Abstract
Pancreatic cancer is an almost uniformly lethal disease, characterized by late diagnosis, early metastasis, resistance to chemotherapy, and early mutation of the Kras oncogene. Here we show that the receptor for advanced glycation endproducts (RAGE) is required for the activation of interleukin 6 (IL-6)–mediated mitochondrial signal transducers and activators of transcription 3 (STAT3) signaling in pancreatic carcinogenesis. RAGE expression correlates with elevated levels of autophagy in pancreatic cancer in vivo and in vitro, and this heightened state of autophagy is required for IL-6–induced STAT3 activation. To further explore the intersection of RAGE, autophagy, and pancreatic carcinogenesis, we created a transgenic murine model, backcrossing RAGE-null mice to a spontaneous mouse model of pancreatic cancer, Pdx1-Cre:KrasG12D/+ (KC). Targeted ablation of Rage in KC mice delayed neoplasia development, decreased levels of autophagy, and inhibited mitochondrial STAT3 activity and subsequent ATP production. Our results suggest a critical role for RAGE expression in the earliest stages of pancreatic carcinogenesis, potentially acting as the “autophagic switch,” regulating mitochondrial STAT3 signaling.
Keywords: oncogenesis, bioenergetics, inflammation, metabolism, high-mobility group box 1
Pancreatic cancer ranks as the fourth leading cause of cancer death, accounting for 6–7% of all cancer-related deaths in the United States, in 2011 (1). Most pancreatic ductal adenocarcinomas (PDA) are thought to arise from well-defined precursor lesions, termed pancreatic ductal intraepithelial neoplasia (PanIN) (2). Many human PanIN lesions do not progress to invasive carcinomas, so defining the events that drive carcinogenesis in the emergent tumor microenvironment is of critical importance. Studies into human pancreatic carcinogenesis have been greatly facilitated by the development of a genetically engineered mouse model that expresses oncogenic Kras under a pancreatic promoter Pdx1-Cre:KrasG12D/+ (KC) (3). A more detailed understanding of how these pathways accelerate pancreatic carcinogenesis may allow improved therapeutic strategies.
The receptor for advanced glycation endproducts (RAGE) is a member of the Ig superfamily. RAGE and its ligands, including high-mobility group box 1 (HMGB1) and S100, are linked to the development and progression of several cancers by facilitating the maintenance of a chronic inflammatory state (4) and/or by promotion of metastases (5). We previously observed that RAGE sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival during chemotherapy and oxidative stress in vivo and in vitro (6, 7). Autophagy is an essential catabolic process by which cells break down old or damaged organelles and proteins (8). Autophagy promotes cell survival and supports metabolism during cell stress (9). Conversely, apoptosis promotes tumor growth early in the development of cancer (10) during periods of inhibition of autophagy with a subsequent switch to suppressed apoptosis, acquired later during the temporal development of more advanced cancers. Pancreatic tumors have elevated autophagy under basal conditions compared with other epithelial cancers (11). Inhibition of autophagy in pancreatic tumor cells augments production of reactive oxygen species, increases DNA damage, and limits effective metabolism with decreased mitochondrial oxidative phosphorylation, resulting in significant inhibition of pancreatic tumor growth (12). Despite the growing body of literature supporting the importance of autophagy and RAGE in pancreatic cancer (6, 7, 12), the role of RAGE-mediated autophagy in pancreatic carcinogenesis and the underlying mechanisms for such a phenomenon have not been addressed.
We demonstrate here that RAGE expression is permissive for the development of early pancreatic neoplasia by enhancing the mitochondrial interleukin 6 (IL-6)/signal transducers and activators of transcription 3 (STAT3) pathway in pancreatic tumor cells. These effects depend on autophagy and lead to enhanced ATP production in vitro and in vivo.
Results
RAGE Promotes Development of PanIN Lesions in the Setting of Kras-Driven Neoplasia.
