.g., refs. 2 and 14).
Study 1
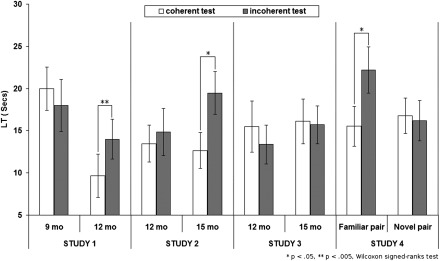
We presented 9- and 12-mo-old infants with short animations depicting the actions of two agents. First, the “subordinate” agent was seen collecting small objects. Then the “dominant” agent entered and started to collect objects while the subordinate one let it succeed (Movie S1). This familiarization demonstrated who prevailed when the two agents' goals conflicted. During the test, the same agents collected objects of a new type, first alone, then competing for the same one. In the “coherent” test movie, the dominant agent took the last object while the subordinate agent backed off; in the “incoherent” test movie, the roles were reversed. Infants’ looking time at the screen was measured from the moment when one of the agents took the last object (Movie S2). The results of studies 1–4 are reported in Fig. 1.
Fig. 1.
Mean looking time (SEM) to the incoherent and to the coherent test events per group and per study.
A three-way ANOVA was performed on looking times with within-subject factor of test coherence (coherent vs. incoherent) and between-subject factors of age (9- vs. 12-mo-old) and order of test trials. A main effect of age was found [F(1,28) = 5.82, P = 0.023]: 9-mo-olds looked overall longer at the test movies than 12-mo-olds. We also obtained a main effect of order [F(1,28) = 6.09, P = 0.02] as infants looked overall longer at the tests when the incoherent movie was presented first than when the coherent movie was presented first. More central to our research question, a significant interaction between age and test coherence [F(1, 28)= 9.2, P = 0.005] indicated that 9- and 12-mo-olds reactions to the tests’ coherence differed from each other. Planned follow-up tests indicated that consistency between familiarizations and test movies had no significant effect on 9-mo-olds’ looking times [19.95 vs. 17.98 s; t(15) = 1.1, P = 0.288; P = 0.44 by Wilcoxon signed ranks test]. In contrast, 12-mo-olds looked significantly longer at the incoherent than at the coherent test movies [9.67 vs. 14.01 s; t(15) = 3.49, P = 0.003; P = 0.017 by Wilcoxon signed ranks test].
These results demonstrate that 12-mo-olds, but not 9-mo-olds, expect that the agent who prevailed against a certain opponent when getting one kind of object should succeed again in obtaining a novel kind of object. The interpretation of the negative results with 9-mo-olds can only be tentative. Interestingly, in studies of infants’ use of size to infer dominance relationships (3), a similar developmental pattern was found: Infants performed above chance only from the age of 10 mo. More generally, in studies on infants’ expectations about social interactions, no positive result was found before 9 mo of age (15–17). At the moment, it is unclear whether young infants fail in these tasks for methodological reasons, or because they lack the capacity to track individuals (18) or to process social relationships. It is nonetheless noteworthy that the available evidence points toward a development of the capacity to track social relationships when children start locomoting autonomously, hence may begin to interact more with people who are not their caregivers.
Twelve-month-olds' success in study 1 suggests that they attribute some stability to the social relations between the observed agents, and this attribution survives certain situational changes. A simple association between objects and agents cannot explain 12-mo-olds' expectations because the objects used in the test and in the familiarization were different. However, because of the similarity between the familiarization and the test situations, infants may have used simple strategies. For example, they may have built up a rule such as “when agents A and B are present, agent A gets the object.” Note that this type of rule processing would still allow infants to predict the outcome of conflicts in a restricted range of new situations. It would however, have a limited power of generalization. By investigating infants' capacity to form expectations for a completely new situation, in study 2 we controlled for the fact that infants might extract local rules to predict the outcome of conflicts.
Study 2
Twelve- and 15-mo-old infants watched familiarization events in which the agents did not collect objects but competed to stay in a little area, the boundaries of which were delimited by walls. First, the subordinate agent entered the area alone. Then the dominant agent arrived and monopolized the little area by repeatedly pushing the subordinate agent away (Movie S3). Infants then watched the same test movies as in study 1.
