Abstract
Gammaherpesvirus infections, such as those caused by EBV, have been suggested to promote the development of autoimmunity. To test this idea, we infected healthy WT and lupus-prone B6.Sle123 mice with an EBV-related and rodent-specific gammaherpesvirus, γHV68. Although acute γHV68 infection increased autoantibody levels for 4 to 6 wk, latent infection inhibited these responses for 1 y. The inhibition of autoantibody expression was only observed in B6.Sle123 females and not in males, which already displayed lower autoantibody titers. Contrary to the initial hypothesis, infection of young B6.Sle123 mice, both male and female, resulted in suppression of lymphoid activation and expansion and of glomerular inflammation and sclerosis, preserving kidney function. Moreover, γHV68 infection led to reduced autoantibody titers, lymphoid activation, and glomerular inflammation whether lupus-prone females were infected before or during disease manifestation. Finally, γHV68 infection also inhibited autoantibody production in the genetically distinct MRL/lpr lupus-prone mice. Our findings indicate that γHV68 infection strongly inhibits the development and progression of lupus-like disease in mice that spontaneously develop this condition mediating its beneficial effects at the humoral, cellular, and organ levels. The mechanisms by which the virus exerts this down-modulatory action are not yet clear, but appear to operate via reduced activation of dendritic cells, T cells, and B cells. Gammaherpesviruses coevolved with the vertebrate immune systems, establishing lifelong infections in humans and other mammals. Our findings that γHV68 infection prevents rather than exacerbates autoimmunity in mice suggest that infection with gammaherpesviruses may be protective rather than pathological in most individuals.
Keywords: antibody, mouse gammaherpesvirus
Autoimmunity is believed to develop through the intersection of genetic and environmental factors that predispose to this collection of diseases. Infection with EBV has been linked to the development of systemic lupus erythematosus and other autoimmune disorders in humans (1–3) and thus may represent an environmental trigger of autoimmunity. Despite the correlation observed between EBV and autoimmunity, 95% of the adult human population is infected with EBV whereas fewer than 2% develop the autoimmune diseases that have been linked to EBV. This suggests that EBV infection does not inevitably lead to autoimmunity.
Because EBV does not infect mice, studying its effects on complex immunological processes in this animal model has been difficult. This problem has been partly circumvented by generating transgenic mice that express isolated EBV genes which, when used in studies of autoimmunity, have supported a role of EBV in this pathological process (4–6). Interpretation of these studies, however, has to take into account the absence of the whole viral genome and the infection program, which might have additional effects. An alternative way to circumvent the absence of a small animal model for EBV infection has been to study the murine gammaherpesvirus 68 (γHV68, also known as murid herpesvirus-4), a herpesvirus with characteristics similar to those of EBV that is able to infect mice (7–9). Infection of WT mice with γHV68 delivered by i.p. injection or intranasal administration of virus particles results in a productive acute infection that manifests with enhanced Ig secretion, lymphoproliferation, and cytokine production resembling the mononucleosis that follows EBV infection in almost half of infected adult human beings (10–13). Similar to the time course of EBV infection in humans, the mouse immune system controls the γHV68 virus within 2 to 3 wk after the initial infection (13–15), and the virus goes latent in B cells, macrophages, dendritic cells (DCs), and epithelial cells (16–21). This chronic infection persists for the rest of the individual's life. Reactivation of the virus can periodically occur, but this is regulated by the host immune system (13, 15, 22–28).
A previous study has shown that γHV68 infection of WT C57BL/6 (B6) mice promotes expression of dsDNA and collagen-reactive IgG autoantibodies (12). These autoantibodies, moreover, persisted as long as 80 d after infection, after the time the virus has entered its latent phase. Because of this persistence and the fact that the autoantibodies were of the IgG class, it was suggested that γHV68 infection might trigger autoimmune disease in mice (12), potentially mimicking EBV in humans. When γHV68 infection was more directly tested in the context of murine autoimmunity, it was shown to delay the onset of diabetes in NOD female mice infected at 8 to 9 wk of age (29), but also to exacerbate experimental arthritis in K/BxN mice (30) and experimental autoimmune encephalomyelitis in SJL mice and in Lewis rats (31). These studies, therefore, suggest that γHV68 infection can increase or decrease symptoms of autoimmunity in mice, perhaps depending on the type of autoimmune disease and the genetics of the host.
The effect of γHV68 infection on the development of murine lupus has not been addressed so far. In this study, we investigated whether γHV68 acute and latent infection promotes autoimmunity in normal and lupus-prone mice. Our results demonstrate that γHV68 infection does not promote autoimmunity in WT B6 mice. Rather, this virus significantly inhibits the development and progression of autoimmunity in lupus-prone mice and its effect manifests at the humoral, cellular, and organ level for as long as 1 y after the initial infection. Reduced numbers of activated T cells, B cells, and DCs were found in chronically infected lupus-prone mice, likely contributing to the decreased burden of autoimmunity.
Results
Chronic γHV68 Infection Does Not Induce Symptoms of Autoimmunity in WT B6 Mice.
WT B6 mice were infected with γHV68 at 6 to 8 wk of age by i.p. injection of 1 × 106 pfu γHV68 to investigate whether this virus triggers the development of autoimmune diseases, as previously suggested (12). As shown in Fig. S1A, infected mice displayed an antibody response to the virus by 3 wk after infection, and this response reached a plateau by 6 mo after infection.
To investigate whether γHV68 infection has the potential to cause autoimmunity in healthy mice, we measured the level of anti-chromatin and anti-Smith autoantibodies, and total IgM and IgG levels in the sera of infected and noninfected B6 mice. This analysis was performed directly following the clearance of acute virus replication (3 wk) and during the latent phase of infection (1 mo after infection and once per month for as long as 11 mo after infection), during which time viral genome is found within cells in the absence of free infectious virus (9, 32). Acute infection of B6 mice caused a rapid increase of total serum IgG levels (Fig. 1A) whereas total serum IgM levels were similar in infected and noninfected mice right after the acute phase and at the beginning of latent phases of infection (Fig. 1A), as previously reported (12, 14).
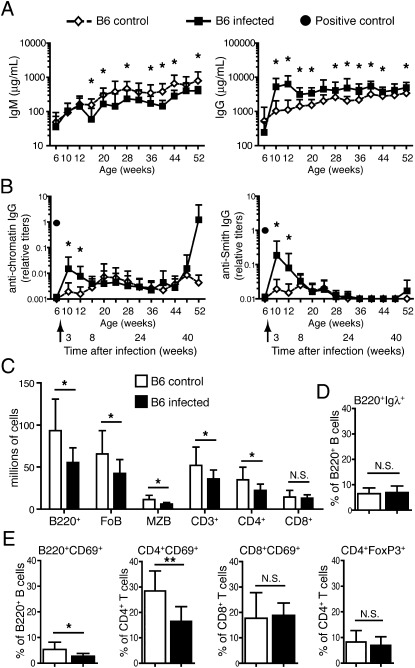
Fig. 1.