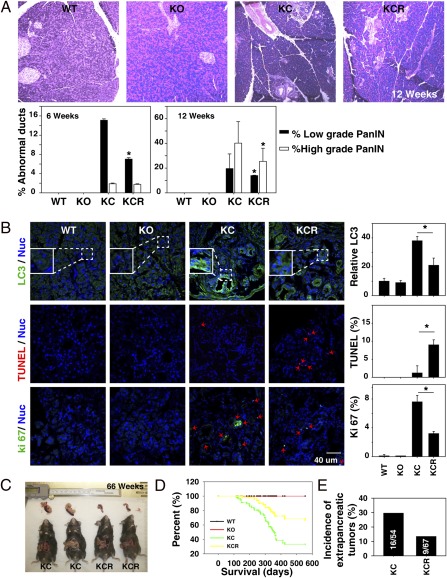
We first examined expression of RAGE in the tumor microenvironment of the genetically engineered KC mice. These mice express a conditional oncogenic Kras mutation in the pancreas, developing PanIN lesions starting around 6 wk of age. As many as 30% of these animals ultimately develop invasive adenocarcinoma of the pancreas (42 wk) in a pattern that recapitulates human pancreatic carcinogenesis with high fidelity (3). We observed increasing RAGE expression concurrent with progression of pancreatic PanIN lesions in these mice. RAGE expression was observed in both the epithelial precursors as well as the stromal components of the developing tumor (Fig. 1). We also observed that RAGE is overexpressed in human pancreatic cancer specimens but not in adjacent normal ducts (Fig. S1). To test the hypothesis that RAGE expression promotes development of neoplasia, we created a transgenic murine strain, Pdx1-Cre:KrasG12D/+:Rage−/− (KCR), backcrossing RAGE-null mice (SVEV129 × C57BL/6) to the genetically engineered KC strain. Targeted ablation of RAGE (KCR) delayed PanIN lesion development and progression (Fig. 2A). Consistent with our previous observations in vitro (6, 7), targeted ablation of RAGE decreased levels of autophagy [microtubule-associated protein light chain 3 (LC3)] in PanIN lesions (Fig. 2B and Fig. S2). We also observed that decreased measures of autophagy correlated with increased evidence of apoptosis (TUNEL) (Fig. 2B and Fig. S2). Decreased autophagy and increased apoptosis are associated with decreased proliferation (Ki67) in the emergent neoplastic microenvironment (Fig. 2B). At the late stage of pancreatic carcinogenesis, we found a difference in animal survival and tumor size between KC and KCR mice that we monitored for 66 wk (462 d) (Fig. 2 C and D). Moreover, there was a significant difference in the incidence of extrapancreatic tumors (Fig. 2E). The incidence of tumors of the face and vulva was 16/54 (29.6%) in KC mice compared with 9/67 (13.4%) in KCR animals.
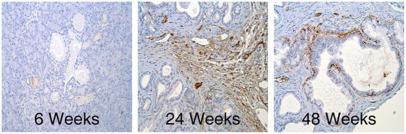
Fig. 1.
Up-regulation of RAGE during pancreatic carcinogenesis. Immunohistochemical staining with anti-RAGE primary antibody in pancreatic specimens from KC animals at 6, 24, and 48 wk of age shows increased RAGE expression in peritumoral stroma and dysplastic ducts as lesions progress.
Fig. 2.
Targeted ablation of RAGE delays pancreatic carcinogenesis. (A) H&E staining of the pancreas in RAGE WT, RAGE KO, KC, and KCR mice at 12 wk of age. Quantitative analysis of abnormal ducts is shown in the graphs below the H&E sections (*P < 0.05, KC vs. KCR). (B) Confocal immunofluorescent microscopic analysis of LC3, TUNEL, and Ki67 stains from pancreatic specimens at 42 wk of age (quantitative analysis drawn from five separate high-power fields; *P < 0.05). TUNEL and Ki67 are shown as percentage of positive cells (red arrows), and LC3 is shown as relative fluorescence intensity (WT set as 10). (C) Pancreatic tumor size in KC and KCR mice at 66 wk. (D) Kaplan–Meier survival curve shows difference in survival between WT, KO, KC, and KCR mice (P = 0.037). (E) Incidence of tumors of the face and vulva in KCR mice is significantly decreased compared with KC.
RAGE Expression Is Associated with Decreased Secretion of IL-6 and IL-6–Driven Proliferation of Pancreatic Tumor Cells.
