Abstract
β-Arrestins are multifunctional proteins that play central roles in G protein-coupled receptor (GPCR) trafficking and signaling. β-Arrestin1 is also recruited to the insulin-like growth factor-1 receptor (IGF-1R), a receptor tyrosine kinase (RTK), mediating receptor degradation and signaling. Because GPCR phosphorylation by GPCR-kinases (GRKs) governs interactions of the receptors with β-arrestins, we investigated the regulatory roles of the four widely expressed GRKs on IGF-1R signaling/degradation. By suppressing GRK expression with siRNA, we demonstrated that lowering GRK5/6 abolishes IGF1-mediated ERK and AKT activation, whereas GRK2 inhibition increases ERK activation and partially inhibits AKT signaling. Conversely, β-arrestin–mediated ERK signaling is enhanced by overexpression of GRK6 and diminished by GRK2. Similarly, we demonstrated opposing effects of GRK2 and -6 on IGF-1R degradation: GRK2 decreases whereas GRK6 enhances ligand-induced degradation. GRK2 and GRK6 coimmunoprecipitate with IGF-1R and increase IGF-1R serine phosphorylation, promoting β-arrestin1 association. Using immunoprecipitation, confocal microscopy, and FRET analysis, we demonstrated β-arrestin/IGF-1R association to be transient for GRK2 and stable for GRK6. Using bioinformatic studies we identified serines 1248 and 1291 as the major serine phosphorylation sites of the IGF-1R, and subsequent mutation analysis demonstrated clear effects on IGF-1R signaling and degradation, mirroring alterations by GRKs. Targeted mutation of S1248 recapitulates GRK2 modulation, whereas S1291 mutation resembles GRK6 effects on IGF-1R signaling/degradation, consistent with GRK isoform-specific serine phosphorylation. This study demonstrates distinct roles for GRK isoforms in IGF-1R signaling through β-arrestin binding with divergent functional outcomes.
Keywords: cancer, ubiquitination, biasing
Insulin-like growth factor type 1 receptor (IGF-1R) regulates multiple cellular functions essential for the malignant phenotype, including cellular proliferation, survival, and metastasis, making this receptor an attractive target for cancer treatment (1). Intriguingly, IGF-1R is rarely confirmed as overexpressed, only in some cancer types, and does not show intrinsic receptor abnormalities (2), indicating other regulatory pathways to be involved. Following the discovery of β-arrestin1 (β-arr1), otherwise known to be involved in the regulation of G protein-coupled receptors (GPCRs), as a key mediator of IGF-1R signaling (3), we demonstrated that β-arr1 serves as an adaptor to bring the E3 ubiquitin-ligase Mdm2 to the IGF-1R (4, 5), with a dual outcome on IGF-1R: ubiquitination and receptor down-regulation as well as IGF-1R/β-arr1–mediated activation of MAPK signaling. The dual regulatory role of β-arr1 in the case of IGF-1R is remarkably similar to the role played by the β-arrs in the case of the GPCR family: while ending G protein activation, β-arrs redirect the signaling to MAPK (6). In the course of GPCR activation, β-arrs bind to the receptor and desensitize G protein signaling only after phosphorylation of specific serine residues by another important regulatory family: the GPCR kinases (GRKs) (7).
Given the high degree of similarity between the functional roles played by β-arrs in the case of IGF-1R and GPCRs, in the present study we aimed to investigate the involvement of GRKs on binding of β-arr to the IGF-1R and the functional consequences.
Results
Screening: Effects of Inhibition of GRKs on IGF1-Stimulated ERK/AKT Signaling.
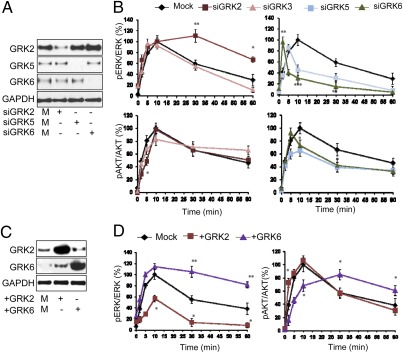
β-Arr1 is required for sustained IGF1-induced ERK activation (5); therefore, in the initial screen we used the temporal characteristics of IGF-1R–induced signaling as a read-out (Fig. 1 and Fig. S1). The roles of GRK2, -3, -5, and -6 on IGF-1R signaling in Human Embryonic Kidney 293T (HEK293T) cells were investigated by suppressing their expression with specific siRNA and monitoring the dynamics of IGF1-mediated activation of the two key downstream IGF-1R signaling pathways, the Ras/Raf/MEK/ERK and PI3K/AKT pathways. Efficiency of GRK2, GRK5, and GRK6 siRNA depletion was confirmed by Western transfer analysis (WB) (Fig. 1A). For GRK3 siRNA, quantitative PCR confirmed a decrease in GRK3-specific mRNA to ≈3% of the normal level. In mock-transfected cells, ERK activation reached a maximum within 10 min of stimulation and decreased to ≈60% of maximal levels after 30 min. GRK3 inhibition demonstrated no major contribution to the IGF1-induced ERK activation. Depletion of GRK5/6 led to substantial inhibition of the ERK phosphorylation, whereas siRNA to GRK2 caused a sustained IGF1-mediated ERK activation (Fig. 1B and Fig. S1A). We also investigated the effects of GRK inhibition on IGF1-mediated AKT phosphorylation (Fig. 1B and