γHV68 promotes autoantibody expression in B6 mice during the acute, but not the latent, phase of infection and does not mediate chronic lymphocyte expansion and activation. Six- to 8-wk-old B6 mice (females and males) were infected i.p. with 106 pfu of γHV68 or left uninfected. (A and B) Blood was collected before infection, at 3 wk postinfection, and monthly as indicated from both groups of mice, and antibodies were analyzed by ELISA. (A) Total IgM and IgG concentration (A) and anti-chromatin and anti-Smith IgG titers (B) in sera of infected (filled square) and noninfected (empty diamond) B6 mice. A filled circle in graphs in B indicates the titer of anti-chromatin or anti-Smith antibodies in serum of a 15-wk-old female MRL/lpr (positive control). Data represent the mean and SD of antibody titers from 10 to 17 mice per group analyzed over the course of at least two separate experiments (*P < 0.05). Arrows indicate the time of infection. (C–E) The infected and not infected B6 mice were euthanized at 11 to 13 mo of age, and spleen cells were analyzed by flow cytometry. Flow cytometric analysis was performed to determine the size of the follicular (CD23highCD21low), marginal zone (CD21highCD1dhigh), Igλ+, and activated (CD69+) B-cell (B220+) populations, and of the CD4, CD8, regulatory (FoxP3+), and activated (CD69+) T-cell populations (representative plots shown in Fig. S2A). (C) Absolute cell numbers of B- and T-cell subsets and (D) percentage of Igλ+ B cells in the B220+ cell population in infected and noninfected B6 mice. FoB, follicular B cells; MZB, marginal zone B cells. (E) Frequency of activated (CD69+) B cells, CD4 and CD8 T cells, and Tregs (FoxP3+). Bar graphs represent arithmetic means and SDs (n = 10–17 from two separate experiments; *P < 0.05, **P < 0.01, and ***P < 0.001).
Gammaherpesvirus 68 infection of B6 mice resulted in detectable levels of anti-chromatin and anti-Smith (Fig. 1B) IgG autoantibodies at 3 wk after infection, and likely as a result of acute infection as previously reported (12). The increase of autoantibodies seen soon after the acute phase of infection was of the order of 10- to 100-fold greater than that of noninfected animals, but significantly lower than the levels observed in mice displaying lupus (Fig. 1B). The kinetics of autoantibody appearance generally followed that of total serum IgG, returning to the background levels observed in noninfected mice by 3 mo after infection (Fig. 1B). In some of the mice older than 8 mo of age, we observed some fluctuations in the levels of the autoantibodies for reasons that are unclear. However, these changes did not cause significant differences between the two groups of mice. Overall, chronic γHV68 infection did not result in increased levels of autoantibodies in B6 mice for at least 1 y of age.
Systemic autoimmunity manifests with lymphoproliferation and activation of B cells and T cells that may precede autoantibody formation. To determine whether γHV68 infection causes alterations in the lymphoid population of WT mice suggestive of an ongoing autoimmune process, we compared the splenic B and T cell populations of the B6 mice at the endpoint (11 mo postinfection). The B-cell population in the spleen of each mouse was analyzed by flow cytometry to determine frequency and numbers of marginal zone and Igλ+ B cells, CD4 and CD8 T cells, and activated B and T cells, gated as shown in Fig. S2A. On average, infected B6 mice exhibited significantly lower numbers of B cells (both follicular and marginal zone) and CD4+ T cells, but not of CD8+ T cells (Fig. 1C). The frequency of Igλ+ B cells was similar in infected and noninfected mice (Fig. 1D), suggesting that γHV68 infection did not significantly change the frequency of secondary Ig gene rearrangement and receptor editing during B-cell development (33). Moreover, chronic γHV68 infection did not mediate accumulation of activated lymphocytes, but actually resulted in a significantly lower frequency of activated (CD69+) B220+ B cells and CD4+ T cells, but not of CD8+ T cells (Fig. 1E). Finally, infection did not significantly alter the frequency of CD4+FoxP3+ regulatory T cells (Tregs; Fig. 1E).
Based on the results of the serological and cellular analyses, we conclude that long-term γHV68 infection does not promote chronic symptoms of autoimmunity in B6 mice.
Latent γHV68 Infection Does Not Increase Autoantibody Titers in Anti-dsDNA B6.56R Mice.
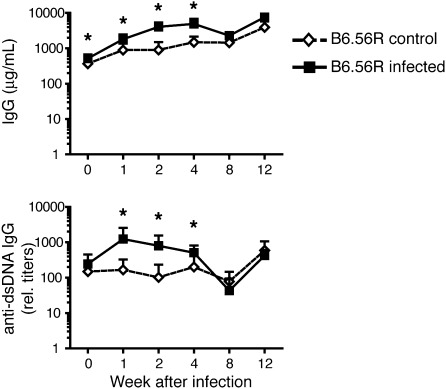
Anti-chromatin B cells are normally silenced by tolerance at several checkpoints during B-cell differentiation from the immature to the activated mature and plasma cell stages (34, 35). The inability to detect anti-chromatin antibodies in latently infected B6 mice may be a result of the normal establishment of tolerance in this mouse strain. To bypass the effect of tolerance and investigate a possible role of γHV68 infection on the differentiation and activation of anti-chromatin B cells, we next infected B6.56R mice, which bear the 56R transgene coding for an anti-DNA Ig heavy chain (36). Central and peripheral tolerance are not particularly stringent in B6.56R mice, and anti-dsDNA B cells reach the mature cell stage and differentiate into anti-dsDNA IgM and IgG antibody secreting cells, although never becoming pathogenic (36). Anti-dsDNA IgG antibody titers in infected B6.56R mice were significantly increased vs. those of noninfected animals during the acute phase of infection and persisting into the latent phase (Fig. 2). However, the autoantibodies in infected mice returned to levels observed in noninfected mice during the latent phase of infection approximately 8 wk after initial infection, and generally following total IgG kinetics (Fig. 2).
Fig. 2.
Chronic γHV68 infection does not exacerbate generation of anti-chromatin antibodies in anti-DNA B6.56R mice. Six- to 8-wk-old B6.56R mice were infected i.p. with 106 pfu of γHV68 or left not infected. Blood was collected before infection (time 0) and at indicated time points after infection to determine total IgG levels (Upper) and anti-chromatin IgG titers (Lower) in sera of infected (filled square) and noninfected (empty diamond) 56R.B6 mice. Data represent the mean and SD of antibody titers from nine mice per group analyzed over the course of two independent experiments (*P < 0.05).
Our data show that chronic γHV68 infection of anti-DNA B6.56R mice do not exacerbate production of anti-chromatin autoantibodies even in mice in which mechanisms of peripheral B-cell tolerance are not particularly stringent.
Chronic γHV68 Infection Significantly Decreases Autoantibody Production in Lupus-Prone B6.Sle123 Mice Before and After Onset of Disease.
To test whether γHV68 infection promotes autoimmunity only in genetically predisposed backgrounds, we acquired the B6.Sle123 mouse strain that bears three distinct genetic loci of lupus susceptibility on the B6 genetic background (37–39) and manifests slow kinetics and 100% penetrance of lupus in both males and females (40).
Groups of B6.Sle123 male (n = 5 per group) and female (n = 16–19 per group) mice were infected with γHV68 (i.p. injection of 1 × 106 pfu) at 6 to 8 wk of age, before the development of detectable symptoms of lupus. Infection induced anti-virus IgG antibody responses (Fig. S1B) similar to those observed in WT B6 mice (Fig. S1A). By performing a limiting dilution nested PCR assay (41), we found that the frequency of viral genome-containing B6.Sle123 B cells was approximately 10- to 20-fold higher than that of B6 B cells (B6.Sle123, approximately 1 in 5,000; B6 extrapolated to be approximately 1 in 100,000; Fig. S1C). A similar difference, although less pronounced, was observed in non-B cells (B6.Sle123, approximately 1 in 30,000; B6 extrapolated to be approximately 1 in 100,000; Fig. S1C).
