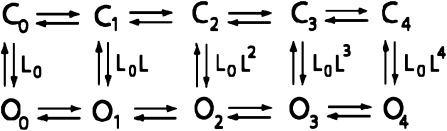Abstract
KCNQ1 (Kv7.1) is a unique member of the superfamily of voltage-gated K+ channels in that it displays a remarkable range of gating behaviors tuned by coassembly with different β subunits of the KCNE family of proteins. To better understand the basis for the biophysical diversity of KCNQ1 channels, we here investigate the basis of KCNQ1 gating in the absence of β subunits using voltage-clamp fluorometry (VCF). In our previous study, we found the kinetics and voltage dependence of voltage-sensor movements are very similar to those of the channel gate, as if multiple voltage-sensor movements are not required to precede gate opening. Here, we have tested two different hypotheses to explain KCNQ1 gating: (i) KCNQ1 voltage sensors undergo a single concerted movement that leads to channel opening, or (ii) individual voltage-sensor movements lead to channel opening before all voltage sensors have moved. Here, we find that KCNQ1 voltage sensors move relatively independently, but that the channel can conduct before all voltage sensors have activated. We explore a KCNQ1 point mutation that causes some channels to transition to the open state even in the absence of voltage-sensor movement. To interpret these results, we adopt an allosteric gating scheme wherein KCNQ1 is able to transition to the open state after zero to four voltage-sensor movements. This model allows for widely varying gating behavior, depending on the relative strength of the opening transition, and suggests how KCNQ1 could be controlled by coassembly with different KCNE family members.
Keywords: allosteric model, fluorescence, IKs potassium channel, KCNE1, cardiac
KCNQ1 (KV7.1) is a member of the superfamily of voltage-gated potassium channels (KV), which contain six transmembrane helices and form functional tetramers with four peripheral voltage-sensing domains surrounding a single potassium-selective pore domain (1). Much study spanning recent decades has focused on the detailed gating mechanisms of these molecular machines, establishing general principles of KV channel gating, as well as unique structural and functional properties that underlie the diverse physiological functions of different family members (2). Within the KV family, KCNQ1 displays a unique flexibility in its gating properties, depending on the tissue where it is expressed and the corresponding β subunit with which it coassembles: in the intestine KCNQ1/KCNE3 channels display voltage-independent current that supports chloride secretion (3), whereas in the heart, KCNQ1/KCNE1 channels display slowly activating voltage-dependent current that is critical to cardiac action potential repolarization (4–6). Remarkably, neither of these physiologically essential phenotypes resembles that of the KCNQ1 channel expressed alone, which activates rapidly over a hyperpolarized range of voltages (4, 5). Still other KCNE proteins coassemble with KCNQ1 to form heteromeric channels with distinct biophysical characteristics (7, 8). This diverse array of gating schema allows this protein to play unique important roles in a vast number of systems in the body, including the heart, brain, inner ear, kidney, lungs, and intestine (3, 9, 10).
To understand what underlies the flexibility of KCNQ1 gating, we sought to characterize the gating mechanisms of this channel, in particular the coupling between the peripheral voltage sensors and the central channel gate. Classic work by Hodgkin and Huxley suggests that voltage-dependent transitions of four independent “voltage-sensing particles” are required for the activation of the squid giant axon potassium current (11). This hypothesis was supported by the discovery that there are four structurally independent voltage sensor domains for each channel pore (1, 12–15), and, indeed, this gating scheme seems to hold for many Kv channels. However, in some voltage-sensitive potassium channels, movement of all four voltage sensors is not required in order for the channel to open. For example, in the calcium- and voltage-gated BK channel, voltage-dependent gating follows an allosteric gating scheme (studied in the absence of Ca2+) wherein voltage-sensor movement acts as an allosteric promoter of gate opening (16, 17).
In our previous study, we showed using voltage-clamp fluorometry (VCF) that in the KCNQ1 potassium channel, the kinetics and steady-state voltage dependence of voltage-sensor transitions appear to closely resemble those of the channel currents, as if multiple voltage-sensor movements are not required to precede channel opening (18). In addition, there is no Cole–Moore shift in KCNQ1 channels (18), in contrast to what would be expected from a Hodgkin–Huxley-type channel. In an attempt to explain this result in light of the predicted tetrameric structure of the KCNQ1 channel, we proposed two hypotheses: (i) All four voltage sensors in KCNQ1 move in concert, so that there is effectively one common voltage sensor transition that leads to channel opening; or (ii) the voltage sensors move relatively independently but that the KCNQ1 channel gate can open before all voltage sensors have moved, perhaps occupying stable subconductance states on the way to a fully open state.