Fig. S1A) and found that the peak activation was moved to 5 min after depleting GRK6 and essentially unchanged after depletion of GRK2 or -3. However, early (2 and 5 min) AKT phosphorylation is decreased by GRK2 siRNA, whereas GRK6 inhibition results in impaired late (30 and 60 min) AKT phosphorylation. GRK3 siRNA has no major effects, whereas GRK5 down-regulation has an overall inhibitory effect on pAKT. Because of the clear and opposing effects of GRK2 and GRK6 on IGF-1R signaling (inhibitory vs. stimulatory on ERK signaling, early- vs. late-phase AKT signaling), we selected them to perform complementary experiments in which they were overexpressed. Overexpression after transfection was confirmed by WB in HEK293T (Fig. 1C). Overexpression of GRK2 markedly reduced the IGF1 activation of ERK as in the case with GRK6 (or GRK5) siRNA (Fig. 1D and Fig. S1B). In the opposite manner, GRK6 overexpression led to a sustained ERK activation pattern, similar to GRK2 inhibition (Fig. 1D and Fig. S1B). We also evaluated the kinetics of AKT phosphorylation as well as IGF-1R activation-loop phosphorylation. As demonstrated in Fig. 1D, early-phase AKT phosphorylation is enhanced by GRK2 overexpression, whereas late-phase AKT phosphorylation is augmented with GRK6 overexpression (Fig. 1D and Fig. S1B). IGF-1R phosphorylation is distinctly reduced after GRK6 over-expression (Fig. S1B), probably secondary to an overall decrease in IGF-1R available at the cell surface (see below). Notably, enhanced IGF1-induced ERK phosphorylation after GRK6 overexpression occurs even with low kinase activity, as represented by low phosphorylated IGF-1R. Quantification of WBs from multiple experiments confirmed altered ERK and AKT activity dynamics for GRK2 and GRK6 (Fig. 1D) and illustrates the opposing effects on IGF-1R signaling pathways. Overall, these data suggest that GRK2 is involved in early AKT activation while limiting the ERK response to IGF1, whereas GRK6 may play a role in prolonging ERK activation and late-phase AKT phosphorylation.
Fig. 1.
Screening: effects of GRKs on IGF1-stimulated ERK/AKT signaling in HEK293T cells. (A) Cells were transfected with either control (M) or the indicated GRK-specific siRNAs. Three days after transfection, cells were lysed and analyzed by WB using GRK-specific antibodies and GAPDH as loading control. (B) Cells transfected with the indicated siRNAs were serum starved and then stimulated with IGF1 (50 ng/mL) for indicated times. The levels of phospho-ERK (pERK), phospho-AKT (pAKT), total ERK 1/2, and total AKT were analyzed by WB. Signals were quantified by densitometry, normalized to total ERK/AKT, and expressed as percentage of the maximum phosphorylated ERK/AKT obtained at 10 min stimulation in control (Mock). Represented as mean ± SEM from four independent experiments. Statistical analysis compared with control transfected: *P < 0.05, **P < 0.01, ***P < 0.005. (C) Cells were transiently transfected with either mock or indicated GRKs-encoding plasmids for 1 d and transfection efficiency analyzed by WB using GRK-specific antibodies. (D) After serum starvation and IGF1 stimulation for the indicated times, the levels of pERK, ERK, pAKT and AKT were quantified as described in A and represent the mean ± SEM from four independent experiments. Statistical analysis compared with control transfected: *P < 0.05, **P < 0.01.
Validation: Effects of GRK2 and -6 on IGF-1R Expression.
The subsequent effect of β-arr1 recruitment to the IGF-1R, decreased levels of the receptor through ubiquitination and degradation, is evident after long-term IGF1 stimulation (8); therefore, we investigated whether GRK2/6 could alter the IGF-1R degradation response.
Depletion or overexpression of GRK2/6 in HEK293T, BE, and DFB cells [two melanoma cell lines previously described to preserve β-arr–dependent IGF-1R degradation mechanisms (4)] was quantified by WB (Fig. S2A). We used 5 min IGF1-induced tyrosine phosphorylation to verify the IGF-1R expression at the cell surface at the beginning of the experiments. Although the cells were maintained in serum-free media, overexpression of GRK6 results in decreased tyrosine-phosphorylated IGF-1R in all three cell lines, suggesting increased receptor internalization (Fig. S2B). Receptor degradation was monitored by WB detection of IGF-1R levels in serum-starved cells stimulated with IGF1 for up to 36 h (Fig. S3A). The IGF-1R degradation rate is increased by GRK2 inhibition or GRK6 overexpression and decreased after GRK2 overexpression or GRK6 inhibition. These trends were confirmed by densitometric quantification of multiple experiments (Fig. S3B). Overall, these experiments indicate that GRK2 protects IGF-1R from ligand-induced degradation, whereas GRK6 accelerates this process.
Functional Validation: IGF-1R as a Substrate for GRKs.