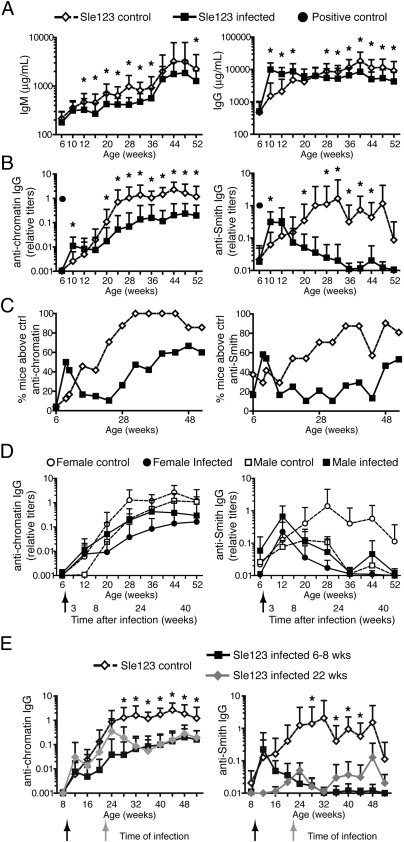
Total serum IgM and IgG levels were affected by γHV68 acute infection in B6.Sle123 mice in a similar fashion as seen in B6 mice (Figs. 1A and 3A). Similar to B6 mice, anti-chromatin and anti-Smith IgG antibody titers in B6.Sle123 mice quickly increased following acute infection, but returned to background levels during the latent phase of infection (Fig. 3B). As expected for mice of this strain (40), B6.Sle123 mice became slowly seropositive for anti-chromatin and anti-Smith autoantibodies (Fig. 3B) and displayed high levels of total IgG (Fig. 3A). The average levels of these antibodies and the fraction of positive mice steadily increased with age, reaching a plateau at 7 to 8 mo of age (Fig. 3 B and C). At this time point, 100% of the mice displayed anti-chromatin and/or anti-Smith autoantibodies greater than those of control (B6) mice (Fig. 3C). The generation of autoantibodies appeared significantly altered by chronic γHV68 infection. Latent γHV68-infected B6.Sle123 females displayed significantly lower levels of anti-chromatin and anti-Smith autoantibodies on average, and some of the mice remained autoantibody-free during the entire time of analysis (Fig. 3 B and C). The effect of infection was particularly evident for anti-Smith antibodies, in which most of the infected mice displayed levels comparable to those of WT mice (Figs. 1B and 3B).
Fig. 3.
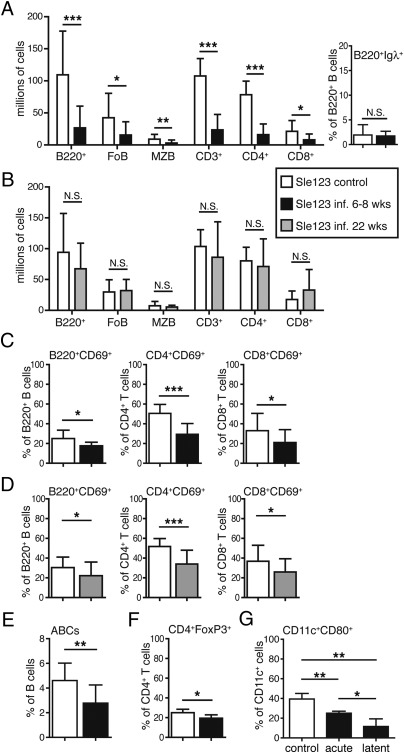
Chronic γHV68 infection significantly decreases autoantibody production in lupus-prone B6.Sle123 female mice. (A–D) Six- to 8-wk-old B6.Sle123 male (n = 5 per group) and female (n = 16–19 per group) mice were infected i.p. with 106 pfu of γHV68 or left uninfected. Blood was collected before infection, at 2 to 3 wk postinfection, and monthly for 11 mo from both groups of mice, and antibodies were analyzed by ELISA. (A) Total IgM and IgG in infected (filled square) and noninfected (empty diamond) B6.Sle123 female mice. (B) Anti-chromatin and anti-Smith IgG titers in serum of infected (filled square) and noninfected (empty diamond) B6.Sle123 female mice. A filled circle indicates the titer of anti-chromatin or anti-Smith antibodies in serum of a 15-wk-old female MRL/lpr (positive control). Data represent the mean and SD of antibody titers from 21 to 24 mice per group analyzed over the course of at least two separate experiments (*P < 0.05). (C) Frequency of B6.Sle123 female mice displaying anti-chromatin or anti-Smith antibody titers above mean titers + 2 SDs of B6 naive (i.e., control) mice. (D) Mean and SD of anti-chromatin and anti-Smith IgG titers in serum of infected (filled symbol) and noninfected (empty symbol) B6.Sle123 male (squares; n = 5 per group) and female (circles; n = 16–19 per group) mice. Arrows indicate the time of infection. (E) B6.Sle123 females were infected i.p. with 106 pfu of γHV68 at 8 wk or 5 to 6 mo (22 wk) of age. A group of females were left uninfected. Blood was collected starting at 6 to 8 wk of age and once per month for the time indicated. Shown are anti-chromatin and anti-Smith IgG titers in serum of B6.Sle123 females infected at 6 to 8 wk (filled black squares), at 22 wk (filled gray diamonds), and not infected (empty diamonds). Graphs represent the mean and SD of antibody titers from 10 to 20 mice per group analyzed in one to two experiments (*P < 0.05 between control and infected at 22 wk).
The Sle1, Sle2, and Sle3 loci promote the development of lupus in males and females, although some of the parameters associated with lupus are more prominently displayed in females (40). In accordance, we found that anti-chromatin and anti-Smith antibody titers were five- to 10-fold and as much as 100-fold lower, respectively, in B6.Sle123 males than in females (Fig. 3D). Interestingly, γHV68 latent infection decreased the autoantibody titers only in female B6.Sle123 mice, whereas those of males remained relatively unchanged (Fig. 3D).
We next asked whether γHV68 infection could inhibit autoantibody formation in female mice in which symptoms of disease had already started. To test this, B6.Sle123 females were infected with γHV68 at 5 to 6 mo (22 wk) of age, at a time when autoantibodies had reached detectable levels in at least 50% of the mice (Fig. 3C), but were still below the maximum typically observed at 1 y of age (Fig. 3B). Autoantibody titers at this time varied from mouse to mouse (Fig. 3E), causing some groups of mice to display lower levels than those of other groups, on average (e.g., anti-Smith titers; Fig. 3E). Nonetheless, we found that γHV68 infection consistently reduced anti-chromatin and anti-Smith autoantibody titers by 4 wk after infection (Fig. 3E). Moreover, the autoantibody titers of B6.Sle123 females infected at 22 wk remained low during the following weeks, similar to those of mice that were infected at 8 wk of age (Fig. 3E).
Similar inhibition of autoantibody production was observed in both males and females of a different lupus-prone mouse strain, the MRL/lpr (Fig. S3), which is genetically different from the B6.Sle123 and develops a much more aggressive form of lupus.
Overall, these data indicate that γHV68 infection strongly inhibits the production of autoantibodies in lupus-prone female mice during the progression of lupus disease and after its initiation.
Chronic γHV68 Infection Prevents Kidney Disease in Lupus-Prone B6.Sle123 Mice.