A three-way (coherence, age, and order of test trials) ANOVA on the looking times did not reveal any main effect or interaction. However, planned tests revealed distinct patterns in each age group. Twelve-month-olds did not look significantly longer at the incoherent than at the coherent test movies [13.48 vs. 14.84 s; t(15) = 0.232, P = 0.82; P = 0.92 by Wilcoxon signed ranks test], whereas 15-mo-olds did [12.66 vs. 19.47 s; t(15) = 2.36, P = 0.032; P = 0.028 by Wilcoxon signed ranks test].
Although 12-mo-olds display some expectations of stable social relations in conditions that require very little generalization (study 1), they do not extend their expectations across two completely different kinds of situations (study 2). Their difficulties can be explained in many ways: limited powers of generalization, difficulties in determining the agents’ goals during test, or a need for more evidence to generalize dominance from one situation to a different one. Our data cannot distinguish among these accounts.
In contrast, 15-mo-olds form expectations from one situation and apply them to a completely different one. This result suggests that 15-mo-olds (i) recognize and memorize who prevailed when two individuals had conflicting goals, and (ii) use this information to predict the outcome of future interaction. (i) Fifteen-month-olds’ recognition of dominance relationships in study 2 is noteworthy for several reasons. First, it suggests that infants can extract dominance relationships on the basis of social interaction alone, in the absence of stable physical differences that may be used to establish dominance relationships such as size or age (for similar results in nonhuman animals, see refs. 19 and 20). Second, in our study, agents do not display any behavior that may intentionally communicate or may have evolved to signal dominance relationships (such as dominance or submission displays). Rather, it is likely that infants use the outcome of the observed conflict situation to establish the dominance relationship between agents. To track who prevails, 15-mo-olds need to process the completion of goals by assessing the match between attributed goals and what they observe. (ii) Fifteen-month-olds form expectations on the basis of the social relationships evidenced during familiarization. Importantly, in the test of study 2, 15-mo-olds recognize the conflict between agents’ goals, even if there is no physical conflict or signal of aggression. This capacity may be important in real social interactions. In many situations in which people’s goals conflict, no physical violence is involved (e.g., from preschool age, many of these situations are negotiated through communication; ref. 21). Moreover, the test of study 2 did not measure infants’ expectation of a stereotypical response from the dominant (aggression) or from the subordinate agent (signal of subordination, flight). Rather it required the ability to process the particular goals that the two agents were trying to achieve.
The results of study 2 thus suggest that 15-mo-old infants represent social dominance in an abstract manner, allowing them to generalize their expectations from one situation to another. Two types of representational format would enable infants to perform this type of generalization. One possibility is that infants represent dominance as a stable individual property of agents (e.g., “agent A is dominant,” “agent B is not dominant”). In this case, after observing agent A being dominant over agent B, infants may come to expect that A may be dominant over another, unknown agent, C, by virtue of A’s individual properties. Alternatively, infants may represent dominance as a relationship characteristic to a particular pair of agents (e.g., “A is dominant over B”). According to this second hypothesis, expectations of dominance should be modulated by whom the agent is confronted. In this view, after observing A being dominant over B, infants should not extend their expectations of dominance when A is facing a novel, unknown agent C, because they would not know the nature of the relations between A and C. Study 3 was designed to distinguish between these two possibilities.
Study 3
We tested 12- and 15-mo-old infants in procedures similar to the ones in which they discriminated coherent and incoherent tests above chance level. Twelve-month-olds were tested with a procedure similar to the one of study 1, and 15-month-olds were tested with a procedure similar to the one of study 2. The only difference from the previous studies was that in study 3, the subordinate agent of the familiarization was replaced by a new agent during the test (Movies S4 and S5). For example, if an infant saw a blue disk dominating a black pentagon in familiarization, (s)he then saw the blue disk facing a red triangle during the test. The agent who was dominant during familiarization remained dominant during the test (i.e., collected the last object) in the coherent sequences of movies, and deferred (i.e., let the new agent collect the last object) in the incoherent sequences of movies.