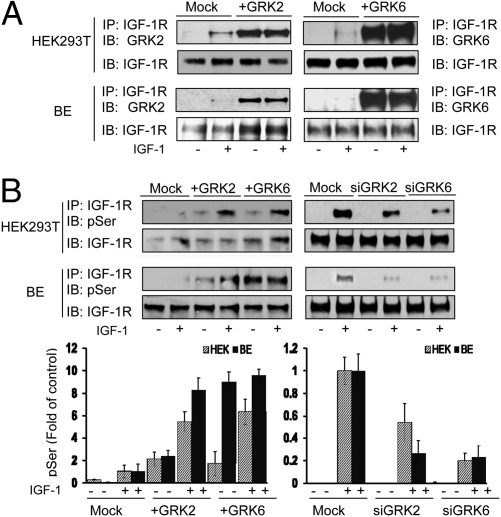
The effects of GRKs on IGF-1R signaling and degradation led us to ask whether GRK2 and GRK6 could be directly associating with the IGF-1R. Direct association was tested by immunoprecipitation (IP) of IGF-1R and detection by WB for the putative associated protein. This was performed in HEK293T, BE, DFB, and mouse embryonic fibroblast (MEF) cells with and without overexpression of the relevant GRK, without and with IGF1 stimulation. In untransfected cells, endogenous levels of GRK2 and -6 associated with IGF-1R only after IGF1 stimulation, indicating a ligand-dependent mechanism (Fig. S4A). After overexpression (transfection efficiency, Fig. S4B), the GRKs could also be detected in unstimulated conditions, suggesting that they are binding to the IGF-1R when overexpressed (Fig. 2A and Fig. S4C).
Fig. 2.
Functional validation: effects of GRKs on IGF-1R serine phosphorylation. (A) Cells were either transfected with mock or indicated GRK-encoding plasmid, serum starved for 12 h, and stimulated or not with IGF1 (50 ng/mL) for 10 min. IGF-1R complexes were isolated by IP, and total IGF-1R and associated GRKs were detected by WB (IB). (B) Upper: Cell lysates were prepared from the indicated siRNA or GRKs-encoding plasmids-transfected cells, stimulated or not with IGF1. Equal amounts of receptors were used for IP and their total and phospho-serine (pSer) levels visualized by WB (IB). Lower: Signals were quantified by densitometry and displayed as fold change of the level in control cells stimulated for 10 min. Data correspond to the mean ± SEM from three independent experiments.
Because the GRKs are serine kinases, the next logical step was to assess whether this direct association leads to IGF-1R serine phosphorylation. The level of IGF-1R serine phosphorylation was detected by WB after IP of the IGF-1R from HEK293T and BE cells, with overexpression or inhibition of the GRKs. In control cells the level of serine phosphorylation in IGF-1R is very low but can just be detected in IGF1-stimulated cells (Fig. 2B). After overexpression of GRK2/6, IGF-1R serine phosphorylation is easily detectable in unstimulated cells and increased substantially in IGF1-stimulated cells (Fig. 2B). In an opposite manner, down-regulation of either GRK2 or GRK6 by siRNA (Fig. S4D) markedly inhibits ligand-induced IGF-1R serine phosphorylation in both cell lines (Fig. 2B).
GRK-Dependent β-Arr1 Recruitment to the IGF-1R.
GRKs generate serine-phosphorylated binding sites on GPCRs, controlling β-arr recruitment; thus, we next directly addressed whether the GRKs regulate β-arr recruitment to the IGF-1R. We used three approaches to investigate this association: co-IP, confocal microscopy to visualize GFP–β-arr recruitment to the plasma membrane after IGF1 stimulation, and FRET in live cells to quantify the dynamics of this association.
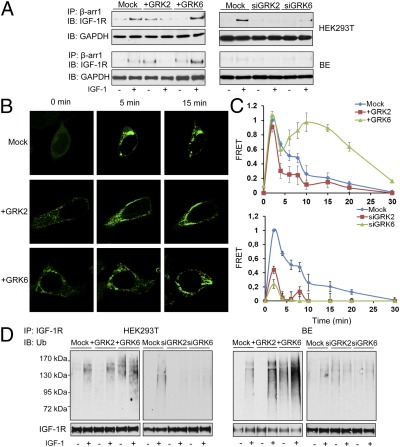
IPs of β-arr1 from HEK293T or BE cells depleted or overexpressing GRK2 or -6 (Fig. S4E) were analyzed for association with IGF-1R by WB. In untreated, unstimulated cells, low levels of β-arr1 are associated with the IGF-1R; however, this association clearly increases after IGF1 stimulation (Fig. 3A). After GRK2 overexpression there is a clear increase in basal levels of β-arr1/IGF-1R association, yet after 10-min stimulation with IGF1, β-arr1 apparently dissociates from this complex. In contrast, overexpression of GRK6 leads to greater IGF1-induced association (Fig. 3A, Left). Similar experiments with GRK2 or -6 siRNA demonstrated decreased β-arr/IGF-1R association, irrespective of ligand presence (Fig. 3A, Right).
Fig. 3.