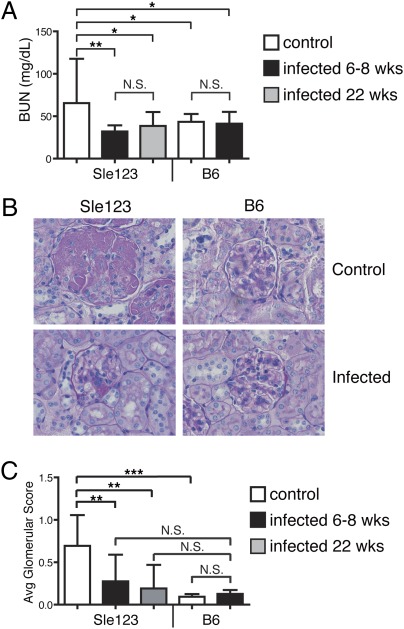
Systemic lupus can affect kidney function, resulting in increased levels of proteinuria, blood urea nitrogen (BUN), and glomerular inflammation, and B6.Sle123 mice have been reported to ultimately die from fatal glomerulonephritis (40). In agreement, BUN levels were significantly elevated in noninfected B6.Sle123 mice relative to B6 controls (Fig. 4A). In contrast, whether they were infected at 6 to 8 or 22 wk of age, chronically infected B6.Sle123 mice exhibited significantly reduced BUN levels that were similar to those of B6 mice (Fig. 4A). To directly investigate kidney function, the glomeruli were examined for signs of inflammation and glomerulosclerosis by microscopy. Most glomeruli from naive B6.Sle123 mice exhibited significant involvement, including proliferative lesions, hyaline deposits, and/or thrombosis (Fig. 4B). In contrast, most glomeruli from infected B6.Sle123 mice were normal or had minimal involvement restricted to segmental lesions (Fig. 4B). The glomeruli were scored blindly, and an average glomerular score was calculated for each mouse group (Fig. 4C). B6.Sle123 mice had an average score of 0.8, indicating that approximately 80% of their glomeruli were severely affected. In contrast, the average glomerular score of γHV68-infected B6.Sle123 mice was 0.2 (20% of glomeruli affected; Fig. 4C), a result not significantly different from those in infected and noninfected B6 mice (Fig. 4C).
Fig. 4.
γHV68 infection prevents kidney disease in lupus-prone mice. Parameters of kidney function were evaluated in B6 and B6.Sle123 mice described in Figs. 1, 3, and 4 at the end of the study (12–13-mo-old mice). (A) BUN levels were measured in serum of mice. Graphs represent the arithmetic mean and SD of BUN levels in serum of 10 to 24 mice per group analyzed over one or two independent experiments. (B) Representative kidney sections stained with periodic acid–Schiff from B6 and B6.Sle123 mice that were not infected or infected with γHV68 at 6 to 8 wk of age. A representative glomerulus is shown in each section (original magnification of ×400). (C) Twenty-five glomeruli for each mouse were evaluated in a blinded analysis in kidney sections. A score of 1 was assigned to each severely affected glomerulus (showing, e.g., proliferative lesions, thrombosis, hyaline deposits), and a score of 0 was assigned to nonaffected or mildly affected (with segmental lesions) glomeruli. An average score was assigned to each mouse. The bar graph represents the average glomerulus score and SD for each group of mice (n = 10–24 mice per group analyzed in two independent experiments; *P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant).
These data indicate that γHV68 infection does not induce kidney pathology in WT mice, and protects lupus-prone B6.Sle123 mice from glomerulonephritis and end-organ damage.
Chronic γHV68 Infection of B6.Sle123 Mice Decreases Frequency of Activated Lymphocytes and DCs.
The development and progression of lupus is driven by abnormal activation of hematopoietic cells and production of cytokines. We first speculated that a strong cytokine imbalance might be mediating at least some of the suppressing effects on autoimmunity observed in infected B6.Sle123 mice. IFN-γ is greatly augmented following viral infection and increased levels of this cytokine have been shown to reduce the burden of autoimmunity in some studies (42, 43). We treated groups of B6.Sle123 young females with either blocking anti–IFN-γ or isotype control antibodies starting at 1 wk after γHV68 infection and for a total of 8 wk (Fig. S4A). Anti-chromatin and anti-Smith autoantibody titers were not significantly different in the two groups of mice, suggesting that IFN-γ does not likely contribute to the inhibition of autoantibody production mediated by γHV68 in B6.Sle123 mice.
B6.Sle123 mice develop splenomegaly and supraphysiological numbers of B cells and T cells, and a higher frequency of activated lymphocytes that drive the development of autoimmunity (40, 44). Chronically infected B6.Sle123 mice were analyzed at approximately 1 y of age to determine the effect of γHV68 virus on the lymphoid population (Fig. S2B). The absolute number of B220+ B cells, and CD3+ T cells were significantly reduced in B6.Sle123 females that were infected at 6 to 8 wk of age, but not in those infected at 22 wk of age, relative to noninfected animals (Fig. 5 A and B). In the B-cell population of B6.Sle123 mice infected at 6 to 8 wk, follicular and marginal zone B cells were significantly reduced in number, whereas the frequency of λ+ B cells was similar in infected and noninfected mice (Fig. 5A). In the T-cell population, both CD4+ and CD8+ T-cell numbers were significantly decreased (Fig. 5A). Moreover, chronically infected B6.Sle123 mice, whether infected at 6 to 8 wk or 22 wk of age, displayed significantly lower frequency of both activated B cells and (CD4 and CD8) T cells than noninfected mice (Fig. 5 C and D). The reduced activation of B cells and CD4 T cells was also observed in infected males (Fig. S4B). Thus, chronic γHV68 infection prevents the lymphoid cell expansion and activation that is associated with lupus development in B6.Sle123 mice, but is unable to restore normal lymphocyte numbers in mice in which cell expansion has already ensued.
Fig. 5.
Chronic γHV68 infection decreases lymphocyte and DC activation in B6.Sle123 mice. (A–F) B6.Sle123 mice described in Fig. 3 that were infected at 6 to 8 wk or at 22 wk of age or left noninfected were euthanized at approximately 12 to 13 mo of age, at which time spleen cells were analyzed by flow cytometry as described in Fig. 1 and shown in Fig. S2B. (A and B) Absolute cell numbers of B- and T-cell subsets in B6.Sle123 female mice that were noninfected (A and B, white bars) or infected at 6 to 8 wk (A, black bars) or 22 wks (B, gray bars) of age. (A) Right: Percentage of Igλ+ B cells in the B220+ cell population of noninfected B6.Sle123 mice relative to mice that were infected at 6 to 8 wk of age. FoB, follicular B cells; MZB, marginal zone B cells. (C and D) Frequency of activated (CD69+) B cells (B220+), CD4, and CD8 T cells of B6.Sle123 female mice infected at 6 to 8 wk (C, black bars) or 22 wk (D, gray bars) of age relative to noninfected mice (white bars; n = 10–20 mice per group from separate experiments). (E) Spleen cells from B6.Sle123 female mice that were not infected (white bar) or infected at 6 to 8 wk of age (black bar) were analyzed by flow cytometry for the detection of ABCs (n = 10–16 mice per group from two separate experiments). ABCs were gated as single live and B220+CD4/CD8−CD11c+ lymphoid cells to determine their frequency. The bar graphs represent the arithmetic mean and SD of the percentage of ABCs in the B220+ B-cell population. (F) Frequency of FoxP3+ Tregs in the CD4+ T-cell population of the spleen of B6.Sle123 mice that were left noninfected (white bar) or infected at 6 to 8 wk of age (black bar; n = 14–24 mice per group from two separate experiments). (G) CD11c+ cells were purified from B6.Sle123 female mice and analyzed by flow cytometry for the expression of CD80 on CD11c+ cells. The graph represents the frequency of CD80+ cells in the total CD11c+B220− cells in the spleen of noninfected (control, white bar) mice and during acute (9 d postinfection) and latent (12 mo postinfection) γHV68 infection (n = 3 mice per group). An additional independent experiment performed on similar groups of mice (n = 3–5) resulted in the following frequencies of CD80+ cells in the CD11c+B220− cell fraction: control, 32.4%; acute, 19.5% (P = 0.01 vs. control); and latent, 11.8% (P = 0.0009 vs. control). Bar graphs represent arithmetic means and SDs (*P < 0.05, **P < 0.01, and ***P < 0.001; NS, not significant).