A three-way (coherence, age, order) ANOVA on the looking times did not yield any main effect or interaction. Moreover, planned tests indicated that 12- and 15-mo-olds did not significantly differentiate between the coherent and incoherent test movies [15.50 vs. 13.37 s; t(15) = 0.229, P = 0.82; P = 0.68 by Wilcoxon signed ranks test for 12-mo-olds; and 16.11 vs. 15.69 s; t(15) = 0.400, P = 0.695; P = 0.88 by Wilcoxon signed ranks test for 15 mo-olds]. To compare infants’ performance in study 3 to studies 1 and 2, repeated-measures ANOVAs were performed with test coherence as a within-subject variable and familiarization movie type (study 1 vs. study 3 for 12-mo-olds, and study 2 vs. study 3 for 15-mo-olds) as a between-subject variable. These analyses showed no main effect of familiarization movie type on infants' looking time during the test, either for 12-mo-olds [F(1,30) = 0.83, P = 0.37], or for 15-mo-olds [F(1,30) = 0.001, P = 0.98], indicating that infants did not look significantly longer at the test movies involving only familiar agents (studies 1 and 2) than at test movies involving a new agent (study 3). However, we found a significant interaction between familiarization movie type and test coherence both for 12-mo-olds [F(1,30) = 12.77, P = 0.001] and for 15-mo-olds [F(1,30) = 4.3, P = 0.047]: Coherence had a stronger effect on infants’ looking times when the previously dominant agent faced an agent that it dominated before (studies 1 and 2), than when it faced a novel agent (study 3).
Infants can form expectations about the test situation by tracking who prevailed in the familiarization (as shown by studies 1 and 2). However, they do not overextend their expectations to a new situation in which the dominant agent faces a new agent. This result raises the possibility that young infants might represent dominance as a social relation between two or more individuals rather than as an individual trait or property. Study 3 rules out the possibility that infants might attribute a universal disposition of dominance to the agent who prevails during the familiarization of studies 1 and 2. However, it does not rule out entirely the possibility that infants might represent dominance as an individual property. In particular, infants might attribute individual dominance ranks to agents and organize them on an ordered scale (such as an ordinal, interval, or a ratio scale). For example, during familiarization, infants might attribute a high level of dominance to the individual who prevails and a low level of dominance to the individual who is subordinate. The new individual, who has never been seen competing with another agent, might be seen as having an intermediate level of dominance, or her level of dominance might remain unknown. This hypothetical scenario would explain why infants develop expectations in the test when the previously dominant agent faces a previously subordinate agent (studies 1 and 2), but not when it faces a novel, unknown agent (study 3). In study 4, we controlled for this possibility.
Study 4
Animals and, arguably, humans form dominance structures that tend often to be linear, in the sense that the alpha individual dominates all of the others, the beta individual dominates all individuals but alpha, and so on (22–24). Moreover, representing and memorizing power structures that have some degree of linearity may offer some cognitive benefits (25, 26). Nonetheless, humans do not necessarily represent social dominance as an individual rank organized on an ordered scale. Ordered scales can only represent strictly linear dominance structures, which may lead to inaccuracies. In many animal species, including nonhuman primates, the linearity of dominance structures may vary depending on species, sex, group, or environment (e.g., refs. 27 and 28). In humans, similarly, there is a variety of social structures, some of which may entail dominance structures that depart from perfect linearity (29, 30). We thus assume that humans may expect dominance structures to have some degree of linearity (and, thus, may expect dominance relations to have some degree of transitivity). However, this expectation of linearity need not be strict and absolute. If dominance was systematically represented as an individual property organized on an ordered scale, humans would have no choice but to represent dominance structures as strictly linear. Representing dominance as a relation offers more room for flexible representation of social dominance structures that depart from linearity. This hypothesis predicts that there are cases where humans represent that A is dominant over B, and B is dominant over C, without necessarily assuming that A is dominant over C.
In study 4, infants were presented with two relationships: Agent A was subordinate to B, and B was subordinate to C. If infants represent dominance as an individual property organized on an ordered scale, they should construct a linear hierarchy with A at the bottom, B in the middle, and C at the top. In this view, infants’ highest expectations should be found when the bottom-ranking individual (A) competes with the top-ranking individual (C). Alternatively, if infants treat dominance as a social relationship, they may track the relationships between A and B, and between B and C, without necessarily inferring any relationship between A and C (see also SI Discussion for more discussion of the computational consequences of representational formats).
