Abstract
Stimulation of cells with physiological concentrations of calcium-mobilizing agonists often results in the generation of repetitive cytoplasmic Ca2+ oscillations. Although oscillations arise from regenerative Ca2+ release, they are sustained by store-operated Ca2+ entry through Ca2+ release-activated Ca2+ (CRAC) channels. Here, we show that following stimulation of cysteinyl leukotriene type I receptors in rat basophilic leukemia (RBL)-1 cells, large amplitude Ca2+ oscillations, CRAC channel activity, and downstream Ca2+-dependent nuclear factor of activated T cells (NFAT)-driven gene expression are all exclusively maintained by the endoplasmic reticulum Ca2+ sensor stromal interaction molecule (STIM) 1. However, stimulation of tyrosine kinase-coupled FCεRI receptors evoked Ca2+ oscillations and NFAT-dependent gene expression through recruitment of both STIM2 and STIM1. We conclude that different agonists activate different STIM proteins to sustain Ca2+ signals and downstream responses.
Keywords: excitation-transcription coupling, transcription
Stimulation of cell-surface receptors that couple to the phospholipase C pathway with physiological concentrations of agonist generally evokes repetitive cytoplasmic Ca2+ oscillations (1). Oscillatory Ca2+ signals enable cytoplasmic Ca2+ to reach high levels transiently, thereby avoiding the deleterious effects of a prolonged, elevated Ca2+ rise. Information is encoded in the oscillatory amplitude and frequency (2) and the spatial profile of the Ca2+ signal (3), each of which can be deciphered by cells to drive selective downstream responses.
Ca2+ oscillations are triggered by inositol trisphosphate (InsP3)-mediated Ca2+ release from intracellular Ca2+ stores, primarily the endoplasmic reticulum (ER) (2). The resulting fall in Ca2+ within the stores opens store-operated CRAC channels in the plasma membrane (4, 5). Ca2+ entry through these channels refills the stores and, thus, sustains InsP3-dependent Ca2+ oscillations (6). In addition to this supportive role, local Ca2+ entry through Ca2+ release-activated Ca2+ (CRAC) channels during oscillatory responses in mast cells, and not the oscillations per se, signals to the nucleus to regulate Ca2+-dependent gene expression (7).
The two main molecular components of store-operated Ca2+ entry are the stromal interaction molecule (STIM) and Orai proteins (reviewed in refs. 8–10). The transmembrane ER proteins STIM1 and STIM2 detect ER Ca2+ content through an EF-hand domain in their respective N-termini, which face the lumen of the store. Loss of luminal Ca2+ leads to STIM aggregation within the ER followed by migration to ER-plasma membrane junctions located just below the plasma membrane. Here, they bind to and activate Orai1, a four transmembrane domain spanning plasma membrane protein, which forms the CRAC channel.
Despite significant homology between STIM1 and STIM2, there are some important differences between them. First, they differ in their respective abilities to activate Orai1. STIM2 activates Ca2+ entry less well than STIM1, for similar levels of Orai1 expression (11). This divergence has been attributed to differences within the amino-terminal domain of the STIM proteins (12) as well as inhibition of STIM2-Orai1 coupling by cytoplasmic calmodulin (13). Second, they exhibit different affinities for Ca2+. STIM2 has an approximately twofold lower affinity for Ca2+ than STIM1 and requires a smaller drop in luminal Ca2+ for activation (14). STIM2 has therefore been proposed both to fulfill a housekeeping role, ensuring that the stores are topped up with calcium in the absence of stimulation, and to activate Ca2+ entry through Orai1 after moderate store depletion (14). By contrast, STIM1 requires a substantial fall in stored Ca2+ for activation and is thought to gate CRAC channels after strong stimulation (11).