Here, we show that KCNQ1 voltage sensors move relatively independently and that channels can indeed open before all voltage sensors have moved. Remarkably, we find that in some KCNQ1 mutant channels, transition to an open state can occurs even in the absence of voltage-sensor movement. In these mutants, we see that voltage sensors move with more depolarized voltage dependence than the channel gate, implying that some voltage-sensor movements can take place after the channel is open. These results lead us to adopt an allosteric gating scheme wherein KCNQ1 is able to transition to the open state after zero to four voltage-sensor movements, with each successive voltage-sensor movement strengthening the opening transition. This gating scheme allows for the flexible gating behaviors seen in channels formed with KCNQ1, which may be controlled in physiological contexts by coassembly with different accessory KCNE proteins.
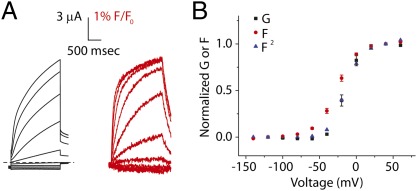
Results
In our previous study, we monitored movement of the KCNQ1 voltage sensor using a pseudo-WT KCNQ1 construct containing two cysteine neutralization mutations and a cysteine introduction at the top of the S4 helix at position 219, which we label with Alexa-488 maleimide (KCNQ1 C214A/C331A/G219C). Here, we refer to KCNQ1 C214A/C331A simply as KCNQ1 and denote the presence of G219C with attached fluorophore by subscript “L,” for labeled with fluorophore. From our previous work, we know that currents from KCNQ1L are similar to the WT KCNQ1 currents with two exceptions: the half-point of voltage-dependent activation is shifted by about −10 mV, and the channels display relatively less inactivation (18). Fluorescence signals from this channel exhibit similar kinetics and steady-state voltage dependence as channel currents, suggesting multiple voltage-sensor movements are not required to precede channel opening. Based on this result, we initially hypothesized that all four KCNQ1 voltage sensors might move in concert to open the channel.
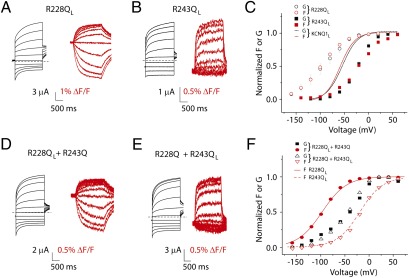
To test for concerted movement of the voltage sensors in KCNQ1 channels, we design experiments wherein KCNQ1 channels contain heterogenous voltage sensors. If voltage sensors move in concert, we expect voltage sensors with different voltage dependence in the same channel to influence each other so that they remain concerted. First, we identify mutations that alter voltage senor movements. A recent study by Wu et al. finds that currents from two voltage-sensor charge mutations, R228Q and R243Q, display very hyperpolarized and very depolarized voltage dependence, respectively, compared with the WT KCNQ1 channel (19). In agreement with previous findings, two-electrode voltage clamp recordings of KCNQ1 R228QL and KCNQ1 R243QL show dramatic hyperpolarizing and depolarizing shifts, respectively, in voltage dependence (Fig. 1 A–C). Using VCF, we find fluorescence signals are similarly shifted, as if the voltage-sensor movement, but not the relationship between voltage sensors and channel gate, is altered by these mutations.
Fig. 1.
KCNQ1 voltage sensors move independently. (A) Current (black traces) and fluorescence (red traces) from R228Q in the KCNQ1L background recorded in ND96. Broken lines represent zero current. Cells are held at −80 mV and stepped between −160 and 60 mV for 2 s, followed by pulse to −40 mV to record tail currents (−80 mV for fluorescence tails). (B) Current (black traces) and fluorescence (red traces) from R243Q in the KCNQ1L background. (C) Steady-state voltage dependence of KCNQ1 R228QL and R243QL current and fluorescence. Lines from KCNQ1L are shown for reference [from Osteen et al. (18)]. (D) Current (black traces) and fluorescence (red traces) from KCNQ1 R228QL and KCNQ1 R243Q coexpression experiments. (E) Current (black traces) and fluorescence (red traces) from KCNQ1 R228Q and KCNQ1 R243QL coexpression experiments. (F) Steady-state voltage dependence of coexpression experiments. Lines from fits of R228QL and R243QL fluorescence (separate expression) are shown for reference. Error bars represent SEM.