Effects of GRKs on β-arrestin recruitment to the IGF-1R and subsequent receptor ubiquitination. (A) Cells transfected as indicated were serum starved then stimulated or not with IGF1 (50 ng/mL) for 10 min, β-arrestin 1 (β-arr1) immunoprecipitated (IP), and associated IGF-1R detected by WB (IB). GAPDH levels in the total protein lysates before IP were used as loading controls. (B) HEK293T cells were transfected with GFP–β-arr1 and indicated GRK-expressing plasmids. After serum starvation, the cells were IGF1 stimulated and GFP–β-arr1 recruitment to the plasma membrane visualized by confocal microscopy. Images displayed are representative of three independent experiments. (C) HEK293T cells coexpressing IGF-1R–YFP (acceptor) and β-arr1–CFP (donor) plasmids had GRK2 or -6 depleted or overexpressed as indicated. Serum-starved cells were excited at 430 nm, and acceptor and donor fluorescent emissions were recorded at indicated time points after IGF1 addition and used to calculate the energy transfer (FRET) between the CFP and YFP as an indicator of IGF-1R/ β-arr1 interaction. Data are represented as ratios of FRET obtained at 2 min of IGF1 stimulation in mock-transfected cells, calculated as described in SI Materials and Methods; mean ± SEM from three independent experiments done in triplicate. (D) Cell lysates were prepared from the indicated siRNA or GRKs-encoding plasmids-transfected cells, stimulated or not with IGF1 for 10 min. Equal amounts of receptors were used for IP, and their total and ubiquitinated (Ub) levels visualized by WB (IB).
Upon agonist stimulation, many GPCRs induce translocation of cytosolic β-arr to the plasma membrane (9), where β-arr forms a complex with the GPCR. The stability of these complexes directs the activated receptor to different intracellular trafficking pathways (6). Class A receptors (e.g., β2-adrenergic receptor), when stimulated, lead to rapid translocation of β-arr to the plasma membrane, transient binding, and β-arr dissociation at or near the plasma membrane, with the majority of receptors being recycled back to the cell surface (10). Class B receptors (e.g., angiotensin II type 1A), in contrast, maintain a stable association and traffic together with β-arr, and the postendocytic receptor sorting is directed mostly toward degradative pathways (10). Likewise, β-arr involvement in IGF-1R endocytosis was reported (11); therefore, confocal microscopy was used to explore whether overexpression of GRK2 or -6 could modify IGF1-induced GFP–β-arr1 redistribution pattern. In unstimulated mock-transfected cells, the GFP–β-arr1 was evenly distributed throughout the cytoplasm (Fig. 3B, 0 min). IGF1 promoted the rapid movement of β-arr1 from the cytosol to the plasma membrane (Fig. 3B, 5 min). After 15 min IGF1, β-arr1 fluorescence was observed both in a punctate pattern at the plasma membrane, similar to the class A GPCR pattern, and localized to endocytic vesicles, similar to the class B pattern. When GRK2 is overexpressed (transfection efficiency, Fig. S4E), the punctate pattern is visible even in basal conditions. Activation of the IGF-1R further increases the redistribution of β-arr1 from the cytoplasm to the receptor at the plasma membrane, with a predominantly “A” pattern. After 15-min exposure to IGF1, a prominent difference in the trafficking of β-arr compared with the untransfected cells is observed: β-arr fluorescence remained at the plasma membrane in a characteristic class A model in cells overexpressing the GRK2 (Fig. 3B, 15 min). In contrast, in GRK6 transfected cells, GFP–β-arr1 was distributed in a class B pattern in unstimulated cells, with IGF1 treatment accentuating the localization of GFP–β-arr1 to endocytic vesicles (Fig. 3B). To quantify the extent of IGF-1R/β-arr1 association we used a FRET assay to measure the nonradiative energy transfer from a CFP donor to a YFP acceptor. We coexpressed IGF-1R–YFP and β-arr1–CFP in HEK293T cells and validated the FRET in single cells using acceptor photobleaching (Fig. S5). The same donor/acceptor system was then used to follow the kinetics of β-arr1 binding to the IGF-1R in cell populations. IGF1 stimulation induces a rapid IGF-1R/β-arr association, indicated by energy transfer that reaches a maximum at ≈2 min and then declines to 50% of maximum energy transfer 8 min after stimulation, implying dissociation of the two proteins (Fig. 3C). There are two distinct phases of this interaction: an initial phase during the first 5 min characterized by fast association followed by equally fast dissociation, and a second phase proceeding for the next 5 min, with much slower dissociation (or reassociation). After GRK2 overexpression (transfection efficiency, Fig. S4E), and consistent with the class A pattern observed in the confocal experiment, the β-arr/IGF-1R association is transient, and after 5 min stimulation only background levels of energy transfer were measured, with complete abrogation of the second phase. In contrast, GRK6 overexpression (Fig. S4E) prolonged β-arr/IGF-1R association, with an amplified second phase and more than 50% of the maximum energy transfer maintained even 25 min after stimulation (Fig. 3C). By using the FRET system we could also measure the β-arr/IGF-1R association in the absence of GRK2/6 (Fig. 3C, Lower). In both cases a substantial decrease of β-arr recruitment to the IGF-1R was observed.