Different agonists evoke different patterns of cytoplasmic Ca2+ signal (1). In the rat basophilic leukemia (RBL)-1 mast cell line, stimulation of the G protein-coupled cysteinyl leukotriene type I (cysLT1) receptor with low concentrations of the agonist LTC4 evokes a series of cytoplasmic Ca2+ oscillations (7), typical of the large all-or-nothing baseline Ca2+ spikes seen in many other cell types. By contrast, activation of FCεRI receptors with antigen or IgE leads to a Ca2+ response that develops after a longer delay and which is punctuated by a series of slow Ca2+ oscillations on an elevated background Ca2+ rise (15, 16). Both types of Ca2+ response depend on Ca2+ influx through CRAC channels: Both agonists activate CRAC channels in RBL cells (16, 17), and exposure to CRAC channel blockers accelerates decline of the Ca2+ signal in response to either cysLT1 (7) or FCεRI receptor activation (18). However, the kinetics of InsP3 production and steady-state InsP3 levels differ between the stimuli. G protein-coupled receptors link rapidly to phospholipase Cβ, producing a fast, albeit transient, rise in InsP3 levels. Tyrosine kinase-coupled FCεRI receptors activate phospholipase Cγ through a series of phosphorylation reactions, leading to a slower rise in InsP3 levels. Fast spikes of InsP3 that lead to the large cytoplasmic Ca2+ oscillations exemplified by cysLT1 receptor activation will lead to large but transient decreases in store Ca2+ content. However, the slower changes in InsP3 levels in response to antigenic stimulation will produce smaller but more prolonged decreases in Ca2+ store content. Therefore, modest store depletion, as seen with IgE, could drive Ca2+ entry through a STIM2 pathway, whereas the G protein-coupled cysLT1 receptor might promote Ca2+ signals through STIM1 instead. Here, we have tested this idea by comparing the effects of selective knockdown of STIM1 or STIM2 on physiological patterns of Ca2+ signal and NFAT-driven gene expression after cysLT1 or FCεRI receptor stimulation. We find that different agonists recruit different combinations of STIM proteins to sustain cytoplasmic Ca2+ signals and thereby signal to the nucleus.
Results
To examine the effects of altering STIM1 expression on CRAC channel activity, we knocked down the protein by using an siRNA approach and then measured protein levels by using Western blot and immunocytochemistry. Western blot analysis revealed protein reduction of ≈60% after knockdown of STIM1 (Fig. 1A). Broadly similar results were obtained with immunocytochemistry (Fig. 1B), where knockdown of STIM1 occurred to an extent (≈80%; Fig. 1C) slightly greater than that seen with Western blot. STIM1 knockdown reduced the rate of rise of the cytoplasmic Ca2+ signal that occurred after readmission of external Ca2+ to cells pretreated with thapsigargin in Ca2+-free solution to deplete stores and open CRAC channels (Fig. 1D; STIM1 knockdown reduced the rate of rise of the Ca2+ signal by ≈85%). Whole cell patch clamp experiments to record ICRAC directly confirmed STIM1 knockdown led to a substantial decrease in the size of the current (Fig. 1E; aggregate data summarized in Fig. 1F).
Fig. 1.
Knockdown of STIM1 reduces Ca2+ influx and CRAC channel activity. (A) Western blot showing significant knockdown (KD) of STIM1 after transfection with an siRNA construct. (Upper) A typical gel. (Lower) Summary of aggregate data from three independent experiments. (B) Confocal microscopy images showing expression of STIM1 in mock-transfected cells and after knockdown of the protein. (C) Histogram depicts aggregate data from three independent experiments (each bar is the average from >30 cells). (D) Store-operated Ca2+ entry is significantly reduced after STIM1 knockdown. Cells were stimulated with thapsigargin in Ca2+-free solution for 10 min and then external Ca2+ was readmitted. Only the Ca2+ entry phase is shown. (E) Whole cell patch clamp recordings compare ICRAC in a control cell and in one in which STIM1 has been knocked down. (F) Aggregate data from 9 control cells and 11 STIM1 KD cells are compared. Pipette contained InsP3 and 10 mM EGTA. *P < 0.05.
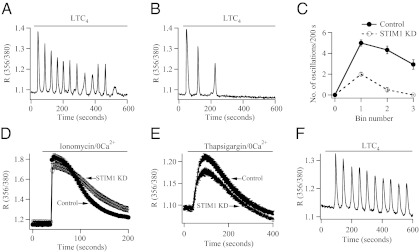
To examine the involvement of STIM1 in physiological patterns of Ca2+ signaling, we measured cytoplasmic Ca2+ oscillations in response to stimulation of cysLT1 receptors with the agonist LTC4 (7). Stimulation with 120 nM LTC4 in the presence of external Ca2+ evoked oscillatory Ca2+ signals in ≈75% of cells (Fig. 2A). The Ca2+ oscillations run down quickly when external Ca2+ is removed (7), (Fig. S1) or when CRAC channels are blocked, demonstrating that Ca2+ entry through the channels is needed to maintain the oscillations (7). After knockdown of STIM1, only a few Ca2+ oscillations occurred in response to LTC4 (Fig. 2B), and the rate of rundown of these oscillations was similar to that seen in Ca2+-free solution (Fig. S1). Aggregate data from several cells is shown in Fig. 2C. Fewer oscillations were evoked in cells in which STIM1 had been knocked down, and the oscillations ran down considerably more quickly than the corresponding controls that were obtained in the presence of external Ca2+.