Next, we coexpress KCNQ1 R228QL and KCNQ1 R243Q to assay currents from coassembled channels but only record fluorescence signals from R228Q-containing subunits. We reason that if voltage sensors are cooperative, then coassembly of R243Q-containing subunits will shift the voltage-dependent movement of R228Q-containing voltage sensors. As shown in Fig. 1 D and F, currents from coexpression of R228QL and R243Q are significantly right-shifted relative to R228QL currents, but the fluorescence signal from R228QL is unchanged. Assuming equal expression and stochastic assembly of expressed subunits, the binomial distribution predicts that 87.5% of channels will assemble with mixed R228QL and R243Q subunits. Therefore, the presence of such a significant shift in currents without any change in R228QL fluorescence strongly suggests voltage sensors are not concerted.
We next coexpress KCNQ1 R228Q and KCNQ1 R243QL and record current and fluorescence. In this experiment, we again find that the voltage dependence of current is distinct from that of fluorescence, which is unchanged from R243QL monomer (Fig. 1 E and F). This shows that whereas currents from coassembled R228Q and R243QL channels are significantly shifted, fluorescence signals from R243Q are not affected by the presence of R228Q, again strongly supporting relatively independent KCNQ1 voltage sensors. The currents from both coexpression experiments lie between the two fluorescence signals (Fig. 1F), as if, in channels containing mixed subunits, currents activate after some, but not all, voltage sensors move. This observation is in agreement with our alternative hypothesis that current in KCNQ1 can activate before all voltage sensors have moved.
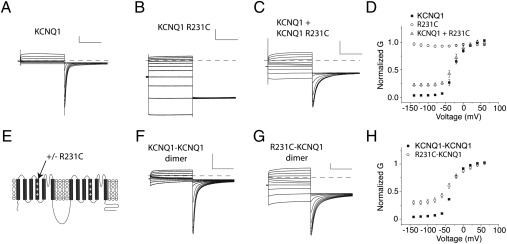
To more rigorously address the possibility that KCNQ1 channels can open before all four voltage sensors move, we explore another voltage sensor mutation, R231C, which leads to a constitutively active channel by locking the voltage sensor in the activated position (20, 21). Again, using coexpression, we explore whether the presence of constitutively active voltage sensors within a coassembled channel of KCNQ1 and KCNQ1 R231C subunits generates voltage-independent (constitutive) current. To distinguish constitutive current from KCNQ1 channels from background currents or leak currents, we perform activation protocols before and after application of 100 μM chromanol, a KCNQ1-specific blocker (22), to isolate chromanol-sensitive current. We perform these experiments in high potassium external solution (see Methods) to better resolve K+ current at hyperpolarized potentials. First, we assay constitutive current through homomeric KCNQ1 and KCNQ1 R231C channels (Fig. 2 A and B). Fig. 2D shows that KCNQ1 channels display 2.3% constitutive current, whereas R231C channels are completely constitutive. When we coexpress KCNQ1 and R231C subunits and assay constitutive current, we find 21% of current to be constitutive (Fig. 2 C and D), according to the average of A1/A2 parameters from Boltzmann fits to each experiment (see Methods). According to the binomial distribution, only 6.25% of channels will assemble with 4 R231C subunits. Experiments extend to −140 mV, where WT KCNQ1 voltage sensors are all in the resting state (18), and we see complete flattening of the G(V) curve at these voltages.
Fig. 2.
KCNQ1 channels can open with only two activated voltage sensors. (A–C) Chromanol-sensitive current traces from experiments expressing KCNQ1 (A), R231C (B), and KCNQ1 + R231C (C). Experiments are performed in 98 mM extracellular potassium (High K+) (see Methods) and cells are held at −80 mV, pulsed to voltages between −140 and 60mV for 2 s, followed by 2-s pulses to −120 mV to collect tail currents. (D) Average conductance-voltage (G-V) relationship for experiments as in A–C. Experiments are normalized to the maximum from Boltzmann fits to the data (see Methods). (E) Schematic showing construction of tandem KCNQ1 dimer and the location of the R231C mutation within the dimer. (F and G) Chromanol-sensitive currents from dimers containing KCNQ1-KCNQ1 (F) and R231C-KCNQ1 (G). Voltage protocol is as in A–C. (H) G-V relationship for the two linked dimers. For all traces, vertical scale bars represent 1 μA and horizontal scale bars represent 1s. Error bars represent SEM.