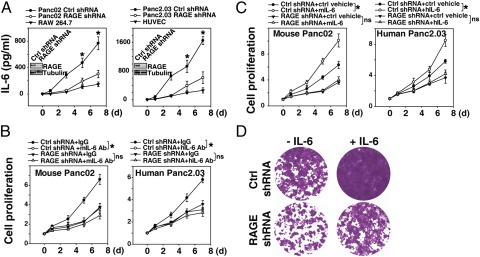
Recent studies have suggested that IL-6 transsignaling from infiltrating myeloid cells contributes to Kras-driven carcinogenesis by promoting phosphorylation of STAT3 (13). We observed decreased levels of serum IL-6 in KCR mice compared with KC mice, suggesting that RAGE promotes IL-6 production in the emerging tumor microenvironment (Fig. S3). To explore the effect of IL-6 and RAGE on pancreatic cancer cells, we suppressed RAGE expression with RAGE-specific shRNA. Knockdown of RAGE significantly inhibited IL-6 autocrine/paracrine secretion relative to control shRNA-expressing cells (Fig. 3A). Moreover, treatment with an IL-6 neutralizing antibody inhibited cell proliferation in RAGE WT but not RAGE knockdown pancreatic cancer cells (Fig. 3B). In addition, knockdown of RAGE inhibited exogenous IL-6–induced cell proliferation (Fig. 3C). Colony-formation assays showed that IL-6 enhanced the long-term survival of RAGE WT Panc02 cells but not RAGE knockdown cells (Fig. 3D). These findings support the notion that RAGE regulates not only IL-6 release but also IL-6–mediated tumor cell proliferation.
Fig. 3.
RAGE expression promotes IL-6 secretion and cellular proliferation. (A) Indicated cells were cultured for 0–7 d, and IL-6 levels in the cell culture medium were assayed by ELISA (n = 3 wells per condition; *P < 0.05, control shRNA vs. RAGE shRNA). (B and C) Indicated cells were cultured with or without IL-6 neutralizing antibody (B, 10 μg/mL) or recombinant IL-6 (C, 10 ng/mL) for 0–7 d, and then the cell proliferation was assayed by Cell Counting Kit-8 (n = 3 wells per condition; *P < 0.05; ns, not significant). IL-6 level on day 0 set as 1, with subsequent days reflecting relative levels compared with day 0. mIL-6, mouse IL-6; hIL-6, human IL-6. (D) Colony-formation assay. Panc02 cells were treated with IL-6 (10 ng/mL) for 72 h, then 1,000 cells were plated into 24-well plates. Colonies were visualized by crystal violet staining 2 wk later.
RAGE Promotes IL-6–Induced Phosphorylation of STAT3 and Its Mitochondrial Localization.
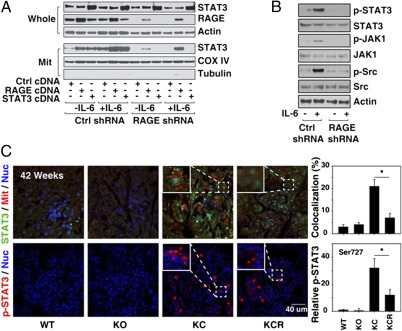
STAT3 mediates IL-6–dependent cell survival and proliferation. STAT3 also functions in mitochondria by regulating complex I activity to modulate cellular respiration (14). We next examined the effect of RAGE knockdown on the localization of STAT3 within cells by isolating mitochondrial and whole-cell lysate fractions. IL-6 significantly increased mitochondrial STAT3 expression in control shRNA-transfected cells but not RAGE shRNA-transfected cells (Fig. 4A). Transfection with RAGE cDNA in RAGE knockdown cells restored IL-6–induced mitochondrial STAT3 localization (Fig. 4A). Phosphorylated Ser727 is important for the mitochondrial localization of STAT3 (14). Consistent with this observation knockdown of RAGE inhibited IL-6–induced STAT3 Ser727 phosphorylation and the upstream signaling pathways of STAT3 such as phosphorylation of Jak1 and Src (Fig. 4B). Next we examined the levels of total and mitochondrial phosphorylated STAT3 (p-STAT3) in vivo in KC and KCR mice. Consistent with our in vitro observations, targeted ablation of RAGE decreased both the overall levels of p-STAT3 as well as the mitochondrial localization (Fig. 4C). These findings suggest that RAGE is required for the mitochondrial localization of STAT3 in pancreatic cancer cells.
Fig. 4.