Fig. 2.
Loss of STIM1 reduces oscillatory Ca2+ signals to LTC4. (A) Oscillatory Ca2+ response after stimulation with 120 nM LTC4 is shown for a control cell. (B) The oscillatory response is greatly reduced after knockdown of STIM1. (C) Aggregate data from 25 control cells and 31 STIM1 KD cells are compared. Bin number represents segments of 200 s, starting as soon as LTC4 was applied. (D) Total store Ca2+ content is not significantly reduced after STIM1 KD. Five micromolar ionomycin was applied. (E) Ca2+ content of the thapsigargin-sensitive store was not reduced after STIM1 knockdown. Thapsigargin was applied at 2 μM. Only upper error bars for Control and lower error bars for STIM1 KD are shown. (F) Typical oscillatory Ca2+ responses are obtained after knock down of STIM1, when LTC4 is applied in 0 Ca2+ plus 1 mM La3+.
The accelerated rundown of Ca2+ oscillations after knockdown of STIM1 could reflect a change in Ca2+ store content or an additional effect of STIM1 independent to Orai1 gating. We assessed store content in two ways: First, we measured the extent of the Ca2+ rise in response to ionomycin (applied in Ca2+-free solution) (19). Ca2+ release was similar between control cells and those in which expression of STIM1 had been reduced (Fig. 2D). Second, we stimulated cells with thapsigargin in Ca2+-free solution and measured the rate and extent of Ca2+ release from the stores (Fig. 2E). Although store content was reduced slightly after knockdown of STIM1, the difference was not significantly different from control cells [for clarity, only upper (control trace) and lower (STIM1 KD trace) error bars are shown in Fig. 2E]. To see whether STIM1 had a role in maintaining Ca2+ oscillations independent of its actions on Ca2+ influx, we stimulated cells with agonist in the absence of external Ca2+ but under conditions where Ca2+ extrusion was prevented. Under these conditions, Ca2+ released from the stores is no longer exported from the cytoplasm and is instead taken back into the stores in readiness for another Ca2+ release cycle (7, 19). With Ca2+ extrusion blocked, stimulation with LTC4 evokes robust and repetitive Ca2+ oscillations (7). Relatively normal responses were still observed after knockdown of STIM1 (Fig. 2F). Collectively, these results demonstrate that both store Ca2+ content and regenerative Ca2+ release are intact after reduced STIM1 expression and, therefore, that the rapid rundown of Ca2+ oscillations in STIM1-deficient cells is due to the loss of Ca2+ entry.
Ca2+ entry through CRAC channels sustains LTC4-driven Ca2+ oscillations. Consistent with this view, knockdown of Orai1 resulted in accelerated rundown of Ca2+ oscillations and significantly reduced gene expression in response to cysLT1 receptor activation (Fig. S2).
To investigate a role for STIM2 in cytoplasmic Ca2+ signaling, we followed a similar tack to that used for STIM1. Transfection with an siRNA construct directed against STIM2 led to a significant fall in protein expression (Fig. 3A, Western blot; Fig. 3 B and C, confocal microscopy). However, cytoplasmic Ca2+ oscillations to LTC4 (at least over a 10-min recording period) were unaffected by the reduction in STIM2 levels (a control response is shown in Fig. 3D, and one taken after STIM2 knockdown is depicted in Fig. 3E). Aggregate data from several experiments is summarized in Fig. 3F. STIM2 knockdown had no significant effect on either the amplitude or frequency of Ca2+ oscillations compared with corresponding controls (Fig. S1). Because STIM2 plays an important role in basal Ca2+ entry (14), we were concerned that loss of STIM2 could reduce the amount of Ca2+ within the stores and, thereby, lower store content to a level where STIM1 might compensate. However, the amount of Ca2+ mobilized by ionomycin or thapsigargin in cells in which STIM2 had been knocked down was similar to control cells (Fig. S3).
Fig. 3.
STIM2 does not contribute to cysLT1 receptor-evoked Ca2+ oscillations over 600 s. (A) Western blot shows significant reduction in STIM2 expression after siRNA transfection. (Lower) Depicts aggregate data from three independent results. (B) Confocal microscopy images comparing protein expression in mock-transfected cells and in those in which STIM2 had been knocked down. (C) Histogram depicts aggregate data from three independent experiments (each bar is the average from >18 cells). (D) The trace represents a typical oscillatory Ca2+ signal to LTC4 in a control cell. (E) The recording depicts a typical oscillatory Ca2+ signal to LTC4 in which STIM2 had been knocked down. (F) Aggregate data from several cells are compared (each point represents 18–46 cells).