This result suggests that channels containing some voltage-dependent and some constitutively active voltage sensors do conduct current. However, because we cannot rule out higher expression of R231C or preferential assembly of channels with four R231C-containing subunits, it is difficult to be quantitative. We, therefore, construct a tandem KCNQ1 dimer containing KCNQ1 R231C and KCNQ1 within a single reading frame (Fig. 2E). Expression of this tandem construct will result in equal expression of these two subunits and may promote assembly of channels containing two KCNQ1 R231C subunits and two KCNQ1 subunits as dimers of dimers. Expression of the tandem dimer R231C-KCNQ1 yields currents that display 29% constitutive current, whereas control tandem dimers containing KCNQ1-KCNQ1 subunits display only 4.4% constitutive current (Fig. 2 F–H). Therefore, if channels assemble as dimers of dimers containing two R231C and two WT voltage sensors, this result suggests that with half its voltage sensors activated, KCNQ1 channels are 29% open.
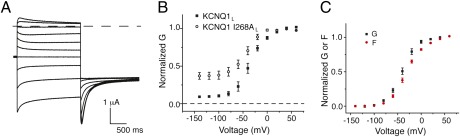
Although the experiments in Figs. 1 and 2 demonstrate that KCNQ1 channels are able to transition to the open state before all voltage sensors have activated, two possible mechanisms can explain this behavior. One possibility is that each independent voltage-sensor movement partially opens a KCNQ1 channel to a stable subconductance state. Alternatively, each voltage-sensor movement might increase the open probability of a channel, so that some percentage of channels with, for example, one activated voltage sensor, would be in the open state. Based on our experiments to this point, it is not possible to distinguish between these possibilities. We gained some insight into this question from the recent study by Ma et al., which identifies a number of mutations in KCNQ1, including I268A, that cause an increase in voltage-independent constitutive current, leading the authors to propose an allosteric gating scheme for KCNQ1 channels wherein the channel can open in the absence of voltage-sensor movement (23). To assay the movement of voltage sensors in one of these mutants, we introduce I268A into the KCNQ1L construct and monitor its fluorescence signal.
Again using high K+ external solution and examining chromanol-sensitive currents, we confirm that I268A introduces a significant amount of constitutive current into KCNQ1 channels (Fig. 3A). We find 37% voltage-independent current in KCNQ1 I268AL vs. 9.2% in KCNQ1L (Fig. 3B). Measurement of the fluorescence signal from I268AL yields an F(V) curve that saturates at positive and negative voltages and is well fit by a Boltzmann equation (Fig. 3C). This result shows that voltage-sensor movement is relatively unperturbed by this mutation and suggests that in the presence of this mutation, a significant number of channels can open in the absence of voltage-sensor movement. Intriguingly, when we plot the voltage dependence of the fluorescence signal from I268AL along with the normalized voltage-dependent portion of current, we find that the fluorescence signal activates with more depolarized voltage dependence than voltage-dependent gate opening (Fig. 3C). This result suggests that voltage sensors can move even after the channel is fully open.
Fig. 3.
The I268A mutation increases the voltage-independent constitutive current in KCNQ1. (A) Chromanol-sensitive I268A KCNQ1L current traces recorded in high K+ . Voltage protocols as in Fig. 2. (B) Chromanol-subtracted G(V)s from KCNQ1L and I268A KCNQ1L. (C) Comparison of G(V) normalized between 0 and 1 (black symbols) and F(V) (red symbols) curves from I268AL. Error bars represent SEM.
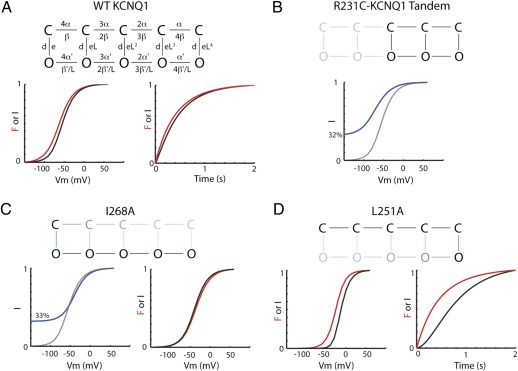
To interpret our results, we adopt an allosteric model of KCNQ1 channel gating wherein lateral movement between states represents movement of a voltage sensor between resting and activated conformations and vertical movement between states represents opening and closing of the channel gate (gating scheme 1) (parameters controlling the opening rate are shown). In this model, successive movement of voltage sensors strengthens the opening transition by a cooperativity factor (L), so that as more voltage sensors move, the probability of transitioning to the open state increases. This simple model is able to explain the 1:1 relationship between voltage-sensor movement and channel opening in KCNQ1 as a balance of rightward and downward movements through states in the model during activation. It also explains how channels can open before all voltage sensors have moved since channel opening is possible with zero to four voltage-sensor movements. Therefore, the effect of I268A is simply to strengthen the downward equilibrium constant (L0) such that more channels open without any activated voltage sensors. In addition, the observation that the voltage-dependent fluorescence signal saturates after all channels are fully open in I268A is interpretable in the allosteric model as voltage-dependent movement of voltage sensors between open states of the channel.