RAGE expression promotes localization of p-STAT3 in mitochondria. (A) RAGE WT (Ctrl shRNA) and knockdown (RAGE shRNA) Panc02 cells were transfected with mouse STAT3 or RAGE cDNA for 48 h, and these cells were treated with IL-6 (10 ng/mL) for 24 h. The expression of STAT3 in whole and mitochondrial (Mit) extracts was analyzed by Western blotting. (B) Representative Western blot showing diminished p-STAT3 (Ser727), p-JAK1 (Tyr1022/1023), and p-Src (Tyr416) in RAGE knockdown Panc02 cells after treatment with IL-6 (20 ng/mL) for 1 h. (C) Confocal immunofluorescent microscopic analysis of colocalization of STAT3 and mitochondria as well as phosphorylation of STAT3 at Ser727 (red arrows) in pancreatic specimens from 42-wk-old mice (quantitative analysis drawn from five separate high-power fields; *P < 0.05).
Autophagy Regulates Mitochondrial Localization and Function of STAT3.
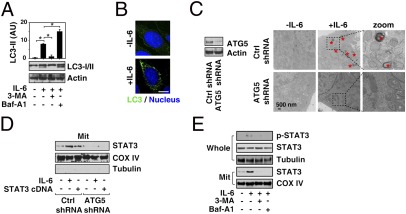
Autophagy is a catabolic pathway used by cells to support metabolism in response to cell stress (9). We have previously demonstrated significant impact of autophagy on mitochondrial synthesis and function (15). Therefore, we next explored the relationship between autophagy and the IL-6/STAT3 signaling pathway. We observed that IL-6 induced LC3-II expression (Fig. 5A) and LC3 spot formation (Fig. 5B), which are both widely used markers of autophagy in murine and human pancreatic tumor cells (16). To characterize the effects of IL-6 on autophagic flux, we assessed the effects of cotreatment with inhibitors of early [3-methyladenine (3-MA)] and late [bafilomycin A1 (Baf-A1)] autophagy on LC3-II formation (Fig. 5A). 3-MA attenuated IL-6–induced LC3-II expression in pancreatic cancer cells. In contrast, an increase in LC3-II protein resulted from blockade of late lysosome–autophagosome fusion and lysosome function (e.g., Baf-A1) (16). To explore whether autophagy regulates the mitochondrial localization and function of STAT3, we suppressed autophagy protein 5 (ATG5) expression by shRNA knockdown in Panc02 cells (Fig. 5C). ATG5 has been previously characterized as a protein specifically required for autophagy (17). Knockdown of ATG5 significantly inhibited IL-6–induced autophagic vacuoles or organelles by transmission electron microscopy (TEM) (Fig. 5C). Notably, knockdown of ATG5 inhibited both IL-6 and STAT3 cDNA-induced STAT3 mitochondrial expression (Fig. 5D). The autophagy inhibitors 3-MA and Baf-A1 significantly reduced IL-6–induced STAT3 serine phosphorylation and mitochondrial STAT3 (Fig. 5E) as well. These findings suggest that autophagy is required for sustaining IL-6–induced mitochondrial localization of STAT3.
Fig. 5.
Enhanced autophagy promotes localization of STAT3 in mitochondria. (A) Panc02 cells were treated with IL-6 (10 ng/mL) for 24 h with or without 3-MA (10 mM) and Baf-A1 (100 nM), and Western blot analysis of LC3 expression was performed (*P < 0.05). (B) Confocal immunofluorescent microscopic analysis of LC3 spot formation in Panc02 cells after treatment with IL-6 (10 ng/mL) for 24 h. (C) Panc02 cells were transfected with ATG5 shRNA, treated with IL-6 (10 ng/mL) for 24 h, and subjected to ultrastructural analysis with TEM. Autophagic vacuoles or organelles are indicated by red asterisks. (D) Western blot analysis of STAT3 expression in ATG5 shRNA-transfected Panc02 cells treated with IL-6 (10 ng/mL for 24 h) or STAT3 cDNA. (E) Western blot analysis of STAT3 and p-STAT3 expression in whole and mitochondrial (Mit) fractions of Panc02 cells treated with IL-6 (10 ng/mL for 24 h) with or without the autophagy inhibitors 3-MA (10 mM) or Baf-A1 (100 nM).
RAGE Permits IL-6/STAT3–Induced Autophagy in Pancreatic Cancer Cells.