A novel B-cell subpopulation, age-associated B cells (ABCs), has been recently described to be composed of B cells expressing CD11b and CD11c (45, 46). ABC numbers are increased with age in female mice and in response to Toll-like receptor (TLR) 7 agonists (45). Moreover, ABCs are also increased with autoimmunity in mice and humans (45). When we analyzed the spleen ABC population, we found that the ABC fraction was significantly diminished in infected B6.Sle123 females relative to that of noninfected individuals (Fig. 5E), potentially contributing to the decreased burden of autoimmunity. The diminished ABC population of infected mice was not likely the result of a general reduced TLR7 signaling in B cells, as B cells from acute and latent infected B6.Sle123 mice up-regulated CD69 to the same extent of those of noninfected animals to a TLR7 agonist in vitro (Fig. S4C).
Lymphocyte activation can be modulated by changes in Treg numbers and phenotype of DCs. Latently infected B6.Sle123 mice displayed frequency and numbers of CD4+FoxP3+ Tregs that were significantly lower than those of noninfected mice (Fig. 5F), suggesting that γHV68 does not mediate inhibition of lupus by generally increasing generation of Tregs. On the contrary, the frequency of CD80+ activated CD11c+ cells in B6.Sle123 mice was significantly diminished by acute and latent γHV68 infection (Fig. 5G). This suggests that the virus might inhibit lymphocyte activation and, consequently, lupus development by modulating the function of DCs.
Discussion
This study was performed to investigate the association between gammaherpesvirus infection and lupus development in the mouse. Here we demonstrate that γHV68 infection neither triggers nor exacerbates lupus disease in healthy and lupus-prone mice, respectively. Instead, γHV68 infection inhibits the development and progression of murine lupus at the humoral, cellular, and organ level.
Gammaherpesvirus 68 infection has previously been associated with autoantibody production in WT mice (12). Our data extend this finding to show that virus-induced autoantibody production is short-lived and transient, with IgG autoantibody kinetics closely mirroring total IgG serum levels and decreasing to preinfection levels during viral latency. Similar results were found in anti-DNA B6.56R mice, which display defects in peripheral B-cell tolerance manifesting with constitutive levels of anti-chromatin autoantibodies. Thus, γHV68-induced autoantibody production may be the result of polyclonal B-cell stimulation, a process previously shown for other viruses (47, 48) and EBV (49, 50), and which resolves with viral latency (10–13).
Our study shows that γHV68 latent infection dramatically inhibits the generation of autoantibodies in lupus-prone mice. Latently infected B6.Sle123 mice, whether infected at 8 or 22 wk of age, had significantly lower levels of autoantibodies compared with noninfected mice of the same age. The reduction of serum autoantibodies mediated by γHV68 was observed in females, but not in males, of the B6.Sle123 strain. In fact, γHV68 infection of B6.Sle123 females caused an autoantibody profile similar to those of males, which display lower autoantibody titers than females. This bias may be a result of differences among sexes in the expression of TLRs and cytokines that affect autoantibody production (51, 52) and indicate the existence of pathways leading to the production of autoantibodies that are not inhibited by the virus. On the contrary, latent γHV68 infection inhibited autoantibody production even in the genetically unrelated lupus-prone MRL/lpr strain, indicating that this virus impinges on a general mechanism of autoantibody formation.
Beyond inhibiting the production of autoantibodies in autoimmune prone mouse strains, γHV68 infection also prevented accumulation of B and T cells when infecting young (both males and females), but not old, B6.Sle123 mice. These latter findings indicate that viral infection inhibits the development of the lymphoid expansion associated with autoimmune development, but does not reverse lymphoproliferation when it has been initiated. B6.Sle123 mice have been described to die from kidney disease starting at 8 mo of age (40), but in our colony, we did not have significant death during our experiments (to 1 y of age). The reduction of BUN and glomerular inflammation we observed in our infected mice, whether infected young (males and females) or at later time (only females), indicates that γHV68 infection inhibits the development of kidney disease. It follows that γHV68 infection might delay the death of B6.Sle123 mice, but we did not protract our observation of the mice long enough to establish a survival curve.
A small fraction of infected B6.Sle123 mice displayed signs of spleen fibrosis by 1 y of age, a phenomenon observed with γHV68 infection of IFNγR−/− mice (53). Fibrosis manifested with darkening and hardening of splenic tissue and severe reduction of splenocyte numbers. The presence of fibrotic spleens raises the possibility that the autoimmune suppression mediated by γHV68 is an indirect consequence of general lymphocyte loss, including autoreactive B cells and T cells. We consider this unlikely, however, as fibrosis was not found in any of the B6.Sle123 mice infected at 22 wk or in most mice infected at 8 wk despite significant inhibition of autoimmunity.
Lupus is a multifactorial disease in which autoantibody production, lymphocyte activation, and changes in pro- and anti-inflammatory cytokine expression all contribute to subsequent tissue and organ damage. Gammaherpesvirus 68 persistent infection could ameliorate autoimmunity by affecting any of these immunological functions. We tested whether IFN-γ, a cytokine strongly up-regulated following viral infection, might play a role in this process. Blocking IFN-γ in B6.Sle123 mice, however, did not prevent the reduction of autoantibodies mediated by γHV68, suggesting that changes in IFN-γ levels do not significantly contribute to the inhibition of humoral autoimmunity in this system. TLR7 signaling has been largely implicated in the development of autoimmunity in lupus-prone mice and, specifically, in the generation of antinuclear antibodies (54–57) and for the production of autoantibodies reactive with ribonuclear proteins such as Smith (55–57). Moreover, TRL7 signaling mediates the accumulation and function of ABCs, which belong to a recently described small subset of autoreactive B cells that accumulate in aged female mice and in some autoimmune conditions (45). The significant decrease of anti-Smith autoantibodies and of ABC numbers in aged B6.Sle123 females with γHV68 infection is compatible with a general inhibition of TLR7 signaling. Although speculative, γHV68 might impinge on TLR7 signaling and, consequently, on ABC accumulation and function and autoimmune development, either by reducing receptor expression as shown for EBV in human B-cell lines (58–60) or by opposing its function via the activation of TLR9 signaling (54, 57, 61, 62). In preliminary studies, we did not find a difference in the response (CD69 up-regulation) of total B cells to a TLR7 agonist in noninfected and γHV68-infected B6.Sle123 mice, suggesting that a general alteration of this pathway in B cells is not likely the cause of the reduced autoimmune phenotype. However, further studies are needed to definitively determine whether changes in TLR signaling in B cells or other hematopoietic cell types play a role in virus-mediated autoimmune inhibition. Another possible mechanism might consider the preference of γHV68 for B cells. Antigen-experienced B cells are a major target of γHV68 (and EBV) infection and a major reservoir of latent virus (9, 19, 63). The much higher frequency of latently infected B cells we observed in B6.Sle123 mice (relative to B6) suggests that γHV68 might frequently infect autoreactive B cells, as these cells are frequent and are self-antigen–activated in lupus-prone mice (64). Latent infection of autoreactive B cells may prevent their further differentiation and activation because of the changes in cell signaling pathways mediated by viral latent genes (65–67), a possibility that requires future exploration.
Chronic γHV68 infection of B6.Sle123 mice resulted in a decrease of B- and T-cell activation in both males and females, suggesting that this virus might inhibit lupus development by inhibiting the effector function of lymphocytes. Our data do not support the idea that γHV68 inhibits lymphocyte activation and autoimmunity by generally increasing Treg cell numbers, as we found reduced numbers of CD4 Tregs in infected B6.Sle123 mice. The virus may otherwise alter the development of autoimmunity by modulating DCs, which are known to contribute to the development of lupus in B6.Sle123 mice by producing large amounts of proinflammatory cytokines and driving high levels of B-cell and T-cell proliferation (68–70). Acute infection of DCs with γHV68 decreases their ability to present antigen and to express proinflammatory cytokines (20, 21, 29, 61, 71). Reduced endocytic function of DCs during γHV68 acute infection was also observed in the context of the autoimmune NOD genetic background (29). We found that, during acute and latent γHV68 infection, B6.Sle123 mice harbor a reduced frequency of activated DCs. Moreover, this frequency is the lowest during latent infection. Thus, it is possible that γHV68 infection permanently alters, directly or indirectly, the function of DCs, contributing to the down-modulation of B-cell and T-cell responses and of autoimmunity, and future studies will test this possibility.
