Abstract
Oxidative stress causes mitochondrial fragmentation and dysfunction in age-related diseases through unknown mechanisms. Cardiolipin (CL) is a phospholipid required for mitochondrial oxidative phosphorylation. The function of CL is determined by its acyl composition, which is significantly altered by the onset of age-related diseases. Here, we examine a role of acyl-CoA:lysocardiolipin acyltransferase lysocardiolipin acyltransferase 1 (ALCAT1), a lysocardiolipin acyltransferase that catalyzes pathological CL remodeling, in mitochondrial biogenesis. We show that overexpression of ALCAT1 causes mitochondrial fragmentation through oxidative stress and depletion of mitofusin mitofusin 2 (MFN2) expression. Strikingly, ALCAT1 overexpression also leads to mtDNA instability and depletion that are reminiscent of MFN2 deficiency. Accordingly, expression of MFN2 completely rescues mitochondrial fusion defect and respiratory dysfunction. Furthermore, ablation of ALCAT1 prevents mitochondrial fragmentation from oxidative stress by up-regulating MFN2 expression, mtDNA copy number, and mtDNA fidelity. Together, these findings reveal an unexpected role of CL remodeling in mitochondrial biogenesis, linking oxidative stress by ALCAT1 to mitochondrial fusion defect.
Oxidative stress causes mitochondrial dysfunction, which has been implicated in aging and age-related diseases (1–3). Although the underlying causes remain elusive, disrupted mitochondrial dynamics by reactive oxygen species (ROS) has been implicated in mitochondrial pathogenesis in these conditions (4–6). The onset of diabetes is associated with mitochondrial fragmentation in islet β-cells, endothelial cells, and neurons attributable to a fusion defect (4–6). Hyperglycemia causes rapid mitochondrial fragmentation concurrently with increased ROS production (7). Consequently, inhibition of mitochondrial fission prevented periodic fluctuation of ROS production during high glucose exposure (7). Furthermore, oxidative stress and mitochondrial fragmentation have also been implicated in neurodegenerative diseases, which are often associated with diabetic complications (8).
Mitofusin 2 (MFN2) encodes a mitochondrial protein that is required for mitochondrial integrity, fusion, metabolism, and tethering with endoplasmic reticulum (ER) (9). Mutations in MFN2 in humans cause peripheral neuropathy and Charcot–Marie–Tooth disease. MFN2 deficiency is also implicated in mitochondrial dysfunction associated with obesity and type 2 diabetes. Muscle MFN2 expression is reduced in type 2 diabetic patients (10). MFN2 expression negatively correlates with obesity and positively correlates with insulin sensitivity (10). Amelioration of insulin resistance in type 2 diabetes by bariatric surgery increases MFN2 expression in muscle. Likewise, moderate physical exercise, which is known to improve insulin sensitivity, significantly increases MFN2 expression and mitochondrial biogenesis (11). Additionally, targeted deletion of MFN2 in mouse skeletal muscle causes mtDNA instability and high mutation rates, leading to severe mtDNA depletion (9).
Cardiolipin (CL) is a key mitochondrial phospholipid required for oxidative phosphorylation. CL is highly sensitive to oxidative damage of its double bonds by ROS because of its rich content in linoleic acid and location near the site of ROS production in the inner mitochondria membrane, a process also known as CL peroxidation (12). CL is the only phospholipid in mitochondria that undergoes early oxidation during apoptosis, which triggers the release of cytochrome c to cytosol, leading to apoptotic consumption (12). Consequently, abnormal CL acyl composition from pathological remodeling has been implicated in the etiology of mitochondrial dysfunction commonly associated with diabetes (13), obesity (2), cardiovascular diseases (14), neurological disorders (5), cancer (15), aging (16), and other age-related diseases (17).
Acyl-CoA:lysocardiolipin acyltransferase 1 (ALCAT1) is a lysocardiolipin acyltransferase that was recently identified to catalyze pathological remodeling of CL in response to oxidative stress in diabetes, obesity, and cardiomyopathy, leading to ROS production, mitochondrial dysfunction, and insulin resistance (2, 18). CL remodeling by ALCAT1 also results in CL deficiency and changes of CL acyl composition that are reminiscent of age-related diseases, including depletion of linoleic acid and enrichment of docosahexaenoic acid (DHA) content in CL. Targeted inactivation of ALCAT1 prevents the onset of diet-induced obesity and its related metabolic complications (2). The present investigation sought to advance our understanding of molecular mechanisms by which ALCAT1 regulates mitochondrial dysfunction associated with oxidative stress. In the process, we describe an unexpected role of ALCAT1 in regulating mitochondrial fusion and mtDNA fidelity, implicating a critical role of ALCAT1 in MFN2 deficiency and mitochondrial fragmentation in age-related diseases.