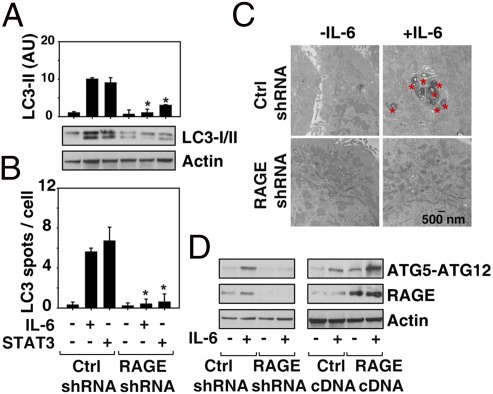
Our previous studies have demonstrated that RAGE is a positive regulator of autophagy during chemotherapy and oxidative stress (6, 7). Consistent with these observations, we found that knockdown of RAGE in pancreatic cancer cells decreased IL-6 and STAT3 cDNA-induced conversion of LC3-I to LC3-II (Fig. 6A) and LC3 spot formation (Fig. 6B). We next monitored IL-6–induced autophagy through detection of autophagic vacuoles or organelles by TEM, the gold standard for assessing autophagy (16). TEM images showed the presence of autophagosomes in IL-6–treated RAGE WT but not RAGE knockdown Panc02 cells (Fig. 6C). The ATG12–ATG5 conjugate has an E3-like ubiquitin ligase activity-promoting protein lipidation in autophagy (18) that is critical for the elongation of the initiating autophagosomal membrane. Overexpression of RAGE increased IL-6–induced ATG12–ATG5 conjugation, whereas targeted knockdown of RAGE decreased this effect (Fig. 6D). Thus, RAGE regulates ATG5-dependent autophagy, which in turn influences mitochondrial localization and function of STAT3.
Fig. 6.
RAGE expression enhances IL-6/STAT3–induced autophagy. (A and B) Control and RAGE shRNA-transfected Panc02 cells were treated with IL-6 (10 ng/mL) or STAT3 cDNA for 24 h and analyzed for LC3 expression by Western blotting and LC3 spot formation and confocal immunofluorescent microscopy (quantitative analysis represents the mean count from three high-power fields; *P < 0.05). (C) In parallel, ultrastructural analysis of autophagic vacuoles and organelles was performed. These are represented by red asterisks. (D) Panc02 cell were transfected with RAGE shRNA or RAGE cDNA for 48 h and then treated with IL-6 (10 ng/mL) for 24 h. ATG12–ATG5 conjugation was assayed by Western blotting.
RAGE Promotes IL-6–Induced ATP Production.
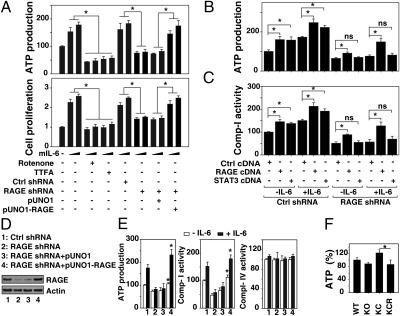
It has been hypothesized that epithelial cells undergoing malignant transformation need to generate significantly more energy in the form of ATP to selectively support their growth and proliferation in the emerging tumor microenvironment (19). Because RAGE and IL-6–induced autophagic flux promote mitochondrial localization of p-STAT3, we evaluated ATP levels. IL-6 stimulation increased intracellular ATP production in pancreatic cancer cells in a dose-dependent manner (Fig. 7A). Small molecular inhibitors that block mitochondrial respiration and ATP production were then used to determine their respective roles in IL-6–induced pancreatic cancer cell proliferation. Inhibition of mitochondrial respiratory chain complex I by rotenone or complex II by 2-thenoyltrifluoroacetone (TTFA) decreased IL-6–induced ATP production and cell proliferation (Fig. 7A). Knockdown of RAGE by shRNA decreased IL-6–induced ATP production, which is accompanied by diminished cell proliferation (Fig. 7A). These findings suggest that RAGE is important for IL-6–mediated cellular proliferation mediated partly through regulation of ATP production. Similarly, transfection with STAT3 cDNA increased IL-6–induced ATP production (Fig. 7B) and complex I activity (Fig. 7C) in control of shRNA-transfected cells but not RAGE shRNA-transfected Panc02 cells, suggesting that RAGE is important in mediating STAT3-mediated cellular respiration. To determine whether changes in IL-6–induced ATP production might be directly attributable to loss of RAGE, we transfected a RAGE-expressing plasmid into RAGE knockdown cells. Reexpression of RAGE restored ATP production and complex I activity but not complex IV activity (Fig. 7D), confirming that RAGE is critical for IL-6–mediated ATP production by regulating mitochondrial respiratory chain complex I activity. We then examined the levels of ATP in KC and KCR mice (Fig. 7E). Overexpression of mutant Kras promotes increased production of ATP compared with WT and RAGE KO nondysplastic controls. Knockdown of RAGE in the setting of mutant Kras expression leads to significantly lower absolute ATP amounts in the pancreas, consistent with our in vitro observations.