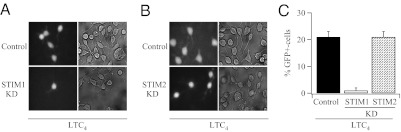
Because T cells from STIM2 knockout mice show impaired NFAT residency in the nucleus with a subsequent decrease in cytokine expression after several hours of stimulation (20), we measured a slow response after cysLT1 receptor activation, namely NFAT-driven gene expression, by using GFP as a reporter gene under an NFAT1 promoter (21). Stimulation with 120 nM LTC4 for 40 min led to robust gene expression 24 h later in ≈20% of the cells (Fig. 4A; aggregate data from several cells is shown in Fig. 4C). Knockdown of STIM1 dramatically reduced the number of responding cells (Fig. 4 A and C). However, knockdown of STIM2 had no significant effect (Fig. 4 B and C).
Fig. 4.
STIM1 is required for NFAT-driven gene expression in response to LTC4. (A) Knockdown of STIM1 reduces NFAT-dependent GFP expression after stimulation with 120 nM LTC4 for 40 min. Control denotes stimulation with LTC4 in mock-transfected cells. (B) Knockdown of STIM2 does not impair gene expression to LTC4. (C) Aggregate data from several experiments is compared.
We investigated the effects of interfering with STIM protein expression on FCεRI-driven Ca2+ signals and downstream gene expression. We therefore applied monomeric IgE acutely to activate the receptors (22).
IgE stimulation of FCεRI receptors evokes a different pattern of Ca2+ oscillation to that elicited after cysLT1 receptor activation. For the latter, the amplitudes of the Ca2+ oscillations are similar, at least for the first few spikes, in the presence or absence of external Ca2+ (Fig. S1). By contrast, the amplitude of Ca2+ oscillations to IgE are reduced by the removal of external Ca2+ (23).
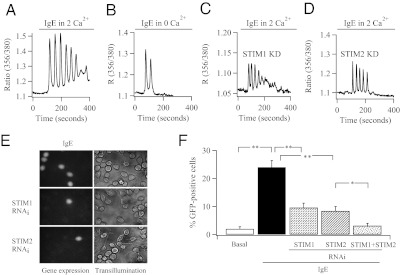
Stimulation with a submaximal concentration of IgE (500 ng/mL; 80 μg/mL leads to a sustained Ca2+ plateau) in the presence of external Ca2+ produced a series of Ca2+ oscillations on a slightly elevated baseline in ≈70% of the cells (Fig. 5A). Both the amplitude and number of Ca2+ oscillations were significantly reduced when cells were challenged with the same concentration of IgE but now in the absence of external Ca2+ (Fig. 5B and Fig. S4). Similar results were seen after knockdown of Orai1 (Fig. S2). Knockdown of either STIM1 or STIM2 altered the Ca2+ response to IgE such that it now more closely resembled the pattern seen in Ca2+-free external solution, (Fig. 5 C and D and Fig. S4). IgE induced robust expression of NFAT-dependent GFP expression (Fig. 5E). Knockdown of either STIM1 or STIM2 led to a significant decrease in the fraction of cells expressing GFP in response to IgE stimulation (Fig. 5F), but there was no significant difference between the STIM1- and STIM2-deficient groups. Knockdown of both STIM1 and STIM2 reduced IgE-driven gene expression to a greater extent than knockdown of either STIM1 or STIM2 alone (Fig. 5F). Knockdown of Orai1 also reduced gene expression to IgE (Fig. S2). It is interesting that Ca2+ entry contributes to the size of the initial IgE-evoked Ca2+ transient (≈30% of the amplitude; Fig. S4), whereas it does not for LTC4-driven oscillations (Fig. S1). Basal Ca2+ entry might be important in helping sensitize InsP3 receptors to the lower levels of InsP3 generated by IgE. Alternatively, tyrosine kinases including Lyn and Syk, which are activated by FCεRI receptors, might facilitate CRAC channel activation (24).
Fig. 5.
FCεRI receptor activation evokes Ca2+ signals and gene expression through STIM1 and STIM2. (A) Typical Ca2+ signal to IgE in a control cell. (B) Ca2+ response to IgE in the absence of external Ca2+. (C) Ca2+ signal to IgE after knockdown of STIM1. (D) Ca2+ signal to IgE after knockdown of STIM2. (E) NFAT-dependent GFP expession after stimulation with IgE in mock-transfected cells (Top) and after knockdown of STIM1 (Middle) or STIM2 (Bottom). (F) Aggregate data from several experiments are compared. *P < 0.05 and **P < 0.01.