We tested two groups of 15-mo-olds in a procedure similar to that of study 2. During familiarization, agent B monopolized access to a target area by pushing agent A away and then yielded the area to agent C (Movie S6). During the test, infants were presented with two agents competing for small objects. These two agents formed a familiar pair for one group of infants (A and B, or B and C; “familiar pair” condition) and an unfamiliar pair for the other group (A and C; “novel pair” condition).
A two-way ANOVA on looking times with test coherence (coherent vs. incoherent) as a within-subject factor, and test pair in the familiar pair condition (A and B vs. B and C) as a between-subject factor showed no interaction between test coherence and test pair [F(1,14) = 0.36, P = 0.56]. Thus, infants tested on the relation between the first pair of agents (A and B) and those tested on the second pair of agents (B and C) did not react differently to the coherence of the test. These two groups were then collapsed together into a single familiar pair condition. A three-way (coherence, condition, order) ANOVA yielded a main effect of order [F(1,28) = 4.60, P = 0.041], suggesting that infants looked overall longer at the tests when the incoherent test was presented first. Moreover, there was a significant interaction between condition (familiar vs. novel pair), and test coherence [F(1, 28) = 4.40, P = 0.045], indicating that infants in the familiar-pair condition reacted more strongly to the coherence of the test than infants in the novel pair condition. Indeed, planned tests indicated that infants in the familiar-pair condition looked significantly longer at the incoherent than at the coherent test movies [15.52 vs. 22.21 s; t(15) = 2.69, P = 0.017; P = 0.014 by Wilcoxon signed ranks test], whereas infants in the novel-pair condition did not [16.76 s vs. 16.20 s; t(15) = 0.441, P = 0.665; P = 0.8 by Wilcoxon signed ranks test].
Our results are inconsistent with the idea that infants represent dominance as an individual property organized on an ordered scale and derive their expectations by comparing individuals’ dominance rank. If they had done so, infants’ expectations should have been as strong (or stronger) for the novel pair condition (in which the difference between the agents’ ranks would have been the largest) than for the familiar pair condition. The data significantly departed from this pattern, thus falsifying this hypothesis. Conversely, our results are consistent with the proposal that infants’ representation of social dominance apply over pairs of agents. Thus, the results of studies 3 and 4 suggest that initially infants represent dominance as a set of relations between agents.
Our results are relevant for the study of representation of social structures, i.e., networks of relations involving at least three individuals. Infants’ performance in the familiar-pair condition shows that 15-mo-olds have the capacity to memorize two relations simultaneously, even when one agent assumes a different role in each relation (B is dominant over A and subordinate to C). Conversely, our results say little about infants’ capacity to transitively infer dominance relations. In study 4, we found no evidence of transitive inferences drawn from the relation between A and B, and between B and C, to a relation between A and C (see SI Discussion for more discussion of the relation between the capacity to draw transitive inferences and our data).
Discussion
The representation of social dominance in human infants evidenced by our results may be appropriately described as belonging to a “naïve sociology” (31–33). It has a conceptual nature and, thus, forms part of an abstract representation of social life. It involves the representation of interactions and relations between goal-directed agents and not just of individual actions. It is represented using a particular format, as a relation between two agents, and not as an individual trait.
This concept posits entities that cannot be described by using a purely spatiotemporal or sensory vocabulary: social interactions such as conflicts between goals, or social roles such as dominant or subordinate. It also supports inferences that go well beyond what is perceived. In our studies, infants expect agents’ relationships to remain stable, even when they no longer perceive the situations that allowed them to recognize the relationships. On the basis of these inferred relations, they form expectations that are abstract enough to be applicable to situations in which the conflicting goals are new.