Fig. 7.
Expression of RAGE enhances ATP production. (A and B) Re-expression of RAGE with full-length cDNA (pUNO1-RAGE) in RAGE knockdown cells (RAGE shRNA) restores IL-6–induced ATP production and cell proliferation. Indicated Panc02 (A) and panc2.03 (B) cells were treated with IL-6 (10 ng/mL or 20 ng/mL) for 24 h with or without rotenone (1 μM) or 2-thenoyltrifluoroacetone (TTFA, 500 μM), and ATP production and cell proliferation were assayed (n = 3; *P < 0.05) mIL-6, mouse IL-6. (C and D) RAGE WT (Ctrl shRNA) and knockdown (RAGE shRNA) Panc02 cells were transfected with mouse STAT3 or RAGE cDNA for 48 h, and these cells were treated with IL-6 (10 ng/mL) for 24 h. ATP production and cell proliferation were assayed (n = 3; *P < 0.05; ns, not significant). (E) Indicated Panc02 cells were treated with IL-6 (10 ng/mL) for 24 h, and ATP production and activation of complexes I and IV were assayed (n = 3 wells per condition; *P < 0.05). (F) ATP levels of pancreas from WT, KO, KC, and KCR mice were harvested at 42 wk and analyzed (n = 3 wells per specimen or three different specimens; *P < 0.05, KC vs. KCR).
Discussion
The present study provides evidence for the importance of RAGE in the early pathogenesis of Kras-driven pancreatic cancer. We demonstrate that RAGE expression appears to amplify a feedforward loop of programmed cell survival that depends on autophagy, autocrine IL-6 production, and IL-6–promoted mitochondrial STAT3 phosphorylation and localization, which in turn promotes enhanced ATP production in pancreatic cancer cells and their progenitors.
RAGE is mainly expressed constitutively in the lung and, following activation, on immune, endothelial, and vascular smooth muscle cells as well as a variety of epithelial malignancies (20). RAGE is expressed in murine and human pancreatic cancer cells in vitro and in vivo. In the current study, we have extended our preliminary observations demonstrating that expression of RAGE promotes the development of PanIN lesions in the genetically engineered KC model of Kras-driven pancreatic carcinogenesis. Our findings support the notion that RAGE expression and functionality are associated with the development of early pancreatic cancer precursors lesions. It will be important to examine the effect of RAGE expression on the progression from late PanIN lesions to invasive PDA and of invasive cancer to metastatic lesions.
RAGE appears to mediate its activity through modulation of the IL-6/STAT3 pathway. STATs are signal messengers and transcription factors that participate in normal cellular responses to cytokines and growth factors. Dysregulated activation of STAT signaling is involved in the development of several human cancers (21). IL-6 is a cytokine with a unique capacity to activate STAT3 in both immune and cancer cells (22) and is elevated in the serum of patients with pancreatic cancer (23). IL-6 transsignaling promotes STAT3 phosphorylation and the subsequent development and progression of PanIN lesions in the KC genetically engineered model (13, 24). Interestingly STAT3-mediated activity is associated with diminished apoptosis and increased proliferation in the emergent tumor microenvironment (13, 24). Knockdown of RAGE in KC mice decreased expression of mitochondrial p-STAT3, increased apoptosis, and decreased proliferation in the tumor microenvironment. Our in vitro studies of murine and human pancreatic tumor cell lines suggested that this effect could be the result of IL-6–dependent induction of autophagy. Based on these observations, we propose that an important and previously unrecognized role of RAGE expression is to serve to amplify the IL-6/STAT3 survival signal within epithelial tumor progenitors. Amplification of mitochondrial p-STAT3 expression, in turn, crucially depends on the ability to increase autophagy within the cell.