Gammaherpesvirus 68 infection has been previously shown to exacerbate experimental autoimmune encephalomyelitis and autoimmune arthritis in rodents (30, 31). In these studies, however, viral influence on autoimmunity was only assessed for as long as 7 wk postinfection, and it is unclear whether chronic virus infection may have inhibited these responses as seen in our studies. In the context of a different autoimmune pathology, γHV68 leads to a delay in the development of diabetes in NOD mice when evaluated through 30 wk after infection (29). The effect of the virus on the onset of diabetes was subtle, resulting in a 5- to 10-wk delay and only if the mice were infected between 8 and 9 wk of age (29). Moreover, these studies suggested that the inhibitory effect was only mediated by the acute phase of infection and not through latency. Our findings that γHV68 infection inhibits lupus development in B6.Sle123 mice for 1 y after the initial infection suggest that the latency (and/or the reactivation) program may be required in this model. The contrasting effects of γHV68 infection observed on different mouse models of autoimmunity suggest that the virus may differentially modulate immune responses depending on the type of response and the genetic background.
Gammaherpesviruses have evolved means to coexist with the mammalian immune system for the entire life of the host (72), and this is partly achieved by down-modulating host immune responses (9, 72). Although there are important genetic differences between γHV68 and EBV that merit consideration, these virus strains belong to the same family and their infections have similar characteristics (9). Moreover, B6.Sle123 mice share lupus-susceptibility loci with people who develop lupus (37–39, 73, 74), suggesting that these mice represent a relevant model for human lupus. Our findings that γHV68 infection prevents rather than exacerbates autoimmunity in mice could explain the disparity between the incidence of EBV infection in humans and the frequency with which individuals become autoimmune. Moreover, a further understanding of how γHV68 suppresses autoimmunity in lupus-prone mice could result in the identification of new preventive and therapeutic targets for lupus.
Materials and Methods
Mice.
WT C57BL/6 (B6) mice and lupus-prone B6.Sle123 (BCN/LmoJ) (40) and MRL/lpr (MRL/MpJ-Faslpr/J) mice were purchased from Jackson Laboratory and then maintained or bred at the Biological Resource Center at National Jewish Health [National Jewish Health (NJH), Denver, CO]. B6.56R mice (36) were a gift from Lenny Dragone (NJH, Denver, CO). Mice were housed under specific pathogen-free conditions until γHV68 infection. Infected mice were housed in BSL2 rooms. Females and males between the ages of 6 wk and 14 mo were used in these studies. All animal experiments were approved by the institutional animal care and use committee.
Virus and Infections.
Viral antigen for ELISA detection of virus-specific antibodies was prepared as follows. Virus particles isolated from infected NIH 3T12 fibroblasts were concentrated by centrifugation at 14,000 × g and purified over a sucrose gradient. Purified virus was resuspended in PBS solution, 0.05% Triton-X buffer by overnight incubation at 4 °C with constant rotation. Virus protein concentration was determined by Bio-Rad RC/DC colorimetric kit for spectrometry. The γHV68 virus was grown and titered as previously described (75). The frequency of spleen B (CD43−) and non-B (CD43+) cells (purified as described later) harboring the viral genome was determined by a limiting-dilution, nested-PCR assay that amplifies the γHV68 gene 50 with single-copy sensitivity, as previously described (41). For infection of mice, animals were anesthetized with isoflurane (no. 398039; Sigma) for 30 s to 1 min just before i.p. injection with 1 × 106 pfu of γHV68 in 500 μL of DMEM or HBSS.
Flow Cytometry, Antibodies, Cell Isolation, and Cell Culture.
Spleen cells were stained with fluorochrome-conjugated antibodies against B220 (RA3-6B2; BD Pharmingen), CD4 (GK1.5; BD Bioscience), CD8 (53-6.7; eBioscience), CD69 (H1.2F3; eBioscience), CD80 (16-10A1; BD Pharmingen), CD21 (7G6; generated in-house), CD23 (B3B4; eBioscience), CD1d (1B1; eBioscience), CD19 (1D3; eBioscience), CD11b (M1/70, eBioscience), CD11c (HL3; eBioscience), and Igλ (goat polyclonal; cat. no. 1065–02; Southern Biotechnology Associates). Stained cells were fixed in PBS solution, 4% (wt/vol) formaldehyde before analysis. Tregs were stained by using a FITC anti-mouse/rat FoxP3 Staining Set (eBioscience). Dead cells were excluded using LIVE/DEAD Fixable Dead Cell Stain Kit (Invitrogen). Cells were analyzed on a CyAn flow cytometer (Beckman Coulter) and with FlowJo 8.8.6 software (Tree Star). Cell analyses were done on a doublet cell excluded (single), live, and lymphoid (based on forward and side scatter) cell gate. B cells were purified by negative selection by using anti-CD43 antibody-conjugated magnetic beads (Miltenyi Biotech). Purified B cells were cultured at 3 × 106 cells/mL in complete RPMI medium (10% FBS) with or without 5 μg/mL of TLR7 agonist 3M-012 (76) for 48 h before staining. For DC isolation, each spleen was first digested in 2 mL of Click medium with 50 mg/mL collagenase D and 50 μg/mL DNase for 30 to 40 min at 37 °C. Equal volume of 0.1 M EDTA was then added, and the tissue was incubated for 5 min at 37 °C. The digested tissue was homogenized and filtered to obtain single-cell suspensions, and erythrocytes were lysed with 0.15 M NH4Cl buffer. DCs were purified by positive selection using anti-CD11c antibody-conjugated magnetic beads (Miltenyi Biotech) and were subsequently stained with fluorescent-conjugated antibodies for flow cytometric analyses.
ELISA for Virus-Specific Antibody.
Nunc-Immuno MaxiSorp plates (Fisher Scientific) were coated with 8 μg/mL γHV68 viral antigen (prepared as described earlier) in PBS solution overnight at 4 °C. Plates were washed with PBS solution, 0.5% Tween-20 and blocked with PBS solution, 1% BSA for 2 h at 37 °C. After washing the plates again, serial dilutions of serum samples (starting at 1:20) in PBS solution, 1% BSA were added to wells. Plates were incubated with serum for 2 h at 37 °C, and then washed three times with PBS solution, 0.5% Tween-20. Bound antibodies were detected with AP-conjugated goat anti-mouse IgG2a antibodies (Southern Biotechnology) diluted 1:2,000 in PBS solution, 1% BSA. After 1 h of incubation at 37 °C, plates were washed three times with PBS solution, 0.5% Tween-20, and color was developed adding p-nitrophenyl phosphate (Sigma) in 1 M diethanolamine, 0.5 mM MgCl2, pH 9.8, buffer. Absorbance values at 405 nm were obtained by reading the plates on a Versamax ELISA reader (Molecular Devices), and the data were analyzed with SoftMax Pro-5 Software (Molecular Devices). Virus-specific antibody titers were determined as the dilution fold at which serum samples displayed the same OD value in the linear part of the curve.
ELISA for Total Serum Ig and Anti-Smith and Anti-chromatin Antibodies.