Human infants’ sensitivity to social dominance appears to be rooted in the naïve psychology of actions, because it appeals to the recognition of agency, goals, and actions. Nonetheless, infants expect that agents' tendency to prevail when their goals conflict with those of others has some stability across different goals. This property is characteristic of a social relationship and is hard to infer just by recognizing the individual goals of agents. Infants thus give a meaning to agents’ interactions that goes beyond what can be established by merely determining what goals agents pursue. They recognize interactions and relations between goal-directed agents—entities that go beyond the mere understanding of individuals’ goals. The precise content of this relation is yet to be determined: It can range from more or less specific notions of influence (such as “A’s actions create constraints for B’s actions”), to notions of prevalence (such as “A prevails over B”), or even to notions close to deference (such as “A gives way to B”). Whatever this precise content, it is “social” in the minimal sense that it includes some reference to the relation between two different goal-directed agents.
Infants’ representation of dominance cannot be reduced to descriptions that include only one individual. In particular, infants’ expectations of stability cannot be accounted for by attribution of individual competence, defined as the capacity of individuals to achieve their goals. Had infants performed such attribution, they should have expected agents’ relations to be transitive in study 4. It thus seems likely that infants represent dominance as a relationship, and our results can be taken as evidence of attributions that characterize not individuals, but groups of individuals, and their interactions. Combined with studies on the representation of affiliation (34), these results thus suggest that by their second year of life, infants represent relations between pairs of individuals.
Whether the characteristics of humans’ intuitions about social dominance identified here are also present in nonhuman primates is an important question for future research. The documented sensitivity to observed dominance relationships in many nonhuman primates species (8, 35) and in young infants raises the possibility that some of the mechanisms dealing with dominance may have evolved in common ancestors to human and present-day nonhuman primates and may be shared by nonhuman species.
In nonhuman animals, deference can have various sources, such as the threat of force, but also genes (when deferring to a kin), fertilizable eggs, or potential social support (2). In humans, the range of sources of deference may be even wider, including almost everything that can serve to sanction or reward others: physical coercion, but also wealth, connections within social networks, valued knowledge, or skills. Additionally, humans may not only defer to others to avoid sanctions or gain rewards, but also because they think that submission is legitimate for moral or other reasons in the given context (36, 37). How young humans come to understand these different sources of social dominance relations is an important question for future research.
Materials and Methods
Participants.
For each study, we recruited 16 infants for each age group and condition (see SI Materials and Methods about excluded infants). In study 1, we had 9-mo-olds (mean age = 247 d; range = 236–258 d) and 12-mo-olds (mean age = 377 d; range = 367–392 d); in study 2, we had 12-mo-olds (mean age = 381 d; range = 367–387 d) and 15-mo-olds (mean age = 471 d; range = 462–478 d); in study 3 we had 12-mo-olds (mean age = 377 d; range = 366–393 d) and 15-mo-olds (mean age = 467 d; range = 453–487 d); and in study 4, we had two groups of 15-mo-olds [familiar-pair conditions: (mean age = 471 d; range = 459–484 d; novel-pair condition: mean age = 466 d; range = 453–477 d)].
Stimuli and Procedure.
We created animations depicting two kinds of goal-directed actions: collecting objects (used as familiarization in study 1 and for the 12-mo-olds in study 3) and occupying a small marked area (used as familiarization in all of the remaining conditions). The objects to be collected became available one-by-one, and the marked area had enough space for only one agent, so both situations presented a conflict when two agents wanted to achieve the same goal. The agents were colored geometrical figures (e.g., a blue circle, a red triangle, a black pentagon) with rudimentary facial features and were varied and counterbalanced across dominance roles and conditions. During familiarization, first the subordinate agent was seen achieving her goal three times before the dominant agent entered and succeeded in collecting the objects or in occupying the marked area three times while the subordinate agent withdrew.
The test phase was the same for all studies. First, the two contrasted agents were seen to achieve their goals (object collection in all studies) alone, and then both agents approached the same object, as if both wanted to obtain it. They stopped moving at the same distance of the object before touching it. Then, one of the agents took the object and pushed it to her side. The other agent simply moved back to her side without the object. From this point, the movie froze. In the coherent test movie, the agent who was dominant (or may have been thought of as dominant) in the familiarization took the last object. In the incoherent test movie, the other agent took the last object. Infants’ looking time at the screen was measured from the moment one of the agents took the last object, up to the point the infants looked away for >2 s, or after 40 s elapsed since the beginning of the measurement.