The pancreatic tumor microenvironment comprises many cell types, including infiltrating immune cells and fibroblasts. Indeed, expression of RAGE and its ligands is required to promote IL-6 production as well as other cytokines in immune cells and fibroblast (25). These intracellular pathways activated in stressed epithelial precursor lesions and tumor progeny are likely also used in these other cells. We have not at this point elucidated the relative importance of RAGE expression on the infiltrating myeloid cells and surrounding stroma.
Tumor cells and their precursors have increased energy requirements. ATP production occurs solely through glycolysis and oxidative phosphorylation within mitochondria. Recently, it was proposed that STAT3 has a role in mitochondrial function, regulating complex I activation and ATP derivation (14), promoting the oncogenic property of Ras-mediated cellular transformation (26). IL-6 promotes STAT3 localization to the mitochondria and increased cellular ATP levels. The mechanism of STAT3 mitochondrial translocation has not been fully characterized, although others, and now we, have confirmed that the phosphorylation of Ser727 on STAT3 appears to be required for its functional activity within the mitochondria (14). In this study, we have further demonstrated that IL-6–dependent mitochondrial localization of p-STAT3 was abrogated when autophagy was inhibited genetically or pharmacologically. Knockdown of critical autophagy genes such as ATG5 or treatment with autophagy inhibitors blocked IL-6–induced STAT3 phosphorylation at Ser727, mitochondrial translocation of STAT3, and subsequent increases in ATP production. Based on these observations, we now propose that autophagy is required for the mitochondrial localization and function of STAT3. The precise means by which STAT3 translocates to the mitochondria is an area of active investigation.
The complex relationship among autophagy, tumorigenesis, and tumor progression depends on tumor type and context. For example, autophagy inhibits the development of breast (27) and liver cancers (28), but it promotes the growth of pancreatic cancer (12) and BCR-Abl–associated leukemia (29). Autophagy can promote both glycolysis and oxidative phosphorylation during Ras-mediated oncogenic transformation and thus augments tumor growth (30, 31). Our findings indicate that RAGE-mediated autophagy is required for enhanced ATP production and proliferation in pancreatic cancer cells, mostly via regulation of mitochondrial STAT3. Our findings may provide the missing link between two factors that are known to drive pancreatic carcinogenesis, enhanced autophagy (mediated by RAGE), and dysfunctional metabolism (mediated by STAT3).
RAGE is a positive regulator of autophagy during both anticancer therapy and pancreatic carcinogenesis. Radiation therapy and some forms of chemotherapy, such as gemcitabine, rely on reactive oxygen species generation and toxicity to eradicate tumor cells. Reactive oxygen species, in turn, increase the activity of NF-κB, resulting in RAGE expression (6). This up-regulation of RAGE protects pancreatic tumor cells from further oxidative injury and increases drug resistance by enhancing autophagy and decreasing apoptosis (6, 7). We found that RAGE increased autophagic flux by regulating ATG12–ATG5 conjugation. RAGE also regulates activation of the mammalian target of rapamycin (mTOR) and Beclin 1–PI3K class III complex (32), suggesting that there are multiple roles for RAGE in the enhancement of autophagy within pancreatic cancer cells. In vivo, targeted deletion of RAGE in the KrasG12D/+ spontaneous KC cancer model decreased Kras-driven autophagy and subsequently mitochondrial STAT3 and ATP production, confirming the interaction between autophagy and STAT3. Interestingly, intracellular HMGB1, the cognate ligand for RAGE, regulates mitochondrial quality via mitophagy (15). Further studies are required to establish the relationship between HMGB1 and RAGE in autophagy-mediated mitochondrial STAT3 activation and their roles within the emergent pancreatic tumor microenvironment. We hypothesize that RAGE expression serves as an ”autophagic switch,” enabling suppression of apoptosis and promotion of survival pathways requisite later during carcinogenesis (10).