Gating scheme 1.
If the I268A mutation has the effect of strengthening the opening transition in our proposed allosteric model, can we find other mutations that weaken the opening transition such that more voltage-sensor movements are needed to observe channel opening? Indeed, we found a mutation like this, F351A, in our previous study (18). F351A homomeric channels activate well after voltage-sensor movement is seen, suggesting that multiple voltage-sensor movements are required before the channel transitions to the open state. This phenotype can be qualitatively explained as a decrease in the strength of the opening equilibrium constant (L0) in the allosteric model. To further explore mutations of this type, we express the S4-S5 linker mutation L251A, which was also previously shown to slow KCNQ1 channel activation (24), in our labeled KCNQ1 background. As shown in Fig. 4, L251AL separates the kinetics of the fluorescence and current, although not to the extent induced by F351A. In agreement with this, steady-state F(V) precedes steady-state G(V) from L251AL such that fluorescence raised to the second power mirrors current (Fig. 4B), whereas in F351AL, we must raise fluorescence to the fourth power to reproduce the voltage dependence of current. We can interpret this result using the allosteric model by assuming that L251A weakens the opening transition but not to the same extent as F351A. Taken together, results from I268A, L251A, and F351A mutants support an allosteric model of KCNQ1 gating wherein the opening transition is highly sensitive to mutation and can be strengthened or weakened to varying degrees by point mutations.
Fig. 4.
KCNQ1 mutant L251AL introduces a brief delay in channel activation and fluorescence indicates that two voltage sensors are needed to cause the channel to open. (A) Current (black traces) and fluorescence (red traces) from L251AL recorded in ND96. Voltage protocols are as in Fig. 1. (B) Steady-state G(V) (black squares) and F(V) (red circles) curves. Fluorescence raised to the second power (blue triangles) mirrors current activation. Error bars represent SEM.
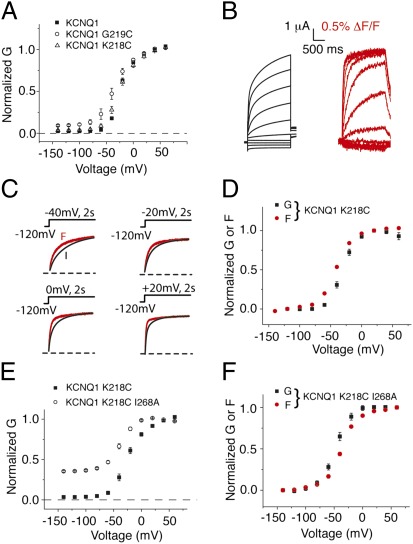
Given the high sensitivity of KCNQ1 channels to mutations, we next ask how closely our labeled construct KCNQ1L resembles the true WT KCNQ1 channel. We compare constitutive current from KCNQ1 and KCNQ1L. Fig. 5A shows that whereas KCNQ1L displays 9.2% constitutive current, KCNQ1 displays only 2.4%. This difference in constitutive current is significant (P < 0.05), and we conclude that the G219C mutation increases constitutive current into the KCNQ1 channel. We, therefore, tested other reporter cysteines to find a construct that would yield a fluorescence signal without increasing constitutive current. Fig. 5 shows that K218C does not significantly increase constitutive current through KCNQ1 and that current and fluorescence signals from labeled KCNQ1 K218C are similar to labeled KCNQ1 G219C (18). The kinetics and steady-state voltage dependence of labeled KCNQ1 K218C show that voltage-sensor movement precedes gate opening in this mutant to a larger extent than in the G219C background (Fig. 5 C and D), suggesting that in K218C, the opening transition is slightly weaker than in labeled KCNQ1 G219C. Given the similarity between constitutive current seen in WT KCNQ1 and KNCQ1 K218C, we take the fluorescence and current seen in this labeling background as the baseline for WT KCNQ1 channels. Finally, to ensure that the observed affects of KNCQ1 mutations on the opening transition are not dependent on G219C, we next retest the effect of I268A in the labeled KCNQ1 K218C background. As shown in Fig. 5 E and F, both the dramatic increase in constitutive current (35%) and the depolarized voltage dependence of fluorescence relative to current are present in the K218C background. The constitutive currents induced by the mutation G219C is most likely also responsible for the small constitutive currents seen in Fig. 1.