Results
ALCAT1 Causes Mitochondrial Fragmentation and mtDNA Instability in C2C12 Cells.
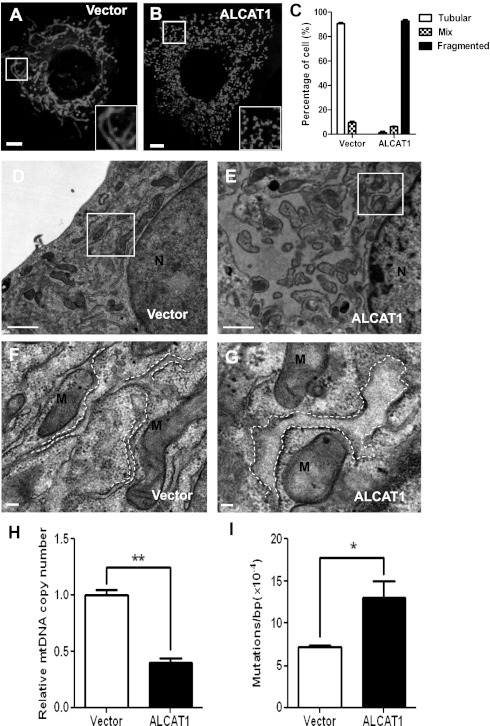
To investigate a role of ALCAT1 overexpression in regulating mitochondria and ER morphology, we generated C2C12 cell lines stably transfected with flag-tagged ALCAT1 cDNA (C2C12-A1) or vector control (C2C12-V). The mRNA expression level of ALCAT1 in the C2C12-A1 cells is only threefold higher than the C2C12-V cells (Fig. S1) and matches the level of ALCAT1 expression induced by the onset of diabetes and obesity (2). Using the stable cells as a cell based model, we showed ALCAT1 overexpression caused mitochondrial fragmentation (Fig. 1 A and B; quantified in Fig. 1C) and mitochondrial swelling (Fig. 1 D and E; highlighted in Fig. 1 F and G), as analyzed by confocal and electron microscopic (EM) analysis. Furthermore, ALCAT1 overexpression also caused severe ER dilation, as highlighted by white dashed lines (Fig. 1 F and G), consistent with previously reported localization of ALCAT1 at mitochondria-associated membrane (MAM) (2). Remarkably, ALCAT1 overexpression also significantly depleted mtDNA copy number in C2C12-A1 cells (Fig. 1H) and increased mtDNA mutation rate (Fig. 1I), suggesting a major role of ALCAT1 in regulating mitochondrial mass and mtDNA stability.
Fig. 1.
ALCAT1 causes mitochondrial fragmentation and mtDNA instability in C2C12 cells. (A and B) Confocal microscopic analysis of mitochondrial network in C2C12 cells stably expressing ALCAT1 (B) or vector control (A) stained with MitoTracker Red. Insets represent magnification of the boxed areas. (Scale bars: 5 μm.) (C) Quantitative analysis of mitochondrial morphology in C2C12 cells expressing ALCAT1 or vector control in three categories: tubular, mix, and fragmented from three independent experiments (n = 300). (D–G) EM analysis of mitochondrial morphology in C2C12 cells stably expressing vector control (D; enlarged in F) or ALCAT1 (E; enlarged in G). ER morphology is highlighted by dashed lines. (Scale bars: 1 μm.) (H and I) RT-PCR analysis of mtDNA copy number (H) and point mutation rate (I) in C2C12 cells stably expressing ALCAT1 or vector control. *P < 0.05; **P < 0.01 (n = 3). N, nucleus; M, mitochondria.
Ablation of ALCAT1 Increases Mitochondrial Mass and mtDNA Fidelity in Mice.