We designed two types of experiments to see whether modest store depletion could activate Ca2+ influx via STIM2. First, we stimulated fura 2-loaded cells with different concentrations of thapsigargin (Fig. S5). All stimuli evoked Ca2+ entry, albeit to different extents, that represented the different levels of store depletion. Knockdown of STIM2 failed to alter the kinetics or extent of the Ca2+ signal over any of these thapsigargin concentrations tested (Fig. S5). We also tested the effect of knockdown of STIM2 on Ca2+ influx evoked by 1 nM thapsigargin. This concentration releases Ca2+ slowly, so that Ca2+ extrusion matches the rate of Ca2+ release and no cytoplasmic Ca2+ rise occurs when cells are stimulated in Ca2+-free external solution (Fig. S5). Nevertheless, 1 nM thapsigargin (applied in Ca2+-free solution) depleted stores by 19 ± 5% (28 cells), as judged by the reduction in response to subsequent challenge with 1 μM ionomycin (also applied in Ca2+-free solution). The rate and extent of Ca2+ influx to 1 nM thapsigargin was unaffected by STIM2 knockdown (Fig. S5).
In a second series of experiments, we depleted stores fractionally to activate ICRAC submaximally. We dialyzed cells with a pipette solution containing 2 mM EGTA, which depletes stores partially and generates ICRAC that is ≈30–50% of maximal (25). Dialysis with 2 mM EGTA activated ICRAC (Fig. S6), and the current amplitude was increased further by stronger store depletion with thapsigargin (Fig. S6). The size of ICRAC in response to 2 mM EGTA was unaffected by knockdown of STIM2 (Fig. S6). Hence, activation of ICRAC by store depletion to a level that provides ≈50% maximal ICRAC is unaffected by a significant fall in STIM2 expression.
Discussion
Although STIM proteins function both as Ca2+ sensors and activators of CRAC channels, the relative roles of STIM1 and STIM2 in supporting physiological Ca2+ signals and downstream Ca2+-dependent responses are unresolved. Because of its lower affinity for ER Ca2+ levels, STIM2 will be recruited preferentially after modest store depletion (14), but its impact will be countered by its lower intrinsic ability to open CRAC channels (11–13). More robust Ca2+ release is required to lower store Ca2+ content sufficiently for STIM1 to activate (26).
In HEK293 cells, knockdown of STIM2 had no resolvable effect either on store Ca2+ content or on the frequency of Ca2+ oscillations in response to muscarinic receptor stimulation (11). By contrast, Ca2+ oscillations ran down quickly in STIM1-deficient cells and disappeared with a time course similar to that seen upon stimulation in Ca2+-free external solution (11). These results convincingly demonstrated that STIM1 and not STIM2 supported physiological levels of Ca2+ signaling in response to a G protein-coupled receptor.
A different conclusion was drawn from functional studies on lymphocytes, monitoring gene expression to the Ca2+-dependent transcription factor NFAT. NFAT-driven interleukin 2 production in CD4+ T cells was less in STIM2-deficient lymphocytes than from those taken from control mice (20). Despite weak effects on the Ca2+ signal (20), STIM2 was nevertheless important for sustaining CRAC channel-dependent gene expression, measured several hours after stimulation. Similarly, B-cell–specific deletion of STIM2 revealed that the protein contributed significantly to NFAT-dependent interleukin 10 synthesis, despite only a modest reduction in store-operated Ca2+ influx (27).
Our results help reconcile these findings by demonstrating that different agonists sustain cytoplasmic Ca2+ signals and gene expression through activation of different STIM proteins. Stimulation of cysLT1 receptors, which evokes large all-or-none baseline Ca2+ spikes, recruits STIM1 to open CRAC channels and, thus, support both repetitive Ca2+ oscillations and NFAT-driven gene expression. Knockdown of STIM2 had no effect on either Ca2+ oscillations or Ca2+-dependent gene expression. Hence, STIM1 is necessary and sufficient to support physiological Ca2+ signals and a functional response after cysLT1 receptor activation. Our findings are in good agreement with the study of Bird et al. (11). These authors have suggested that the primary function of a large Ca2+ oscillatory signal might be to ensure sufficient store depletion to activate CRAC channels robustly through STIM1, leading to activation of downstream effectors. In agreement with this view, we have shown that spatially restricted Ca2+ signals near open CRAC channels, but not the Ca2+ oscillations per se, activate gene expression in response to two different transcription factors [c-fos and NFAT (7, 21)].