In all studies, there were two familiarization trials and two test trials per subject, presented in the following order: Familiarization-Test-Familiarization-Test. Each child was presented with a coherent sequence (a familiarization followed by a coherent test), and with an incoherent sequence (a familiarization followed by an incoherent test) with the order of presentation counterbalanced across participants. Two different sets of geometrical shapes (representing agents) and backgrounds were used, one for each of the sequences. The association between one set of agent/background and the coherence of the sequence was counterbalanced across infants. For each sequence, whether the dominant agent was on the left or on the right side of the screen was also counterbalanced across participants.
Coding and Data Analysis.
The same coding and data analysis procedure was used in studies 1, 2, 3, and 4. Inclusion criteria and details about coding are given in SI Materials and Methods. All statistical tests used in this paper were two-tailed. Preliminary analyses indicated that data departed from normality in the coherent and incoherent conditions of study 1 for 12-mo-olds (respectively, W = 0.75, P < 0.01 and W = 0.86, P < 0.05, Shapiro–Wilks tests). Subsequently, parametric statistics were performed throughout all of the studies on log-transformed data to better approximate a normal distribution. For ease of reading, the means before log-transformation (in seconds) are reported on Fig. 1. The means for the log-transformed data are reported in Table S1. For the effects of main interest, nonparametric statistics are also reported.
Supplementary Material
Acknowledgments
We thank the families who participated in this study; Borbala Kollod, Agnes Volein, and Maria Toth for helping with data collection; all the members of the Cognitive Development Center of Central European University; and Nicolas Baumard, Alessandra Geraci, Laurence Kaufmann, Olivier Morin, and Hugo Viciana for their support and for invaluable inputs at all stages of this research. This research was supported by grants from Central European University and the Fyssen Foundation and European Commission Grant PIEF-GA-2010-276077 (to O.M.). ANR ANR-09-BLAN-0327 SOCODEV from the French Agence Nationale pour la Recherche covered travel expenses related to this project.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113194109/-/DCSupplemental.
References
- 1.Hinde RA. Individuals, Relationships and Culture. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- 2.Hand JL. Resolution of social conflicts: Dominance, egalitarianism, spheres of dominance, and game theory. Q Rev Biol. 1986;61:201–220. [Google Scholar]
- 3.Thomsen L, Frankenhuis WE, Ingold-Smith MC, Carey S. Big and mighty: Preverbal infants mentally represent social dominance. Science. 2011;331:477–480. doi: 10.1126/science.1199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber M. In: Max Weber: Essays in Sociology. Gerth HH, Wrigh Mills C, editors. Oxford: Routledge; 1946. pp. 180–195. [Google Scholar]
- 5.Boehm C, Flack JC. In: The Social Psychology of Power. Guinote A, Vescio TK, editors. New York: Guilford; 2010. pp. 46–86. [Google Scholar]
- 6.Lewis RJ. Beyond dominance: The importance of leverage. Q Rev Biol. 2002;77:149–164. doi: 10.1086/343899. [DOI] [PubMed] [Google Scholar]
- 7.Henrich J, Gil-White FJ. The evolution of prestige: Freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evol Hum Behav. 2001;22:165–196. doi: 10.1016/s1090-5138(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 8.Cheney DL, Seyfarth RM. How Monkeys See the World: Inside the Mind of Another Species. Chicago: Univ of Chicago Press; 1992. [Google Scholar]
- 9.Hawley PH. The ontogenesis of social dominance: A strategy-based evolutionary perspective. Dev Rev. 1999;19:97–132. [Google Scholar]