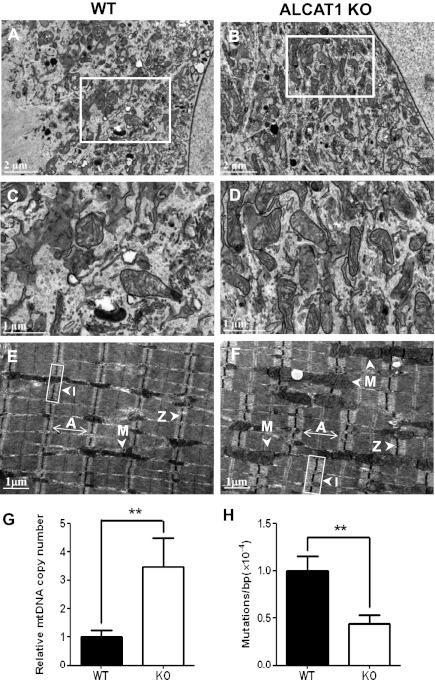
Using ALCAT1 knockout (KO) mice recently generated in our laboratory (2), we analyzed the effect of ALCAT1 deficiency on mitochondrial morphology, mtDNA mass, and mtDNA mutation rate in isolated mouse embryonic fibroblasts (MEFs) and skeletal muscles by EM analysis. In support of the findings in the C2C12 cells, ALCAT1 deficiency significantly increased mitochondrial density (Fig. 2 A and B; highlighted in Fig. 2 C and D). Ablation of ALCAT1 significantly increased thickness of the skeletal muscle fiber, as evidenced by the enlarged dark A bands and light I bands (Fig. 2 E and F; quantified in Fig. S2). Furthermore, ablation of ALCAT1 significantly increased mtDNA copy number and mtDNA fidelity, as evidenced by significantly lower mtDNA mutation rate in the isolated MEFs from ALCAT1 KO mice (Fig. 2 G and H).
Fig. 2.
ALCAT1 deficiency significantly increases mitochondrial mass and improves mtDNA fidelity. (A–D) EM analysis of mitochondrial morphology of MEF cells isolated from wild-type (WT) control mice (A; enlarged in C) and from ALCAT1 KO mice (B; enlarged in D). (E and F) EM analysis of the tibialis anterior longitudinal section from WT mice (E) and ALCAT1 KO mice (F). Arrows indicate A band, I bands, Z line, and mitochondria (M), respectively. (G and H) RT-PCR analysis of mtDNA copy number (G) and point mutation rate (H) in MEF cells isolated from KO mice and the WT controls. **P < 0.01 (n = 3).
Key Role of ALCAT1 in Regulating MFN2 Expression.
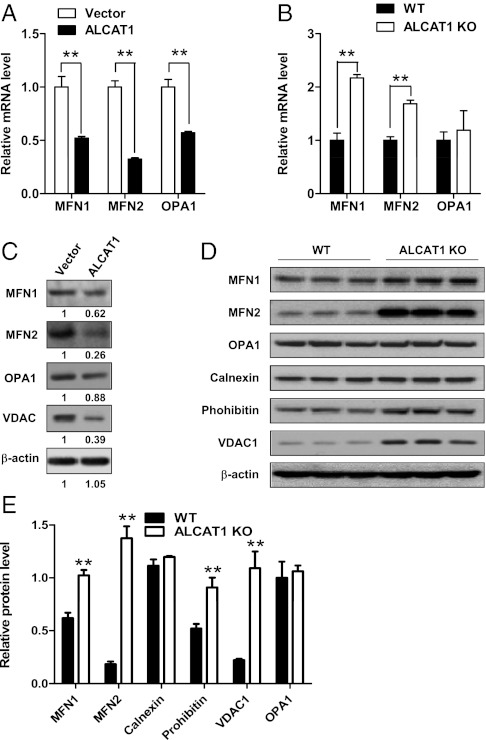
MFN2 is required for mitochondrial and ER morphology and tethering as a functional bridge (19). MEFs from MFN2−/− mice display fragmented mitochondria and dilated ER cisternae that resemble the defects caused by in ALCAT1 overexpression (19). We, thus, asked whether ALCAT1 overexpression and deficiency would reciprocally affect the expression of MFN2 and other regulators of the mitochondrial fusion process. As shown in Fig. 3A, ALCAT1 overexpression caused a >70% depletion of MFN2 mRNA and a 50% reduction of both MFN1 and optic atrophy (OPA)1 mRNAs in the C2C12-A1 stable cell line compared with vector control. Conversely, ALCAT1 deficiency dramatically increased transcription of MFN2 mRNA concurrently with a significant increase in MFN1 mRNA level in isolated MEFs from ALCAT1 KO mice (Fig. 3B). However, ALCAT1 deficiency did not have any effect on mRNA expression of OPA1, which is required for the fusion of inner mitochondrial membrane.
Fig. 3.
ALCAT1 regulates biogenesis of MFN1 and MFN2. (A and B) Real-time PCR analysis of mRNA expression levels of MFN1, MFN2, and OPA1 in C2C12 cells stably expressing ALCAT1 or vector control (A) and in MEFs isolated from WT and KO mice (B). GAPDH expression was used as an internal control. (C) Western blot analysis of MFN1, MFN2, OPA1, and VDAC1 protein levels in C2C12 cells expressing ALCAT1 or vector control. Relative density of each band was shown under each lane. (D) Western blot analysis of protein levels of MFN1, MFN2, OPA1, calnexin, prohibitin, and VDAC1 in MEFs isolated from KO and WT control mice (n = 3 fetuses in each group). (E) Quantitative analysis of data shown in E after normalization with the expression level of OPA1. **P < 0.01 compared with vector control or WT control mice.