Fig. 5.
Labeled KCNQ1 K218C is a more representative reporter of WT KCNQ1 voltage-sensor movement. (A) Chromanol (100 μM)-subtracted currents from KCNQ1, KCNQ1 G219C, and KCNQ1 K218C in high K+ solution. Whereas G219C increases voltage-independent constitutive current through KCNQ1, K218C does not. Voltage protocols are as in Fig. 2. (B) Current (black traces) and fluorescence (red traces) from labeled KCNQ1 K218C recorded in ND96. Voltage protocols are as in Fig. 1. (C) Comparison of activation kinetics of current and fluorescence signals from labeled KCNQ1 K218C in response to the voltage protocols shown recorded in ND96. (D) Steady-state G(V) (black squares) and F(V) (red circles) curves for KCNQ1 K218C. (E) Chromanol-subtracted data for KCNQ1 K218C and KCNQ1 K218C I268A recorded in high K+ solution. Voltage protocols are as in Fig. 2. (F) Comparison of normalized G(V) (black symbols) and F(V) (red symbols) curves from KCNQ1 K218C I268A. Error bars represent SEM.
To achieve a more quantitative description of allosteric gating in KCNQ1, we fit experimental data to a model based on gating scheme 1 (Fig. S1). As shown in Fig. 6A, KCNQ1L is well fit by this model. (See Table S1 for parameter values.) Using these parameters and removing states with three or four voltage sensors in the resting state, the model predicts 32% constitutive current, in close agreement with experiments from R231C/WT linked heterodimer experiments showing 29% constitutive current from channels presumably assembled with two constitutively activated and two WT voltage sensors (Figs. 2 and 6B). This allosteric model also reproduces our fluorescence and current data from the R228Q and R243Q coinjection experiments (Fig. S2). Additionally, data from the I268A mutation is well fit by this gating scheme (Fig. 6C) by increasing the opening transition with respect to the WT (L0 in gating scheme 1, which is the ratio of rates d and e in Fig. 6). Finally, to fit experimental data from KCNQ1 L251A, we mainly decrease the opening transition (Fig. 6D). This decrease in the favorability of the opening transition causes channels to transition through multiple closed state voltage sensor transitions before opening, allowing for separation between voltage-sensor movement (fluorescence) and current in both kinetics and steady-state voltage dependence.
Fig. 6.
An allosteric model can reproduce biophysical properties of KCNQ1 mutants. (A) Allosteric gating scheme, along with rates used to define transitions. Model simulation for current and fluorescence activation at +20 mV and simulated G(V) and F(V) curves are shown for KCNQ1L. (B) Schematic showing states (black) occupied in channels containing two R231C and two WT subunits. Simulated G(V) (blue) for tandem R231C-WT channels based on rates fitted to KCNQ1L and assuming states with 0 and 1 activated voltage sensor are never occupied. The model predicts 32% constitutive current for tandem R231C-WT channels. Simulated G(V) for KCNQ1L shown in gray. (C) Schematic showing predominant activation pathway for I268A mutant. Model simulation showing percent constitutive current (blue) and normalized G(V) and F(V) data for I268A. Simulated G(V) for KCNQ1L shown in gray. (D) Schematic showing predominant activation pathway for L251A mutant to reproduce separation in fluorescence and current. Model simulation for activation kinetics of current (black) and fluorescence (red) at 20 mV and G(V) and F(V) for L251A with fitted rates. See SI Text for values of rates from fits to various mutants.
We previously suggested that KCNE1 alters the KCNQ1 channel such that all four S4s need to be activated to open the channel (18). To test this hypothesis, we coexpressed KCNE1 with the linked WT-R231C KCNQ1 dimer construct, which by itself exhibited around 29% constitutive currents (Fig. 2H). In contrast, the linked WT-R231C KCNQ1 dimer coexpressed with KCNE1 exhibited very small constitutive currents (Fig. S3), as if activating two S4s are not enough to open the channel significantly. This can be explained in our allosteric model by reducing L0 equilibrium constant (even more than for the mutation L251A), so that the channel does not open significantly with two S4s activated.