Because one major outcome of β-arr1 binding to the IGF-1R is receptor ubiquitination (4, 5), to functionally validate the effects of GRK-mediated β-arr recruitment to the IGF-1R we investigated receptor ubiquitination. The IGF-1R was immunoprecipitated from the same samples described in Fig. 3A, and the ubiquitinated receptors were detected by WB. Whereas GRK2 overexpression augmented IGF-1–induced ubiquitination, GRK6 overexpression amplified both basal and ligand-stimulated IGF-1R ubiquitination. GRK2/6 depletion by siRNA drastically reduced IGF-1R ubiquitination (Fig. 3D).
Identification and Validation of the GRK Phosphorylated Serine Residues.
Having shown that GRK2 and GRK6 cause serine phosphorylation of the IGF-1R, with subsequent β-arr recruitment, we aimed to identify those residues involved in β-arr binding. Previous data indicate that IGF-1R truncated at position 1245 (Δ1245) lacks the ability to bind β-arr (5). To functionally confirm the Δ1245 IGF-1R in the context of GRKs overexpression, we used MEF cells expressing either WT IGF-1R or Δ1245 IGF-1R (Fig. S6). As in the other cell lines, GRK2 overexpression stabilized whereas GRK6 increased ligand-dependent degradation of full-length IGF-1R. In contrast, C terminus-truncated IGF-1R was insensitive to GRK2/6 overexpression, thus indicating that the residues involved in GRK phosphorylation are within the C-terminal 100 amino acids of the IGF-1R (Fig. S6B). Given that there are nine serine residues within this domain, using prediction algorithms, we next evaluated which ones are likely to be phosphorylated. Using different algorithms, the three serines predicted with highest probability were at 1248, 1272, and 1291 (Fig. S6C); accordingly, we created mutant receptors with these residues mutated to alanine (A), unable to be phosphorylated, or to aspartic acid (D) to mimic constitutive phosphorylation.
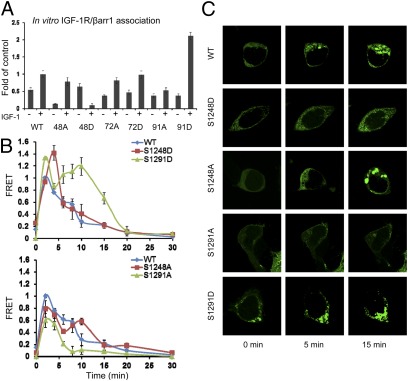
Next we tested the ability of serine IGF-1R mutants to bind β-arr. To minimize competition with the endogenous proteins, we chose to use an in vitro system. β-Arr1-flag–coated beads were used to capture IGF-1R–YFP or mutants, separately expressed in serum-starved HEK293T cells, stimulated or not for 10 min with IGF1. The bound IGF-1R–YFP was detected by WB with an apparent β-subunit molecular mass of ≈125 kDa and densitometric quantification shown (Fig. 4A). In cells expressing WT IGF-1R–YFP there is a clear increase of the YFP-chimeric receptors captured by the β-arr1 after IGF1 stimulation. S1248A can bind β-arr; however, to a lesser extent in comparison with the WT IGF-1R, and the binding is dependent on IGF1 stimulation (Fig. 4A). S1248D, mimicking serine phosphorylation, is captured by the β-arr1 in the unstimulated condition, but this binding decreased after IGF1 stimulation. The S1272 has no effects on β-arr1 binding: both S1272A and S1272D behave like the WT IGF-1R–YFP. Intriguingly, mutation of S1291 to alanine abrogates the β-arr1 binding, whereas the positive S1291D mutant demonstrates increased affinity for β-arr1 only after ligand stimulation (Fig. 4A). Taken together, this experiment indicates S1248 and S1291 of IGF-1R as β-arr1 binding sites.
Fig. 4.
Identification of the GRK phosphorylated serine residues. (A) Cell lysates prepared from serum-starved HEK293T, transfected with indicated IGF-1R–YFP serine mutants, stimulated or not with IGF1 (50 ng/mL) for 10 min, and normalized for equal YFP fluorescence were incubated with equal amounts of purified β-arr1-flag-agarose beads. After overnight incubation, the beads were collected by centrifugation and bound receptors analyzed by WB using IGF-1R–specific antibodies. Signals were quantified by densitometry and displayed as fold change compared with IGF1-stimulated WT IGF-1R–YFP. Data represent the mean ± SEM from three independent experiments. (B) HEK293T cells were cotransfected with IGF-1R–YFP WT or serine mutants (acceptor) and β-arr1–CFP (donor) plasmids and serum starved for 12 h. The cells were excited at 430 nm and acceptor and donor fluorescent emissions recorded at various time points after IGF1 addition and FRET calculated as described in Fig. 3C. Data are represented as ratios of FRET obtained at 2 min of IGF1 stimulation in WT IGF-1R–YFP transfected cells; mean ± SEM from three independent experiments. (C) HEK293T cells expressing GFP–β-arr1 were transfected with WT IGF-1R–YFP or serine mutants as indicated. After serum starvation, the cells were IGF1 stimulated and GFP–β-arr1 recruitment to the plasma membrane visualized by confocal microscopy. Images displayed are representative of three independent experiments.