Consistent with decreased mRNA levels, expression all of the three proteins were also significantly down-regulated by ALCAT1 overexpression (Fig. 3C). Conversely, MFN2 protein level was up-regulated by >600% in MEFs isolated from the ALCAT1 KO mice when normalized by the expression of OPA1, which is used as a marker for mitochondrial mass (Fig. 3D; quantified in Fig. 3E). ALCAT1 deficiency also significantly increased the expression of MFN1 and prohibitin, a mitochondrial membrane chaperone protein required for optimal mitochondrial morphology and respiration. In contrast, ALCAT1 deficiency did not affect the expression of OPA1 or calnexin, an ER resident protein (Fig. 3E). Finally, consistent with a causative role of ALCAT1 in oxidative stress, ALCAT1 negatively regulated the expression of voltage-dependent anion channel (VDAC)1, a voltage-dependent anion channel protein in the outer mitochondrial membrane that regulates ROS release from the intermembrane space to the cytosol (20) (Fig. 3 C and D).
ALCAT1 Impairs Mitochondrial Fusion Through MFN2 Depletion.
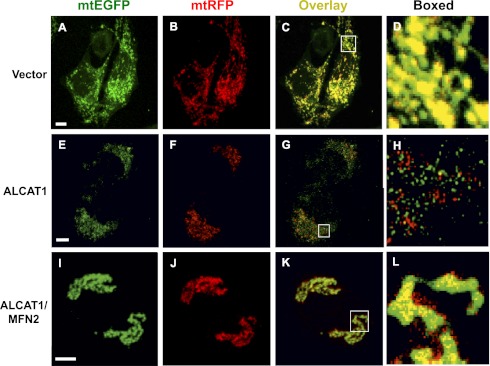
The findings that ALCAT1 causes MFN2 depletion and mitochondrial fragmentation prompted us to investigate a role of ALCAT1 in regulating mitochondrial fusion. Mitochondrial matrix-targeted EGFP (mtEGFP) or red fluorescent protein (mtRFP) was individually transfected in C2C12-A1 and C2C12-V2 cells, followed by fusion analysis by measuring overlay of the EGFP and RFP under a confocal microscope. As shown in Fig. 4 A–C, mitochondria in the vector control C2C12 cells demonstrated normal mitochondrial fusion, as evidenced by the yellow color of the merged image in Fig. 4C (highlighted in Fig. 4D), which suggests a complete fusion. In contrast, ALCAT1 overexpression in C2C12 cells caused a severe fusion defect (Fig. 4 E–H), as evidenced by well-separated green and red colors of the merged image in Fig. 4G (highlighted in Fig. 4H). In direct support of a causative role of MFN2 deficiency in the fusion defect, transient expression of MFN2 in C2C12-A1 cells completely rescued the fusion defect, as supported by the yellow color of the merged image in Fig. 4K (highlighted in Fig. 4L). MFN2 expression also caused super fusion of mitochondria, which is supported by thicker mitochondrial tubular network in C2C12-A cells overexpressing MFN2 (Fig. 4 I–K). The results are consistent with the previously reported effect of MFN2 on super fusion in various cell types (21).
Fig. 4.
ALCAT1 impairs mitochondrial fusion, which can be rescued by MFN2 expression in C2C12 cells. C2C12 cells stably expressing ALCAT1 or vector control were transiently transfected with expression plasmid for mitochondrial-targeted GFP or DsRed (mtRFP), coplated, and fused with PEG-1500, followed by confocal microscopic imaging. (A–D) Confocal images of mitochondria in C2C12 cells stably expressing vector control, which demonstrated complete fusion, as evidenced by the yellow color in panel C and highlighted in D. (E–H) Images of mitochondria in C2C12 cell stably expressing ALCAT1, which exhibited a fusion defect, as shown by the separated green and red colors in G and highlighted in H. (I–L) Transient expression of MFN2 (MFN2-YFP) rescued the fusion defect in C2C12 cells stably expressing ALCAT1 (K; highlighted in L). (Scale bar: 5 μm.)
MFN1/2, but Not OPA1, Rescues Mitochondrial Fragmentation Caused by ALCAT1.