Discussion
Here, we provide evidence for allosteric voltage-dependent gating in the KCNQ1 channel. We first rule out concerted movement of KCNQ1 voltage sensors by showing that coassembly of subunits containing heterogeneous voltage sensors does not significantly affect the behavior of each voltage sensor. We next test for channel opening with fewer than four activated voltage sensors using a voltage sensor mutation R231C. In channels coassembled from KCNQ1 and KCNQ1 R231C channels, we see a large constitutive current both in coexpression and tandem dimer experiments, as if channels containing two activated voltage sensors have a significant probability of opening (∼29%). We next identify a mutation, I268A, which displays a large constitutive current but normal voltage-sensor movement, leading us to propose an allosteric gating scheme for KCNQ1.
Among the questions arising from this study is that of how generalizable this allosteric model is to voltage-gated potassium channels in general. Although this is the only member of the Kv channel family to be shown to gate in this way, the related BK channel does use its voltage sensors as allosteric regulators of the opening transition, along with binding of Ca2+. HCN channels, close relatives to Kv channels, also gate by an allosteric mechanism (25, 26).
Our data not only suggests that KCNQ1 channels gate according to an allosteric scheme but also that this channel is highly sensitive to mutation-induced changes to the strength of the opening transition. Changes in the opening transition relative to the voltage-sensor transitions cause the KCNQ1 channel to open through different pathways in the allosteric scheme, resulting in very different current outputs of the mutant channels (compare L251A and I268A). That KCNQ1 constitutes a highly allosteric protein sensitive to mutations is underlined by the observation that the labeling mutation G219C, located in the S3-S4 extracellular loop and far from the channel gate, seems to alter the opening transition, thereby causing a small increase in constitutive current.
Our allosteric model of KCNQ1 provides a starting point from which to look for structures and interaction points important in regulation of channel gating. We use this allosteric gating scheme to interpret mutations that alter the relationship between voltage-sensor movement and channel opening by exploring how changing specific rates within this gating scheme can alter channel behavior to reproduce our experimental results. By mapping the location of these mutations, we can begin to see what structures may be involved in altering specific rates. Our model provides a basis to understand which parameters, and, therefore, which structures and gating processes, are affected by mutations or coassembly of β subunits. Thus, this work provides a valuable tool not only for understanding how KCNQ1 behaves in physiological contexts but also for understanding the molecular mechanisms of disease-causing mutations that alter gating.
For example, KCNE1 coassembly drastically slows current activation, which may be mostly attributable to a relative weakening of the opening transition in the allosteric model [although the movement of the voltage sensor itself is also affected (18)]. Indeed, we show here that KCNE1 decrease the amount of constitutive current in the linked WT-R231C KCNQ1 dimer, as if KCNE1 weakens the opening transition so that two permanently activated S4s (because of R231C) are not enough to significantly open coassembled KCNQ1/KCNE1 channels. In contrast, the constitutive phenotype of KCNQ1-KCNE3 and KCNQ1-KCNE2 channels might be attributable to a stabilization of the activated state of the voltage sensor or simply a large increase in the strength of the opening transition such that all channels are open before voltage sensors move. The flexibility in gating behaviors afforded by an allosteric framework, therefore, uniquely suits the widely variable physiological roles of the KCNQ1 channel.
Methods
Molecular Biology.
Human KCNQ1 and KCNE1 were subcloned into the pGEM-HE oocyte expression vector. Mutations were introduced using Quikchange site-directed mutagenesis kit (Qiagen) and were fully sequenced to ensure incorporation of intended mutations and the absence of unwanted mutations (sequencing by Genewiz). In vitro transcription of cRNA was performed using mMessage mMachine T7 RNA Transcription Kit (Ambion).
VCF Recordings.
A total of 50 ng of KCNQ1 RNA was injected into defolliculated Xenopus laevis oocytes. VCF experiments were performed 2–7 d after injection: oocytes were labeled for 30 min with 100 μM Alexa-488 maleimide (Molecular Probes) in high K+ ND96 solution [98 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM Hepes (pH 7.6) with NaOH] at 4 °C. Following labeling, they were kept on ice to prevent internalization of labeled channels. Oocytes were placed into a recording chamber animal pole “up” in nominally Ca2+-free solution [96 mM NaCl, 2 mM KCl, 2.8 mM MgCl2, 5 mM Hepes (pH 7.6) with NaOH] or in high K+ ND96 solution. In experiments requiring hyperpolarized voltages, 100 μM LaCl3 is used to block endogenous hyperpolarization activated currents. At this concentration, La3+ does not affect G(V) or F(V) curves from KCNQ1 (18). In chromanol-subtraction experiments, 100 μM chromanol 293B was introduced into the bath by pipetting. High K+ experiments are performed in labeling solution (see above).