Next we investigated the kinetics of β-arr binding to the IGF-1R. We again used FRET to quantify the interaction between β-arr1–CFP and WT IGF-1R–YFP or serine mutants. As shown in Fig. 4B and consistent with the experiment presented in Fig. 3C, the WT IGF-1R–YFP/β-arr1–CFP interaction displayed a two-phase FRET pattern, with a quick β-arr1 recruitment followed by rapid dissociation during the first 5 min and a second, slow dissociation phase reaching 50% of maximum energy transfer 8 min after stimulation. With the S1248D, similar to GRK2 overexpression, the first phase is amplified as regards association/dissociation rates, with a half-maximum energy transfer ≈5 min after stimulation, indicating a transient β-arr binding with the receptor analogous to class A GPCRs (Fig. 4B). S1291D recapitulates the GRK6 overexpression pattern, with an exaggerated second phase and dissociation to half-maximum energy transfer ≈18 min after stimulation, indicating a more stable, class B association pattern (Fig. 4B). Conversely, the mutants S1248A and S1291A considerably prevent the β-arr recruitment, with S1291A being more effective for the second phase whereas S1248A predominantly dampens the first phase (Fig. 4B, Lower).
Next we used confocal microscopy to follow GFP–β-arr1 recruitment to the IGF-1R–YFP mutants expressed in HEK293T cells. Before agonist stimulation, uniform GFP–β-arr1 distribution was observed in WT IGF-1R–YFP transfected cells. Upon IGF1 stimulation, GFP–β-arr1 was rapidly recruited to the membrane (Fig. 4C, WT, 5 min). After 15 min stimulation, GFP–β-arr1 was distributed to visible endocytic vesicles and also located at the plasma membrane (Fig. 4C, WT, 15 min). GFP–β-arr1 in S1248D and S1291A cells shows a predominantly punctate pattern at 0 min, enhanced 5 min after stimulation, with no endocytic vesicle formation after 15 min stimulation (Fig. 4C). This class A GPCR pattern is consistent with the FRET data and is also similar to the pattern of β-arr distribution after GRK2 overexpression (Fig. 3B). Conversely, S1291D and S1248A showed preferential vesicular trafficking (Fig. 4C), consistent with observed β-arr1 recruitment demonstrated by FRET analysis. Taken together, these data suggest that S1248 phosphorylation, similar to GRK2, results in transient IGF-1R/β-arr1 association, promoting a class A receptor behavior, whereas S1291 phosphorylation, similar to GRK6, results in more stable IGF-1R/β-arr1 interaction similar to a class B receptor.
Effects of Serine Mutation on Receptor Trafficking/Degradation and Signaling.
We extended the mutation analysis, to reveal the functional consequences of receptor serine phosphorylation, by investigating the kinetics of IGF-1R degradation. WT IGF-1R degrades over 36 h, in a linear manner and almost exclusively in the presence of IGF1 (Fig. S7). The S1248A mutant displayed a slightly increased degradation rate on IGF1 stimulation; however, the basal down-regulation was not affected. Mutation to S1248D was able to partially rescue the ligand-induced degradation, but this mutant displayed an increased agonist-independent degradation rate. Overall, the degradation pattern of S1248 mutants closely resembles the kinetics of GRK2-induced degradation. For the S1291 mutants a completely different behavior was observed: S1291A stabilizes the IGF1-induced degradation, whereas S1291D mutant increases both the basal and IGF1-dependent degradation rates. Taken together, the S1291 mutants displayed similar characteristics, with GRK6 overexpression increasing the degradation rate (S1291D) or GRK6 inhibition, limiting the ligand-induced degradation (S1291A).
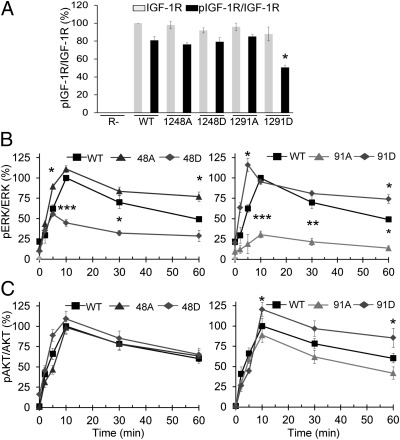
Finally, we investigated the functional effects of S1248 and S1291 mutations on IGF-1R signaling (Fig. 5 and Fig. S8). To avoid signaling interference from endogenous IGF-1R, we expressed the mutants in IGF-1R knockout MEF (R-) cells. The transfection efficiency was verified 48 h after transfection by WB for IGF-1R, whereas tyrosine phosphorylation of the activation loop (Y1135) induced by 5 min ligand stimulation was used as an indicator of cell surface receptor expression. As demonstrated in Fig. 5A, all mutant receptors are being expressed at comparable levels, and all mutants are Y1135 tyrosine phosphorylated to a level similar to the WT IGF-1R–YFP, except IGF-1R S1291D. Consistent with the pattern observed with GRK6 overexpression, the tyrosine phosphorylation of S1291D is approximately 30% lower than the WT IGF-1R, suggesting a higher receptor internalization rate in serum-free conditions.