In contrast to G protein-coupled receptors, oscillatory Ca2+ signals, and NFAT-driven gene expression in response to FCεRI receptor activation requires both STIM1 and STIM2. Knockdown of either protein reduced both the amplitude of the Ca2+ spikes and the number of cells expressing an NFAT-dependent reporter gene. How might two agonists, which both stimulate the phospholipase C pathway to generate Ca2+ oscillations and open CRAC channels, activate different combinations of STIM proteins to sustain CRAC channel activity? Like most G protein-coupled receptors, the Ca2+ oscillations evoked by cysLT1 receptor activation are large, attaining a global amplitude of ≈1 μM. With a cytoplasmic Ca2+ binding capacity of ≈100 (28) and with the ER occupying ≈10% of the cytoplasmic volume, 1.25 mmol/L Ca2+ would have to be mobilized from the ER to generate a typical Ca2+ oscillation. With the total amount of Ca2+ stored within the ER estimated to be 2–5 mmol/L, a sizeable fraction of the total available Ca2+ pool within the organelle would be mobilized during each Ca2+ spike. Each Ca2+ oscillation evoked by LTC4 would therefore cause a substantial, albeit transient, drop in ER Ca2+, which would exceed the threshold required for STIM1 activation. Hence, in response to cysLT1 receptor stimulation, and in good agreement with ref. 11, Ca2+ oscillations, the subsequent opening of CRAC channels, and the local Ca2+ entry that signals to the nucleus to activate gene expression all depend on STIM1 and not STIM2. Unlike G protein-coupled receptors that activate phospholipase Cβ rapidly through a heterotrimeric G protein and, therefore, produce a precipitious but transient drop in ER Ca2+, FCεRI receptor stimulation leads to slower build up of InsP3 through tyrosine kinase-dependent activation of phospholipase Cγ. In the case for IgE, a steady state will therefore arise when store content has been partially reduced, reflecting a balance between InsP3 production and breakdown. Hence, a few STIM1 and many STIM2 proteins would probably be activated with this stimulus, which would be in agreement with the functional study from (20).
An interesting puzzle is why STIM2 contributes to IgE-evoked Ca2+ signals and gene expression but not to those elicited by cysLT1 receptors. It is unlikely that STIM2 is needed for STIM1 trafficking because knockdown of STIM2 does not reduce the response to cysLT1 receptor activation. Nor does STIM2 gate Orai1 so poorly that the channels do not open, because loss of STIM2 had significant effect on the IgE-evoked Ca2+ signal and gene expression. One possibility is that a signal derived from activated cysLT1 receptors, but not FCεRI receptors, destabilizes STIM2–Orai1 interaction. Alternatively, Parvez et al. have reported that STIM2 is prevented from activating Orai1 by calmodulin (13). It is possible that the larger Ca2+ release generated by cysLT1 receptors (ratio of 0.24 ± 0.01; Fig. S1) might stabilize this inhibitory interaction, whereas the smaller Ca2+ release signal to IgE (0.19 ± 0.025 for IgE; Fig. S4) results in a weaker interaction. Resolution of this issue requires further work.
Our cytoplasmic Ca2+ measurements in response to different concentrations of thapsigargin and patch clamp data both suggest that STIM2 plays little role in supporting Ca2+ influx after ≥20% store depletion (Fig. S4) or when ICRAC is activated beyond ≈30–50% of its maximal amplitude (Fig. S5). These findings support the view that STIM2 plays a housekeeping role, ensuring the stores are replete with Ca2+ before stimulation and contributes to Ca2+ signals in response to low levels of receptor stimulation that deplete stores modestly (14). Stronger levels of stimulation, which would cause a larger cytoplasmic Ca2+ rise, switches Orai1 gating to a STIM1-based mechanism.
Materials and Methods
Cells.
RBL-1 cells were purchased from ATCC (via UK supplier LGC) and were cultured at 37 °C with 5% (vol/vol) CO2 in DMEM supplemented with 10% (vol/vol) FBS and 1% (vol/vol) penicillin/streptomycin.
Plasmid cDNA Constructs and RNA Silencing.
The EGFP-based reporter plasmid (pNFAT-TA-EGFP) was a kind gift from Yuriy Usachev (University of Iowa, Iowa City, IA). siRNA against STIM1 and STIM2 was from Invitrogen.
Transfection.