Total serum levels of IgG and IgM were determined by sandwich ELISA by using similar wash, incubation, and blocking steps described earlier for the virus-specific antibody ELISA. Plates were coated with 2 μg/mL of goat anti-mouse IgG or IgM antibodies (Southern Biotechnology). Bound Ig was detected with AP-conjugated goat anti-mouse IgG or IgM (Southern Biotechnology). Serum concentration of IgG and IgM was calculated relative to mouse IgM and IgG standards of defined concentration (Southern Biotechnology).
For the detection of anti-Smith and anti-chromatin serum IgM and IgG titers, plates were coated at 4 °C overnight with 1 μg/mL of Smith antigen (Meridian Life Science) or 5 to 10 μg/mL of chromatin, both from calf thymus. Chromatin, which was also used for the detection of anti-dsDNA antibodies, was either a generous gift of Larry Wysocki (NJH, Denver, CO) or was prepared by 20 s sonication of DNA sodium salt (D1501; Sigma). Antigen-specific IgM and IgG antibodies were detected by using AP-conjugated goat anti-mouse IgM and IgG antibodies (Southern Biotechnology). Absorbance at 405 nm was read after incubating Smith-specific plates for 24 h at 37 °C and chromatin (and dsDNA)-specific plates for 18 h at 4 °C. Positive control serum was prepared from a 15-wk-old female MRL/lpr mouse and stored at −20 °C in aliquots. Positive control serum was added to each anti-chromatin and anti-Smith ELISA plate as a reference value for ELISA performed at different times. Antibody titers were determined as the dilution fold at which serum samples displayed the same OD value in the linear part of the curve. Antibody titers were normalized with respect to positive control values obtained at the time the ELISAs were performed.
Analysis of Kidney Parameters.
BUN levels were measured by using an ACE Chemistry Analyzer (Alfa Wassermann). Kidneys were harvested from B6 and B6.Sle123 noninfected and infected mice and were stored in formalin until paraffin wax preparation, sectioning, and subsequent staining with periodic acid–Schiff. To assess histologic injury of the kidneys, 25 glomeruli from each kidney were examined by a blinded observer by using a BX51 microscope (Olympus).
Statistical Analysis.
Statistical significance was calculated with Prism software (GraphPad Software) by using a one-tailed Student t test with equal variance (with Welch correction when appropriate). P values lower than 0.05 were considered significant. Data are represented as arithmetic means ± SD.
Supplementary Material
Acknowledgments
We thank Dr. L. Wysocki [National Institutes of Health (NIH)] for the gift of chromatin, Drs. L. Dragone and L. Peterson (NJH) for the gift of B6.56R mice, and Dr. R. Kedl [University of Colorado Denver (UCD)] for the gift of 3M-012. We also thank A. Holland-Neidermyer (UCD, Aurora, CO) for measuring blood urea nitrogen and M. Glogowska (UCD, Aurora, CO) for generating the kidney slides. We also thank Drs. A. Getahun and J. C. Cambier and members of the R.P. and R.M.T. laboratories for helpful discussions. This work was supported by NIH Grant P01 AI022295 and in part by NIH Grants R01 AI052310 (to R.P.), R01 AI052157 (to R.M.T.), and DK076690 (to J.M.T.). J.D.L. was supported in part by T32 NIH Training Grant T32-AI07405.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 6804 (volume 109, number 18).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203019109/-/DCSupplemental.
References
- 1.James JA, Harley JB, Scofield RH. Epstein-Barr virus and systemic lupus erythematosus. Curr Opin Rheumatol. 2006;18:462–467. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 2.Posnett DN, Yarilin D. Amplification of autoimmune disease by infection. Arthritis Res Ther. 2005;7:74–84. doi: 10.1186/ar1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lünemann JD, Münz C. Epstein-Barr virus and multiple sclerosis. Curr Neurol Neurosci Rep. 2007;7:253–258. doi: 10.1007/s11910-007-0038-y. [DOI] [PubMed] [Google Scholar]
- 4.Peters AL, Stunz LL, Meyerholz DK, Mohan C, Bishop GA. Latent membrane protein 1, the EBV-encoded oncogenic mimic of CD40, accelerates autoimmunity in B6.Sle1 mice. J Immunol. 2010;185:4053–4062. doi: 10.4049/jimmunol.0904065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson-Mungerson MA, Caldwell RG, Bultema R, Longnecker R. Epstein-Barr virus LMP2A alters in vivo and in vitro models of B-cell anergy, but not deletion, in response to autoantigen. J Virol. 2005;79:7355–7362. doi: 10.1128/JVI.79.12.7355-7362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, et al. EBV latent membrane protein 2A induces autoreactive B cell activation and TLR hypersensitivity. J Immunol. 2006;177:2793–2802. doi: 10.4049/jimmunol.177.5.2793. [DOI] [PubMed] [Google Scholar]
- 7.Blackman MA, Flaño E, Usherwood E, Woodland DL. Murine gamma-herpesvirus-68: A mouse model for infectious mononucleosis? Mol Med Today. 2000;6:488–490. doi: 10.1016/s1357-4310(00)01813-x. [DOI] [PubMed] [Google Scholar]
- 8.Virgin HW, Speck SH. Unraveling immunity to gamma-herpesviruses: A new model for understanding the role of immunity in chronic virus infection. Curr Opin Immunol. 1999;11:371–379. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 9.Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: Lessons from the mouse. Annu Rev Immunol. 2011;29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 10.Sarawar SR, et al. Cytokine production in the immune response to murine gammaherpesvirus 68. J Virol. 1996;70:3264–3268. doi: 10.1128/jvi.70.5.3264-3268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson PG, Doherty PC. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J Virol. 1998;72:943–949. doi: 10.1128/jvi.72.2.943-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sangster MY, et al. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol. 2000;164:1820–1828. doi: 10.4049/jimmunol.164.4.1820. [DOI] [PubMed] [Google Scholar]
- 13.Flaño E, Woodland DL, Blackman MA. A mouse model for infectious mononucleosis. Immunol Res. 2002a;25:201–217. doi: 10.1385/IR:25:3:201. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson PG, Cardin RD, Christensen JP, Doherty PC. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J Gen Virol. 1999;80:477–483. doi: 10.1099/0022-1317-80-2-477. [DOI] [PubMed] [Google Scholar]
- 15.Ehtisham S, Sunil-Chandra NP, Nash AA. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart JP, Usherwood EJ, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weck KE, Kim SS, Virgin HW IV, Speck SH. B cells regulate murine gammaherpesvirus 68 latency. J Virol. 1999b;73:4651–4661. doi: 10.1128/jvi.73.6.4651-4661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weck KE, Kim SS, Virgin HW IV, Speck SH. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999a;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaño E, Kim IJ, Woodland DL, Blackman MA. Gamma-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J Exp Med. 2002b;196:1363–1372. doi: 10.1084/jem.20020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaño E, Kayhan B, Woodland DL, Blackman MA. Infection of dendritic cells by a gamma2-herpesvirus induces functional modulation. J Immunol. 2005;175:3225–3234. doi: 10.4049/jimmunol.175.5.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochreiter R, Ptaschinski C, Kunkel SL, Rochford R. Murine gammaherpesvirus-68 productively infects immature dendritic cells and blocks maturation. J Gen Virol. 2007;88:1896–1905. doi: 10.1099/vir.0.82931-0. [DOI] [PubMed] [Google Scholar]