Fig. 5.
Effects of serine residue phosphorylation on IGF1-mediated ERK and AKT signaling. (A) Cell lysates were prepared from the indicated IGF-1R–YFP WT or serine mutants transfected IGF-1R knockout MEF cells (R-), stimulated or not with IGF1 (50 ng/mL) for 5 min and analyzed by WB for phospho-IGF-1R (pIGF-1R) and total IGF-1R levels. Signals were quantified by densitometry, pIGF-1R normalized to the total IGF-1R and displayed as percentage of the total IGF-1R level in WT transfected cells. Data correspond to the mean ± SEM from three independent experiments. Statistical analysis compared with control pIGF-1R WT transfected cells: *P < 0.05, **P < 0.01. (B and C) R- cells were transfected as in A, serum starved, and IGF1 stimulated for the indicated times. The levels of total and phosphorylated ERK and AKT were detected by WB, signals quantified by densitometry, normalized to total ERK/AKT, and expressed as percentage of the maximum phosphorylated ERK/AKT obtained at 10 min stimulation in WT IGF-1R transfected cells. Represented as mean ± SEM from three independent experiments. Statistical analysis: single mutants compared with WT, double mutants compared with control single mutants: *P < 0.05, **P < 0.01, ***P < 0.005.
Knowing the relative expression at the cell surface for various mutants, the cells were IGF1 stimulated and the time–response course for pERK and pAKT quantified. The WT IGF-1R–YFP activates ERK in response to IGF1 stimulation, reaching a maximum 10 min after stimulation and declining to approximately 50% of the maximum after 1 h stimulation (Fig. 5B and Fig. S8). The S1248A enhanced and prolonged ERK activation, whereas S1248D has the opposite outcome, resembling the effects of GRK2 down-regulation and overexpression, respectively (Fig. 5B and Fig. S8). Conversely, recapitulating the effects of the GRK6 modulation, the S1291A mutant demonstrates low efficiency in sustaining ERK phosphorylation, whereas the S1291D mutant both enhanced and sustained the ERK phosphorylation (Fig. 5B and Fig. S8). Although for the ERK activation clear opposite effects were observed for 1248 vs. 1291 mutants, both S1248D and S1291D are more efficient in activating AKT, with S1248D particularly increasing and S1248A decreasing the early-phase AKT phosphorylation, whereas S1291D, despite its decreased expression at the cell surface, enhanced and sustained pAKT, with the S1291A predominantly decreasing the same late-phase AKT phosphorylation (Fig. 5C and Fig. S8). The S1248 mutants closely resemble the pAKT pattern obtained in conditions whereby GRK2 was overexpressed (S1248D) or inhibited (S1248A), whereas S1291 mimicked the conditions of GRK6 alteration.
Discussion
From a functional point of view, because of their interaction with the G proteins, receptor tyrosine kinases (RTKs) could be considered to some extent GPCRs (12). This is also the case for the IGF-1R/IR, which have long been known to use G protein signaling components (12, 13). In the present study we reveal the missing links that would fully functionally portray a prototypical RTK, the IGF-1R, as a GPCR: GRK-dependent phosphorylation of IGF-1R serine residues as the underlying mechanism for β-arr binding to these residues.
The first key finding of the present work is identification of the GRK2 and GRK6 as serine kinases for the IGF-1R. Three lines of evidence support this conclusion: first, by co-IP we demonstrated the ligand-dependent interaction between the IGF-1R and endogenous or overexpressed GRK2 and -6; second, we demonstrated the IGF-1R serine phosphorylation dependency on GRK expression; finally, we established that both IGF-1R signaling and degradation are under the control of these GRKs.
The second key finding of the present report is confirmation of the GRK-dependent IGF-1R serine phosphorylation as a prerequisite for the β-arr binding and the subsequent identification of serines 1248 and 1291 as the major serine phosphorylation sites of the IGF-1R.
Mutation of these two residues demonstrated clear differences in behavior that mirrored the alterations observed after manipulation of the GRKs expression. This unambiguous correspondence between the effects from specific GRKs inhibition/overexpression and mutation analysis of specific serine residues advocates substrate specificity at the level of individual residues: GRK2 phosphorylates S1248, whereas GRK6 phosphorylates 1291. This is supported by (i) parallel records between GRK modulation and serine mutation from FRET and confocal data on β-arr recruitment, and (ii) the IGF-1R functional outcomes of receptor degradation and signaling, with both exaggerating and abrogating manipulations: overexpression vs. depletion of GRKs and alanine vs. aspartic acid mutations.