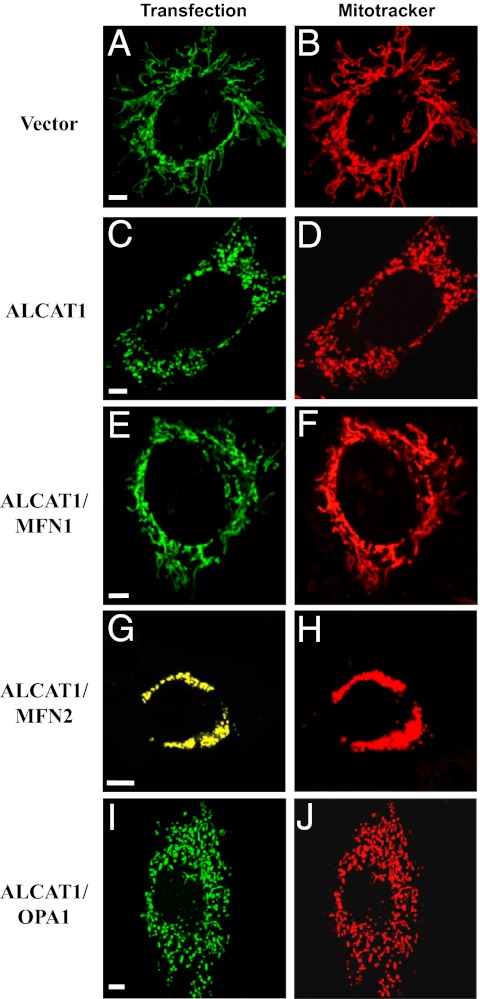
Because MFN2 can rescue the fusion defect caused by ALCAT1, we questioned whether other members of the dynamin-related family, such as MFN1 and OPA1, could also rescue fusion defect by restoring mitochondrial network in the C2C12-A1 cells. In contrast to C2C12-A1 cells, which exhibit mitochondrial fragmentation (Fig. 5 C and D), transient expression of MFN1 in C2C12-A1 cells almost completely rescued the fusion defect, as shown by the recovery of the tubular mitochondria (Fig. 5 E and F) similar to vector control cells (Fig. 5 A and B). Again, transient expression of MFN2 completely rescued the fusion defect, leading to superfusion of mitochondria (Fig. 5 G and H). In contrast, transient expression of OPA1 failed to rescue any of the fusion defect (Fig. 5 I and J), which is consistent with a lack of any effect of ALCAT1 deficiency on OPA1 expression in MEFs (Fig. 3 B–E). The results suggest that the ALCAT1-mediated fusion defect is limited to the outer mitochondrial membrane, which is closely associated with MAM, where ALCAT1 is localized (2).
Fig. 5.
Overexpression of MFN1 and MFN2, but not OPA1, restores mitochondrial network in C2C12-A1 cells. (A–D) Confocal image analysis of mitochondrial network in C2C12 cells stably expressing vector control (A and B) or ALCAT1 (C and D). The stable C2C12 cell lines were transiently transfected with mitochondrial-targeted EGFP expression vector (A and C) and stained with MitoTracker Red (B and D) before imaging. (E–J) Confocal image analysis of mitochondrial network in C2C12 cells stably expressing ALCAT1 and transiently transfected with expression vectors for Myc-tagged MFN1 (E and F), MFN2-YFP (yellow fluorescence protein) (G and H), or Myc-tagged OPA1 (I and J), respectively. For the Myc-tagged proteins, cells were first stained with MitoTracker Red and then fixed, followed sequentially by immunostaining with mouse anti-Myc antibodies and goat anti-mouse FITC-conjugated secondary antibodies (green). (Scale bar: 5 μm.)
MFN2 Rescues Mitochondrial Respiratory Defects Caused by ALCAT1 Overexpression.
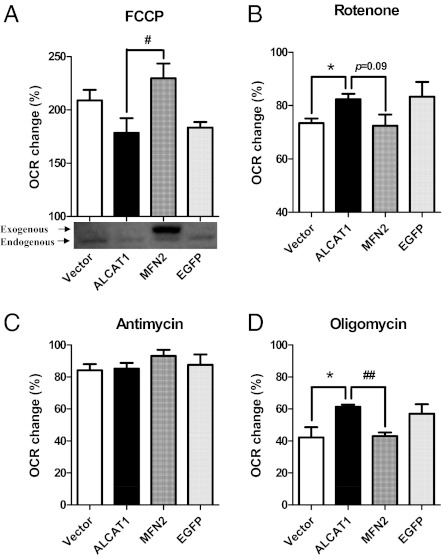
ALCAT1 causes mitochondrial dysfunction and mitochondrial proton leakage, which is likely a compensatory response to oxidative stress, leading to increased oxygen consumption rate (OCR) (2). We, therefore, interrogated a role of MFN2 expression in regulating OCR of cultured C2C12-A1 cells in response to treatments with different mitochondrial inhibitors. Using the Seahorse Extracellular Flux (XF-24; Seahorse Bioscience) analyzer, we show transient expression of MFN2, but not EGFP vector control, completely restored mitochondrial respiratory capacity, as evidenced by the recovery of OCR relative to vector control in response to FCCP (Fig. 6A). Consistent with complex I as the major site for ROS production, ALCAT1 overexpression significantly increased OCR in response to treatment with rotenone, an inhibitor of complex I (NADH CoQ1 reductase), which is also normalized by transient expression of MFN2 in C2C12-A1 cells (Fig. 6B). Consistent with increased proton leakage, ALCAT1 overexpression also significantly increased OCR in response to treatment with oligomycin, a mitochondrial ATPase inhibitor. Likewise, such a defect can also be completely normalized by transient expression of MFN2 (Fig. 6D). In contrast, neither ALCAT1 nor MFN expression had any effect on OCR from complex III, in response to treatment with antimycin, a complex III inhibitor, as previously reported (2).