VCF experiments were carried out as previously reported (18).
Data Analysis.
Steady-state voltage dependence of current was calculated from exponential fits of tail currents following different test potentials. The fit of the tails were extrapolated to the beginning of the tail pulse to avoid contamination by the “hook,” which results from inactivation that is removed within hundreds of milliseconds at the −40-mV tail potential. Each G(V) experiment was fit with a Boltzmann equation:
where A1 and A2 are the minimum and maximum, respectively, V1/2 is the voltage at which there is half-maximal activation, and K is the slope. Data were normalized between the A1 and A2 values of the fit. Fluorescence signals were bleach-subtracted, and data points were averaged over tens of milliseconds at the end of the test pulse to reduce errors from signal noise. This data are fit with a Boltzmann and normalized between A1 and A2 parameters for each experiment.
For experiments in high K+ solution, tail currents are measured at −120 mV following 2-s test pulses to voltages between −140 and 60 mV. Experiments are performed before and after application of 100 μM chromanol 293B. G(V) curves are measured by fitting the tail currents with single exponential functions and extrapolated to the beginning of the tail pulse, again to eliminate contamination from inactivation, which is rapidly relieved at −120 mV. G(V) experiments are fit with a Boltzmann and normalized only to the A2 parameter and averaged. Constitutive current is calculated as A2/A1 for each experiment.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL-364418 (to R.S.K.), P01-HL 076849-07 (to R.S.K.), and HL-095920 (to H.P.L.); and American Heart Grant 10GRNT4150069 (to H.P.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201582109/-/DCSupplemental.
References
- 1.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 2.Catterall WA. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder BC, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 4.Sanguinetti MC, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 5.Barhanin J, et al. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 6.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 7.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Um SY, McDonald TV. Voltage-gated potassium channels: Regulation by accessory subunits. Neuroscientist. 2006;12:199–210. doi: 10.1177/1073858406287717. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, Kass RS. Long QT syndrome: From channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: From gene to physiological function. Physiology (Bethesda) 2005;20:408–416. doi: 10.1152/physiol.00031.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papazian DM, Timpe LC, Jan YN, Jan LY. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- 13.Zagotta WN, Hoshi T, Aldrich RW. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zagotta WN, Hoshi T, Dittman J, Aldrich RW. Shaker potassium channel gating. II: Transitions in the activation pathway. J Gen Physiol. 1994;103:279–319. doi: 10.1085/jgp.103.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: Structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 16.Horrigan FT, Aldrich RW. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca(2+) J Gen Physiol. 1999;114:305–336. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horrigan FT, Cui J, Aldrich RW. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca(2+) J Gen Physiol. 1999;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osteen JD, et al. KCNE1 alters the voltage sensor movements necessary to open the KCNQ1 channel gate. Proc Natl Acad Sci USA. 2010;107:22710–22715. doi: 10.1073/pnas.1016300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu D, Pan H, Delaloye K, Cui J. KCNE1 remodels the voltage sensor of Kv7.1 to modulate channel function. Biophys J. 2010;99:3599–3608. doi: 10.1016/j.bpj.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartos DC, et al. R231C mutation in KCNQ1 causes long QT syndrome type 1 and familial atrial fibrillation. Heart Rhythm. 2011;8:48–55. doi: 10.1016/j.hrthm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panaghie G, Abbott GW. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J Gen Physiol. 2007;129:121–133. doi: 10.1085/jgp.200609612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busch AE, et al. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflugers Arch. 1996;432:1094–1096. doi: 10.1007/s004240050240. [DOI] [PubMed] [Google Scholar]
- 23.Ma LJ, Ohmert I, Vardanyan V. Allosteric features of KCNQ1 gating revealed by alanine scanning mutagenesis. Biophys J. 2011;100:885–894. doi: 10.1016/j.bpj.2010.12.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labro AJ, et al. The S4-S5 linker of KCNQ1 channels forms a structural scaffold with the S6 segment controlling gate closure. J Biol Chem. 2011;286:717–725. doi: 10.1074/jbc.M110.146977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altomare C, et al. Integrated allosteric model of voltage gating of HCN channels. J Gen Physiol. 2001;117:519–532. doi: 10.1085/jgp.117.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Männikkö R, Pandey S, Larsson HP, Elinder F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J Gen Physiol. 2005;125:305–326. doi: 10.1085/jgp.200409130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.