The functional analysis of IGF-1R highlights the functional antagonism of GRK2 and GRK6 for IGF-1R: increased GRK2/S1248D protects the receptor from degradation, decreases ERK activation, and slightly increases pAKT (early phase), whereas GRK6/S1291D increases ERK activation and AKT late-phase phosphorylation. For ERK signaling, similar antagonism was previously reported for the angiotensin II receptor and V2 vasopressin receptor (14, 15). Applying the model proposed for the angiotensin II receptor (14) to the IGF-1R, increased ERK activation by GRK6 or S1291D could be recognized as β-arr–mediated ERK signaling. The stable nature of the β-arr/IGF-1R association, increased ERK phosphorylation in conditions with impaired IGF-1R kinase activity (as demonstrated by low tyrosine phosphorylation), fully supports this mechanism. Equally, increased AKT signaling after GRK6 overexpression or S1291D mutation can also be explained as β-arr–mediated signaling in agreement with a previous report demonstrating that β-arr1 is able to activate PI3K pathways in response to IGF1 (3). This mechanism is active even in conditions with impaired IGF-1R kinase activity (3), therefore our data demonstrating enhanced AKT activation in conditions with decreased IGF-1R tyrosine phosphorylation fully support this scenario.
A general inhibition of IGF-1R kinase activity by GRK2 might explain the decrease of ERK signaling, but the increase of early phase AKT phosphorylation contradicts this. Another alternative is that phosphorylation of distinct serine residues governs the stability of the β-arr/receptor binding and controls the β-arr functions (14, 16). This scenario could fully explain the equivalence between GRKs expression/depletion and serine residue mutations, as well as dissociation of the AKT and ERK signaling induced by GRK2 modulation or mutation of S1248.
The further perspective for an RTK opened by the present study, already largely accepted for GPCRs, is that the receptor conformation activating the kinase cascade is distinct from that which interacts with β-arrs, as demonstrated by the serine mutants, degraded even in the absence of the ligand. This model is validated by studies demonstrating that IGF-1R signaling could be activated in a “biased manner” via β-arr by IGF-1R inhibitors as well as by natural “biased” agonists (17, 18). By searching for therapies inhibiting only one type of IGF-1R activity (e.g., kinase activity), many potential drugs that cause alternative downstream effects, the “biasing agonists” have not yet been considered. Potentially β-arr–specific or now GRK-specific drugs might have novel therapeutic properties and perhaps more restricted side effects.
With the present study we are building on previous results regarding cross-talk between the IGF-1R and GPCR at the level of GRKs. Moreover, we demonstrate for an RTK that individual GRKs generate distinct phosphorylation patterns resulting in different functional activities of recruited β-arrs. These findings are in line with, and extend to RTKs, the recent study demonstrating that for β(2)-adrenergic receptor, distinct GRK isoform-dependent serine phosphorylation establishes a “barcode” encoding differential functions of β-arrestin (16).
Materials and Methods.
Cell lines, materials, and procedures for the mutation analysis, WB, in vitro binding assay, FRET, RT-PCR and confocal microscopy are described in SI Materials and Methods. FRET validation is described in Fig. S5. Algorithms for prediction of phosphorylated serine residues are described in Fig. S6.
Supplementary Material
Acknowledgments
This study received grant support from the Swedish Cancer Society, Swedish Medical Council, Swedish Childhood Cancer Foundation, Crown Princess Margareta’s Foundation for the Visually Impaired, Welander Finsen Foundation, King Gustaf V Jubilee Foundation, Vinnova, Stockholm Cancer Society, Stockholm County, and Karolinska Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118359109/-/DCSupplemental.
References
- 1.Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92:2097–2101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchamp MC, Yasmeen A, Knafo A, Gotlieb WH. Targeting insulin and insulin-like growth factor pathways in epithelial ovarian cancer. J Oncol. 2010;2010:257058. doi: 10.1155/2010/257058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- 4.Girnita L, et al. beta-Arrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by actng as adaptor for the MDM2 E3 ligase. J Biol Chem. 2005;280:24412–24419. doi: 10.1074/jbc.M501129200. [DOI] [PubMed] [Google Scholar]
- 5.Girnita L, et al. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem. 2007;282:11329–11338. doi: 10.1074/jbc.M611526200. [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 7.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 8.Girnita L, Girnita A, Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA. 2003;100:8247–8252. doi: 10.1073/pnas.1431613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oakley RH, et al. The cellular distribution of fluorescently labeled arrestins provides a robust, sensitive, and universal assay for screening G protein-coupled receptors. Assay Drug Dev Technol. 2002;1:21–30. doi: 10.1089/154065802761001275. [DOI] [PubMed] [Google Scholar]
- 10.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 11.Lin FT, Daaka Y, Lefkowitz RJ. beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J Biol Chem. 1998;273:31640–31643. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- 12.Hupfeld CJ, Olefsky JM. Regulation of receptor tyrosine kinase signaling by GRKs and beta-arrestins. Annu Rev Physiol. 2007;69:561–577. doi: 10.1146/annurev.physiol.69.022405.154626. [DOI] [PubMed] [Google Scholar]
- 13.Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem. 2001;276:15688–15695. doi: 10.1074/jbc.M010884200. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren XR, et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobles KN, et al. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasilcanu R, et al. Insulin-like growth factor type-I receptor-dependent phosphorylation of extracellular signal-regulated kinase 1/2 but not Akt (protein kinase B) can be induced by picropodophyllin. Mol Pharmacol. 2008;73:930–939. doi: 10.1124/mol.107.040014. [DOI] [PubMed] [Google Scholar]
- 18.Girnita A, Zheng H, Grönberg A, Girnita L, Ståhle M. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene. 2012;31:352–365. doi: 10.1038/onc.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.