Fig. 6.
MFN2 restores the mitochondrial respiratory capacity in C2C12 cells stably expressing ALCAT1. C2C12 cells stably expressing ALCAT1 were transiently transfected with expression vectors for MFN2 or EGFP and were compared with vector control for changes in OCR in response to treatment with various mitochondrial inhibitors, including: FCCP, an mitochondrial uncoupler (A); rotenone, a complex I inhibitor (B); antimycin, a complex III inhibitor (C); and oligomycin, an ATPase inhibitor (D). The exogenous and endogenous MFN2 protein levels were analyzed by Western blot analysis using MFN2 antibodies, as shown in A. OCR was analyzed by Seahorse X-24 analyzer and calculated from at least three independent measurements for each chemical treatment. *P < 0.05 compared with control; #P < 0.05 and ##P < 0.01 compared with nontransfected ALCAT1-expressing cells.
ALCAT1 Links Oxidative Stress to Mitochondrial Fragmentation and MFN2 Deficiency.
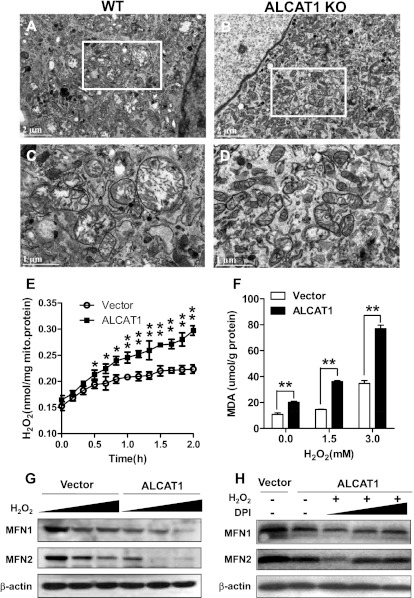
Impaired mitochondrial fusion from oxidative stress causes mitochondrial swelling by opening the mitochondrial permeability transition pore (22). Damaged mitochondria often generate more ROS which further exacerbate mitochondrial dysfunction, leading to a vicious cycle (23). Because CL remodeling by ALCAT1 causes oxidative stress, we questioned whether ALCAT1 played a role in the vicious cycle, which was tested in isolated MEFs from ALCAT1 KO and control mice. As shown in Fig. 7A (highlighted in Fig. 7C), MEFs from control mice exhibit severe mitochondrial swelling in response to treatment with H2O2, as evidenced by enlarged mitochondria and damaged cristae. Likewise, ALCAT1 overexpression also caused severe mitochondrial swelling in one of the C2C12 stable cell lines that exhibits the highest expression of ALCAT1 relative to the vector controls (Fig. S3). Because of this extreme feature, this C2C12 cell line was not used for the current studies. In contrast, ALCAT1 deficiency completely prevented mitochondrial swelling (Fig. 7B; highlighted in Fig. 7D) and mitochondrial fragmentation in isolated MEFs in response to treatment of H2O2 (Fig. S4).
Fig. 7.
Oxidative stress by ALCAT1 links ROS production to MFN2 deficiency. (A–D) EM analysis of mitochondrial morphology in MEF from WT mice (A; highlighted in C) or KO mice (B; highlighted in D) treated with 1 mM H2O2. (E) Real-time production of H2O2 from isolated mitochondria from C2C12 cells overexpressing vector or ALCAT1. (F) C2C12 cells were treated with indicated doses of H2O2 for 1 h, followed by analysis for the level of MDA, a lipid peroxidation product from oxidative stress. (G) C2C12 cells stably expressing vector control or ALCAT1 were treated with increasing doses of H2O2 for 1 h as indicated in F, followed by Western blot analysis for MFN1 and MFN2 expression. (H) Western blot analysis of MFN1 and MFN2 expression in C2C12 cells stably expressing vector, ALCAT1, or ALCAT1 but were pretreated for 1 h with increasing doses (0, 1, and 5 μM) of DPI, an NADPH oxidase inhibitor, followed by treatment with 1.5 mM H2O2 for 1 h. *P < 0.05; **P < 0.01 compared with vector control.