RBL-1 cells were transfected by using the Amaxa system, as described (21). Cells were used 24–36 h after transfection.
Western Blotting.
Total cell lysates (50 μg) were separated by SDS/PAGE on a 10% gel and electrophoretically transferred to nitrocellulose membrane, as described (21). Membranes were blocked with 5% (wt/vol) nonfat dry milk in TBS plus 0.1% Tween 20 (TBST) buffer for 1 h at room temperature. Membranes were washed with TBST three times and then incubated with primary antibody overnight at 4 °C. Anti-STIM1 and -STIM2 antibodies were obtained from Cell Signaling Technology and used at 1:1,000 dilution. Total ERK antibody was used at a dilution of 1:5,000. The membranes were then washed with TBST again and incubated with a 1:2,500 dilution of goat anti-rabbit secondary antibody IgG from Santa Cruz Biotechnology for 1 h at room temperature. After washing with TBST, the bands were developed for visualization by using ECL-plus Western blotting detection system (GE Healthcare). Gels were quantified by using the UN-SCAN-IT software package (Silk Scientific). Total ERK2 is widely used as a control for gel loading. The antibody does not discriminate between phosphorylated (and hence active) and nonphosphorylated ERK2 and therefore detects the total amount of this protein, regardless of whether the kinase has been activated. The extent of STIM protein was therefore normalized to the total amount of ERK2 present in each lysate, to correct for any differences in amount of cells used for each experiment.
Immunocytochemistry.
Cells were fixed in 4% (wt/vol) paraformaldehyde in phosphate buffer for 30 min at room temperature (29). All of the washes used 0.01% PBS (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 1 mM KH2PO4). The cells were blocked with 2% (wt/vol) BSA and 10% (vol/vol) goat serum for 1 h. Anti-STIM1 and -STIM2 were used in carrier (0.2% (wt/vol) BSA, 1% (vol/vol) goat serum) and left overnight at 4 °C and were purchased from Cell Signaling. The secondary anti-rabbit IgG was a HandL chain specific (goat) fluorescein conjugate (Alexa Fluor 568, excitation at 578 nm, emission at 603 nm wavelength) from Invitrogen. This antibody was used in PBS for 2 h at room temperature. The cells were mounted in Vectashield mounting medium. Images were obtained by using a Leica confocal microscope, as described. Protein expression was quantified by computing fluorescence in three regions of interest (of identical size) in each cell, using ImageJ software, averaged and pooled together for each condition. Overexpression of STIM1 increased fluorescence approximately two- to fourfold above levels seen in wild-type cells, indicating that the latter level was not close to saturation of the detection system.
Gene Reporter Assay.
Twenty-four to 36 hours after transfection with the EGFP-based reporter plasmid that contained an NFAT promoter, cells were stimulated with either leukotriene C4 or IgE, and the percent of cells expressing EGFP was measured. Gene expression was defined as fluorescence 3× SD > cell autofluorescence, measured in nontransfected cells. Cells were stimulated in culture medium and maintained in the incubator.
Cytoplasmic Ca2+ Measurements.
Cytoplasmic Ca2+ measurements were carried out at room temperature by using the IMAGO charge-coupled device camera-based system from TILL Photonics (29). Cells were alternately excited at 356 and 380 nm (20-ms exposures), at 0.5 Hz. Cells were loaded with Fura-2/AM (1 μM) for 40 min at room temperature in the dark and then washed three times in standard external solution composed of 145 mM NaCl, 2.8 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM d-glucose, and 10 mM Hepes at pH 7.4, with NaOH. Cells were left for 15 min to allow further deesterification. Ca2+-free solution had the following composition: 145 mM NaCl, 2.8 mM KCl, 2 mM MgCl2, 10 mM d-glucose, 10 mM Hepes, and 0.1 mM EGTA at pH 7.4 with NaOH. Ca2+ signals are plotted as R, which denotes the 356/380 nm ratio. Rmin was 0.3 and Rmax was 2.2.
Electrophysiology.
Whole cell patch clamp experiments were carried out as described (25). Sylgard-coated, fire-polished pipettes were filled with internal solution containing the following: 145 mM Cs+-glutamate, 8 mM NaCl, 1 mM MgCl2, 1 mM Mg-ATP, 2 mM EGTA, and 10 mM Hepes at pH 7.2 with CsOH. Bath solution contained the following: 140 mM NaCl, 4 mM KCl, 10 mM CaCl2, 2 mM MgCl2, 10 mM CsCl, 10 mM Hepes, 10 mM d-glucose, pH 7.2 with NaOH. Pipette resistances were ≈4 MΩ and series resistance was <10 MΩ. ICRAC was measured at −80 mV from voltage ramps spanning −100 to + 100 mV (50-ms duration) at 0.5 Hz. Holding potential was 0 mV.