We have previously established that knockdown of RAGE with shRNA significantly alters the biology of PDA (6, 7). DiNorcia et al. have recently shown in an accelerated compound p16-deleted Kras mutant tumor model that RAGE ablation limits development of frank carcinoma (33). No mechanisms for this finding were proposed, and our study extends and confirms their study, now showing the critical role of the IL-6/STAT3 signaling pathway. Interestingly, we have not been able to derive a PDA from a RAGE-null animal at this point but will continue to try. The principle findings relate not to the phenotype of PDA in the setting of RAGE knockdown (6, 7) but rather its link to early precursor formation. We believe the observation that knockdown of RAGE attenuates development of PDA precursor lesions through an IL-6/STAT3 autophagic pathway is important, suggesting unique therapeutic strategies.
In summary, we demonstrate here that RAGE is a critical mediator of pancreatic carcinogenesis through its ability to amplify IL-6–induced autophagic translocation of STAT3 to the mitochondria. Ultimately, this amplification leads to increased ATP production by fully transformed tumor cells and early epithelial precursor lesions. We hypothesize that the RAGE/IL-6–dependent programmed cell survival pathways provide a means for epithelial tumor precursor cells to gain a selective growth advantage within the tumor microenvironment.
Materials and Methods
Cell Lines and Reagents.
Human (Pan2.03) or mouse (Panc02) pancreatic tumor cells, mouse RAW264.7 cells, and human umbilical vein endothelial cells were purchased from American Type Culture Collection. The neutralizing antibodies to human or mouse IL-6 and RAGE were obtained from R&D Systems. The antibodies to STAT3, p-STAT3 (Ser727), p-JAK1 (Tyr1022/1023), JAK1, p-Src (Tyr416), Src, COX IV, and ATG12–ATG5 were obtained from Cell Signaling Technology. The antibodies to LC3 and ATG5 were obtained from Novus. The antibodies to actin, tubulin, and RAGE were obtained from Sigma. The antibody to Ki67 was from Abcam. Human and mouse IL-6 protein and ELISA kit were obtained from R&D Systems. Other chemical reagents were from Sigma.
Human Samples.
Human pancreatic adenocarcinoma specimens were collected from patients who underwent surgical procedures. Samples were obtained according to an institutional review board-approved clinical protocol at the University of Pittsburgh Medical Center.
Full methods and associated references are available in the SI Materials and Methods.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113865109/-/DCSupplemental.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Hruban RH, Adsay NV. Molecular classification of neoplasms of the pancreas. Hum Pathol. 2009;40:612–623. doi: 10.1016/j.humpath.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 4.Gebhardt C, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taguchi A, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 6.Kang R, et al. The Receptor for Advanced Glycation End-products (RAGE) protects pancreatic tumor cells against oxidative injury. Antioxid Redox Signal. 2011;15:2175–2184. doi: 10.1089/ars.2010.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang R, et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–676. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Klionsky DJ. Eaten alive: A history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang D, Lotze MT, Kang R, Zeh HJ. Apoptosis promotes early tumorigenesis. Oncogene. 2011;30:1851–1854. doi: 10.1038/onc.2010.573. [DOI] [PubMed] [Google Scholar]
- 11.Fujii S, et al. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci. 2008;99:1813–1819. doi: 10.1111/j.1349-7006.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesina M, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Wegrzyn J, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang D, et al. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13:701–711. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuma A, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 18.Hanada T, et al. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. 2007;282:37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 19.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Sparvero LJ, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada S, et al. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–15. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda A, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 26.Gough DJ, et al. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 28.Takamura A, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman BJ, et al. Autophagy is essential to suppress cell stress and to allow BCR-Abl-mediated leukemogenesis. Oncogene. 2011;30:1855–1867. doi: 10.1038/onc.2010.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lock R, et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. 2011;22:165–178. doi: 10.1091/mbc.E10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo JY, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang R, Tang D, Loze MT, Zeh HJ. Apoptosis to autophagy switch triggered by the MHC class III-encoded receptor for advanced glycation endproducts (RAGE) Autophagy. 2011;7:91–93. doi: 10.1038/cdd.2009.149. [DOI] [PubMed] [Google Scholar]
- 33.DiNorcia J, et al. RAGE gene deletion inhibits the development and progression of ductal neoplasia and prolongs survival in a murine model of pancreatic cancer. J Gastrointest Surg. 2012;16:104–112, discussion 112. doi: 10.1007/s11605-011-1754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.