In direct support of a causative role of ALCAT1 in oxidative stress, ALCAT1 overexpression significantly increased H2O2 production in C2C12-A1 cells (Fig. 7E). Consequently, ALCAT1 overexpression also caused severe lipid peroxidation, which was further exacerbated in response to treatment with increased doses of H2O2, as shown by elevated level of malondialdehyde (MDA), an end product of lipid peroxidation (Fig. 7F). It was previously reported that mitochondria undergo rapid fragmentation concomitantly with an increase in ROS production (7). In support of oxidative stress by ALCAT1 as the primary cause of MFN depletion, treatment of C2C12-A1 and the vector control cells with H2O2 dose-dependently depleted MFN1 and MFN2 expression (Fig. 7G), leading to a complete loss of MFN2 expression in C2C12-A1. Finally, in direct support of oxidative stress by ALCAT1 as the primary cause of MFN depletion, preincubation of the C2C12 cells with diphenyleneiodonium (DPI), an antioxidant, dose-dependently prevented the loss of MFN expression caused by H2O2 in C2C12-A1 cells (Fig. 7H) and in vector control cells (Fig. S5).
Discussion
Dynamic networks are formed when mitochondria undergo a fusion event that causes the compartments of participating mitochondria to become continuous. The fusion event allows the constituents of each network to share solutes, metabolites, and proteins. Consequently, disruption of such networks causes oxidative stress and mitochondrial fragmentation, which has been implicated in the etiology of aging and age-related diseases (4–8, 24, 25). However, little is known about the underlying mechanisms. The current studies investigated a role of ALCAT1 in regulating oxidative stress and mitochondrial fragmentation commonly associated with age-related metabolic diseases. We demonstrate a critical role for ALCAT1 in regulating mitochondrial biogenesis and mtDNA fidelity. Accordingly, ALCAT1 overexpression severely impairs mitochondrial fusion, leading to mitochondrial fragmentation and mtDNA depletion. Conversely, targeted inactivation of ALCAT1 in mice significantly increases mitochondrial mass and protects mitochondria from ROS-induced mitochondrial swelling and fragmentation. Strikingly, we also demonstrate a key role of ALCAT1 in regulating mtDNA fidelity, which is corroborated by previous studies that mitochondrial fusion is required to safeguard mtDNA integrity (9).
In support of a role for ALCAT1 in mitochondrial fusion, the current studies identified MFN2 as a downstream target of ALCAT1-mediated mitochondrial dysfunction. MFN2 is required for mitochondrial fusion, tethering with ER, energy metabolism, and mtDNA fidelity in mammals (9, 19). We show that ALCAT1 overexpression severely depleted MFN2 expression in C2C12 cells, whereas ALCAT1 deficiency dramatically increased MFN2 expression in isolated MEFs from ALCAT1 KO mice. In contrast, ALCAT1 deficiency did not have any effect on expression of OPA1, which is required for fusion of the inner mitochondrial membrane (26). The results suggest that ALCAT1 only affects the fusion of the outer mitochondrial membrane which is closely associated with MAM, where ALCAT1 is localized (2). The finding is further corroborated by a recent report that 25% CL is also localized in the outer membrane where active CL remodeling takes place (27). Consistent with MFN2 as the downstream target of ALCAT1-mediated fusion defect, targeted inactivation of MFN2 in mice causes multiple mitochondrial defects that are reminiscent of ALCAT1 overexpression, including high mtDNA mutation rate, mtDNA depletion, fragmented mitochondria, and a profound loss of tethering between mitochondria and ER (19, 28). A further study showed that MFN2 deficiency also caused skeletal muscle atrophy, which is consistent with our finding that ALCAT1 deficiency significantly increased skeletal mass in ALCAT1 KO mice (2, 9). In direct support of MFN2 as downstream target of ALCAT1, the mitochondrial fusion defect caused by ALCAT1 can be rescued by expression of either MFN1 or MFN2, but not by OPA1. Furthermore, MFN2 expression also restored the mitochondrial respiratory capacity and reduced oxidative stress in C2C12 cells overexpressing ALCAT1. These results are consistent with a previous report that MFN2 deficiency significantly increases proton leakage (29), whereas overexpression of MFN2 blocks hyperglycemia-induced ROS production (7).