Statistical Analysis.
Data are presented as the mean ± SE. Statistical significance was determined by using the Student t test, except in Fig. 5F, where an ANOVA followed by a post hoc Newman–Keuls multiple comparison test was used (*P < 0.05 and **P < 0.01).
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Council.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201204109/-/DCSupplemental.
References
- 1.Thomas AP, Bird GS, Hajnóczky G, Robb-Gaspers LD, Putney JWJ., Jr Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci. 2011;36:78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 5.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 6.Wedel B, Boyles RR, Putney JW, Jr, Bird GS. Role of the store-operated calcium entry proteins Stim1 and Orai1 in muscarinic cholinergic receptor-stimulated calcium oscillations in human embryonic kidney cells. J Physiol. 2007;579:679–689. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Capite J, Ng S-W, Parekh AB. Decoding of cytoplasmic Ca(2+) oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–858. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 8.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parekh AB. Store-operated CRAC channels: Function in health and disease. Nat Rev Drug Discov. 2010;9:399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]
- 10.Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird GS, et al. STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–1729. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, et al. The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J Biol Chem. 2009;284:19164–19168. doi: 10.1074/jbc.C109.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parvez S, et al. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 2008;22:752–761. doi: 10.1096/fj.07-9449com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TD, Eddlestone GT, Mahmoud SF, Kuchtey J, Fewtrell C. Correlating Ca2+ responses and secretion in individual RBL-2H3 mucosal mast cells. J Biol Chem. 1997;272:31225–31229. doi: 10.1074/jbc.272.50.31225. [DOI] [PubMed] [Google Scholar]
- 16.Parekh AB, Penner R. Depletion-activated calcium current is inhibited by protein kinase in RBL-2H3 cells. Proc Natl Acad Sci USA. 1995;92:7907–7911. doi: 10.1073/pnas.92.17.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Capite JL, Shirley A, Nelson C, Bates G, Parekh AB. Intercellular Ca2+ wave propagation involving positive feedback between CRAC channels and cysteinyl leukotrienes. FASEB J. 2009;23:894–905. doi: 10.1096/fj.08-118935. [DOI] [PubMed] [Google Scholar]
- 18.Ng S-W, di Capite JL, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 19.Bird GS, Putney JWJ., Jr Capacitative calcium entry supports calcium oscillations in human embryonic kidney cells. J Physiol. 2005;562:697–706. doi: 10.1113/jphysiol.2004.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kar P, Nelson C, Parekh AB. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2011;286:14795–14803. doi: 10.1074/jbc.M111.220582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandey V, Mihara S, Fensome-Green A, Bolsover S, Cockcroft S. Monomeric IgE stimulates NFAT translocation into the nucleus, a rise in cytosol Ca2+, degranulation, and membrane ruffling in the cultured rat basophilic leukemia-2H3 mast cell line. J Immunol. 2004;172:4048–4058. doi: 10.4049/jimmunol.172.7.4048. [DOI] [PubMed] [Google Scholar]
- 23.Kuchtey J, Fewtrell C. Protein kinase C activator PMA reduces the Ca(2+) response to antigen stimulation of adherent RBL-2H3 mucosal mast cells by inhibiting depletion of intracellular Ca(2+) stores. J Cell Physiol. 1999;181:113–123. doi: 10.1002/(SICI)1097-4652(199910)181:1<113::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Chung SC, Limnander A, Kurosaki T, Weiss A, Korenbrot JI. Coupling Ca2+ store release to Icrac channel activation in B lymphocytes requires the activity of Lyn and Syk kinases. J Cell Biol. 2007;177:317–328. doi: 10.1083/jcb.200702050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fierro L, Parekh AB. On the characterisation of the mechanism underlying passive activation of the Ca2+ release-activated Ca2+ current ICRAC in rat basophilic leukaemia cells. J Physiol. 1999;520:407–416. doi: 10.1111/j.1469-7793.1999.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto M, et al. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34:703–714. doi: 10.1016/j.immuni.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995;34:1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- 29.Ng S-W, Nelson C, Parekh AB. Coupling of Ca(2+) microdomains to spatially and temporally distinct cellular responses by the tyrosine kinase Syk. J Biol Chem. 2009;284:24767–24772. doi: 10.1074/jbc.M109.011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.