- 22.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks JW, et al. Requirement for CD40 ligand, CD4(+) T cells, and B cells in an infectious mononucleosis-like syndrome. J Virol. 1999;73:9650–9654. doi: 10.1128/jvi.73.11.9650-9654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparks-Thissen RL, Braaten DC, Kreher S, Speck SH, Virgin HW., 4th An optimized CD4 T-cell response can control productive and latent gammaherpesvirus infection. J Virol. 2004;78:6827–6835. doi: 10.1128/JVI.78.13.6827-6835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braaten DC, Sparks-Thissen RL, Kreher S, Speck SH, Virgin HW., 4th An optimized CD8+ T-cell response controls productive and latent gammaherpesvirus infection. J Virol. 2005;79:2573–2583. doi: 10.1128/JVI.79.4.2573-2583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparks-Thissen RL, et al. CD4 T cell control of acute and latent murine gammaherpesvirus infection requires IFNgamma. Virology. 2005;338:201–208. doi: 10.1016/j.virol.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Barton ES, Lutzke ML, Rochford R, Virgin HW., 4th Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J Virol. 2005;79:14149–14160. doi: 10.1128/JVI.79.22.14149-14160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gargano LM, Forrest JC, Speck SH. Signaling through Toll-like receptors induces murine gammaherpesvirus 68 reactivation in vivo. J Virol. 2009;83:1474–1482. doi: 10.1128/JVI.01717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith KA, Efstathiou S, Cooke A. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J Immunol. 2007;179:7325–7333. doi: 10.4049/jimmunol.179.11.7325. [DOI] [PubMed] [Google Scholar]
- 30.Yarilin DA, Valiando J, Posnett DN. A mouse herpesvirus induces relapse of experimental autoimmune arthritis by infection of the inflammatory target tissue. J Immunol. 2004;173:5238–5246. doi: 10.4049/jimmunol.173.8.5238. [DOI] [PubMed] [Google Scholar]
- 31.Peacock JW, Elsawa SF, Petty CC, Hickey WF, Bost KL. Exacerbation of experimental autoimmune encephalomyelitis in rodents infected with murine gammaherpesvirus-68. Eur J Immunol. 2003;33:1849–1858. doi: 10.1002/eji.200323148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speck SH, Virgin HW. Host and viral genetics of chronic infection: A mouse model of gamma-herpesvirus pathogenesis. Curr Opin Microbiol. 1999;2:403–409. doi: 10.1016/s1369-5274(99)80071-x. [DOI] [PubMed] [Google Scholar]
- 33.Pelanda R, Torres RM. Receptor editing for better or for worse. Curr Opin Immunol. 2006;18:184–190. doi: 10.1016/j.coi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Sekiguchi DR, et al. Development and selection of edited B cells in B6.56R mice. J Immunol. 2006;176:6879–6887. doi: 10.4049/jimmunol.176.11.6879. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen C, Limaye N, Wakeland EK. Susceptibility genes in the pathogenesis of murine lupus. Arthritis Res. 2002;4(suppl 3):S255–S263. doi: 10.1186/ar583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: Multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z, Morel L. Genetics of systemic lupus erythematosus: Contributions of mouse models in the era of human genome-wide association studies. Discov Med. 2010;10:71–78. [PubMed] [Google Scholar]
- 40.Morel L, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Dyk LF, Virgin HW, 4th, Speck SH. Maintenance of gammaherpesvirus latency requires viral cyclin in the absence of B lymphocytes. J Virol. 2003;77:5118–5126. doi: 10.1128/JVI.77.9.5118-5126.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicoletti F, et al. Dichotomic effects of IFN-gamma on the development of systemic lupus erythematosus-like syndrome in MRL-lpr / lpr mice. Eur J Immunol. 2000;30:438–447. doi: 10.1002/1521-4141(200002)30:2<438::AID-IMMU438>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J Immunol. 2002;169:2694–2700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 45.Rubtsov AV, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao Y, O'Neill PJ, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lardans V, et al. Polyclonal B lymphocyte activation induced by mouse hepatitis virus A59 infection. J Gen Virol. 1996;77:1005–1009. doi: 10.1099/0022-1317-77-5-1005. [DOI] [PubMed] [Google Scholar]
- 48.Hunziker L, et al. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol. 2003;4:343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan ME, Tan EM. Antinuclear antibodies in infectious mononucleosis. Lancet. 1968;1:561–563. doi: 10.1016/s0140-6736(68)92831-6. [DOI] [PubMed] [Google Scholar]
- 50.Sutton RN, Emond RT, Thomas DB, Doniach D. The occurrence of autoantibodies in infectious mononucleosis. Clin Exp Immunol. 1974;17:427–436. [PMC free article] [PubMed] [Google Scholar]
- 51.McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Curr Mol Med. 2009;9:1058–1079. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- 52.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dutia BM, Clarke CJ, Allen DJ, Nash AA. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J Virol. 1997;71:4278–4283. doi: 10.1128/jvi.71.6.4278-4283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 56.Deane JA, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nickerson KM, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Gent M, et al. EBV lytic-phase protein BGLF5 contributes to TLR9 downregulation during productive infection. J Immunol. 2011;186:1694–1702. doi: 10.4049/jimmunol.0903120. [DOI] [PubMed] [Google Scholar]
- 59.Buisson M, et al. A bridge crosses the active-site canyon of the Epstein-Barr virus nuclease with DNase and RNase activities. J Mol Biol. 2009;391:717–728. doi: 10.1016/j.jmb.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 60.Covarrubias S, Richner JM, Clyde K, Lee YJ, Glaunsinger BA. Host shutoff is a conserved phenotype of gammaherpesvirus infection and is orchestrated exclusively from the cytoplasm. J Virol. 2009;83:9554–9566. doi: 10.1128/JVI.01051-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guggemoos S, et al. TLR9 contributes to antiviral immunity during gammaherpesvirus infection. J Immunol. 2008;180:438–443. doi: 10.4049/jimmunol.180.1.438. [DOI] [PubMed] [Google Scholar]
- 62.Santiago-Raber ML, et al. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2010;34:339–348. doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Flaño E, Husain SM, Sample JT, Woodland DL, Blackman MA. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J Immunol. 2000;165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 64.Liang Z, Chang S, Youn MS, Mohan C. Molecular hallmarks of anti-chromatin antibodies associated with the lupus susceptibility locus, Sle1. Mol Immunol. 2009;46:2671–2681. doi: 10.1016/j.molimm.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boname JM, de Lima BD, Lehner PJ, Stevenson PG. Viral degradation of the MHC class I peptide loading complex. Immunity. 2004;20:305–317. doi: 10.1016/s1074-7613(04)00047-0. [DOI] [PubMed] [Google Scholar]
- 66.Pires de Miranda M, et al. The Gammaherpesvirus m2 protein manipulates the Fyn/Vav pathway through a multidocking mechanism of assembly. PLoS One. 2008;3:e1654. doi: 10.1371/journal.pone.0001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodrigues L, et al. Termination of NF-kappaB activity through a gammaherpesvirus protein that assembles an EC5S ubiquitin-ligase. EMBO J. 2009;28:1283–1295. doi: 10.1038/emboj.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu J, et al. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Invest. 2005;115:1869–1878. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 70.Wan S, Zhou Z, Duan B, Morel L. Direct B cell stimulation by dendritic cells in a mouse model of lupus. Arthritis Rheum. 2008;58:1741–1750. doi: 10.1002/art.23515. [DOI] [PubMed] [Google Scholar]
- 71.Weslow-Schmidt JL, et al. Type I interferon inhibition and dendritic cell activation during gammaherpesvirus respiratory infection. J Virol. 2007;81:9778–9789. doi: 10.1128/JVI.00360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 73.Tsao BP. Update on human systemic lupus erythematosus genetics. Curr Opin Rheumatol. 2004;16:513–521. doi: 10.1097/01.bor.0000132648.62680.81. [DOI] [PubMed] [Google Scholar]
- 74.Lindqvist AK, et al. A susceptibility locus for human systemic lupus erythematosus (hSLE1) on chromosome 2q. J Autoimmun. 2000;14:169–178. doi: 10.1006/jaut.1999.0357. [DOI] [PubMed] [Google Scholar]
- 75.Virgin HW, 4th, et al. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gorden KB, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]