- 10.Gergely G, Csibra G. Teleological reasoning in infancy: The naïve theory of rational action. Trends Cogn Sci. 2003;7:287–292. doi: 10.1016/s1364-6613(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 11.Warneken F, Tomasello M. Altruistic helping in human infants and young chimpanzees. Science. 2006;311:1301–1303. doi: 10.1126/science.1121448. [DOI] [PubMed] [Google Scholar]
- 12.Marr D. Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- 13.Pylyshyn ZW. Computation and Cognition: Toward a Foundation for Cognitive Science. Cambridge, MA: MIT Press; 1986. [Google Scholar]
- 14.Rowell TE. The concept of social dominance. Behav Biol. 1974;11:131–154. doi: 10.1016/s0091-6773(74)90289-2. [DOI] [PubMed] [Google Scholar]
- 15.Hamlin JK, Wynn K, Bloom P. Social evaluation by preverbal infants. Nature. 2007;450:557–559. doi: 10.1038/nature06288. [DOI] [PubMed] [Google Scholar]
- 16.Schöppner B, Sodian B, Pauen S. Encoding action roles in meaningful social interaction in the first year of life. Infancy. 2006;9:289–311. doi: 10.1207/s15327078in0903_2. [DOI] [PubMed] [Google Scholar]
- 17.Rochat P, Striano T, Morgan R. Who is doing what to whom? Young infants’ developing sense of social causality in animated displays. Perception. 2004;33:355–369. doi: 10.1068/p3389. [DOI] [PubMed] [Google Scholar]
- 18.Xu F, Carey S. Infants’ metaphysics: The case of numerical identity. Cognit Psychol. 1996;30:111–153. doi: 10.1006/cogp.1996.0005. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Y-Miño C G, Bond AB, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- 20.Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- 21.Shantz CU. Conflicts between children. Child Dev. 1987;58:283–305. [Google Scholar]
- 22.Wilson EO. Sociobiology: the New Synthesis. Cambridge, MA: Harvard Univ Press; 2000. [Google Scholar]
- 23.Strayer FF, Strayer J. An ethological analysis of social agonism and dominance relations among children. Child Dev. 1976;47:980–989. [Google Scholar]
- 24.Savin-Williams RC. Dominance hierarchies in groups of early adolescents. Child Dev. 1979;50:923–935. [Google Scholar]
- 25.Seyfarth RM, Cheney DL. In: Animal Social Complexity. DeWaal F, Tyack PL, editors. Cambridge, MA: Harvard Univ Press; 2004. pp. 207–229. [Google Scholar]
- 26.Zitek EM, Tiedens LZ. The fluency of social hierarchy: The ease with which hierarchical relationships are seen, remembered, learned, and liked. J Pers Soc Psychol. 2012;102:98–115. doi: 10.1037/a0025345. [DOI] [PubMed] [Google Scholar]
- 27.de VRIES. Finding a dominance order most consistent with a linear hierarchy: A new procedure and review. Anim Behav. 1998;55:827–843. doi: 10.1006/anbe.1997.0708. [DOI] [PubMed] [Google Scholar]
- 28.Vehrencamp SL. A model for the evolution of despotic versus egalitarian societies. Anim Behav. 1983;31:667–682. [Google Scholar]
- 29.Archer J. Ethology and Human Development. Savage, MD: Barnes & Noble Books; 1992. [Google Scholar]
- 30.Boehm C. Hierarchy in the Forest. Cambridge, MA: Harvard Univ Press; 1999. [Google Scholar]
- 31.Jackendoff RS. Languages of the Mind. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- 32.Fiske AP. The four elementary forms of sociality: Framework for a unified theory of social relations. Psychol Rev. 1992;99:689–723. doi: 10.1037/0033-295x.99.4.689. [DOI] [PubMed] [Google Scholar]
- 33.Hirschfeld LA. In: Mapping the Mind: Domain Specificity in Cognition and Culture. Hirschfeld LA, Gelman SA, editors. Cambridge, UK: Cambridge Univ Press; 1994. pp. 201–233. [Google Scholar]
- 34.Kuhlmeier VA. In: The Development of Social Cognition. Banaji M, Gelman SA, editors. Oxford: Oxford Univ Press; [Google Scholar]
- 35.Cheney DL, Seyfarth RM. Baboon Metaphysics: The Evolution of a Social Mind. Chicago: Univ of Chicago Press; 2007. [Google Scholar]
- 36.Bourdieu P. In: Handbook of Theory of Research for the Sociology of Education. Richardson JE, editor. Westport, CT: Greenwood; 1986. pp. 241–258. [Google Scholar]
- 37.French JR, Raven B. In: Studies in Social Power. Cartwright D, editor. Ann Arbor, MI: Inst for Social Res; 1959. pp. 150–167. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.