It has been shown previously that mitochondrial fission/fusion machinery controls acute and chronic production of ROS in hyperglycemia-associated disorders (7). Mitochondria undergo rapid fragmentation concomitantly with an increased ROS production in response to oxidative stress, which can be mitigated through inhibition of fission or stimulation of the fusion process (30). The present studies identified ALCAT1 as a missing link between mitochondrial fusion defect and ROS production in metabolic diseases, which is supported by multiple lines of evidence. First, CL remodeling by ALCAT1 significantly increased DHA content in CL, leading to proton leakage and oxidative stress (2, 18). This is likely the primary mechanism by which ALCAT1 regulates oxidative stress, because double bond content in mitochondrial membrane phospholipids inversely correlates with lifespan and positively correlated with ROS production and lipid peroxidation index in mammals (31). Hence, increased DHA content in CL increases lipid peroxidation index, which has been implicated in mitochondrial dysfunction in aging and age-related diseases (13, 14, 16, 32–34). Second, we show that ALCAT1 overexpression significantly increased H2O2 production rate in C2C12 cells. Consequently, ALCAT1 overexpression also caused severe lipid peroxidation in C2C12 cells, which was further exacerbated by oxidative stress. Third, in direct support of oxidative stress by ALCAT1 as the primary cause of the fusion defect, ALCAT1 overexpression significantly depleted MFN2 expression, which can be mitigated by pretreatment of C2C12 cells with an antioxidant. Finally, in support of a causative role of ALCAT1 in the onset of age-related diseases, ALCAT1 expression is up-regulated by oxidative stress and by the onset of age-related metabolic diseases (2). Together, these findings support a key role for ALCAT1 in linking ROS production to mitochondrial fragmentation through depletion of MFN2 in age-related diseases, as depicted by a schematic model in Fig. S6. Therefore, it can be envisaged that development of chemical inhibitors for ALCAT1 may provide a potential treatment for aging and age-related diseases in the future.
Materials and Methods
The generation of the ALCAT1 KO mice and measurement of OCR were as described previously (2). All experiments used littermate control of matched age and sex and in accordance with approval of institutional animal care and use protocols according to National Institutes of Health guidelines (35). Confocal microscopy, EM analysis, mtDNA mutation assay, mitochondrial fusion assay, MEF preparation, statistical analysis, and all of the reagents used in this study were described in details in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Mr. Roland Myers for EM analysis and Dr. Jaeseok Han for technical help with MEF isolation. This study was supported, in part, by National Institutes of Health Grants DK076685 (to Y.S.) and GM062967 (to D.C.C.) and a scholarship from the Chinese National Scholarship Council (to J.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120043109/-/DCSupplemental.
References
- 1.Haigis MC, Yankner BA. The aging stress response. Mol Cell. 2010;40:333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010;12:154–165. doi: 10.1016/j.cmet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan DC. Mitochondria: Dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Edwards JL, et al. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;53:160–169. doi: 10.1007/s00125-009-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayir H, et al. Selective early cardiolipin peroxidation after traumatic brain injury: An oxidative lipidomics analysis. Ann Neurol. 2007;62:154–169. doi: 10.1002/ana.21168. [DOI] [PubMed] [Google Scholar]
- 6.Molina AJ, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach D, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: Effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 11.Cartoni R, et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagan VE, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 13.Han X, et al. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: A shotgun lipidomics study. Biochemistry. 2007;46:6417–6428. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- 15.Sandra F, et al. Tumor necrosis factor-related apoptosis-inducing ligand alters mitochondrial membrane lipids. Cancer Res. 2005;65:8286–8297. doi: 10.1158/0008-5472.CAN-04-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H-J, Mayette J, Rapoport SI, Bazinet RP. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids Health Dis. 2006;5:2. doi: 10.1186/1476-511X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claypool SM, Koehler CM. The complexity of cardiolipin in health and disease. Trends Biochem Sci. 2012;37:32–41. doi: 10.1016/j.tibs.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao J, Liu Y, Lockwood J, Burn P, Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J Biol Chem. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- 19.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 20.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 21.Choi SY, et al. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 22.Peng TI, Jou MJ. Mitochondrial swelling and generation of reactive oxygen species induced by photoirradiation are heterogeneously distributed. Ann N Y Acad Sci. 2004;1011:112–122. doi: 10.1007/978-3-662-41088-2_12. [DOI] [PubMed] [Google Scholar]
- 23.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bindokas VP, et al. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem. 2003;278:9796–9801. doi: 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- 25.Shenouda SM, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebert N, et al. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: Implications for Barth syndrome. Curr Biol. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach D, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 30.Barsoum MJ, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 32.Watkins SM, Carter LC, German JB. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J Lipid Res. 1998;39:1583–1588. [PubMed] [Google Scholar]
- 33.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic Biol Med. 2010;48:1286–1295. doi: 10.1016/j.freeradbiomed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y. Emerging roles of cardiolipin remodeling in mitochondrial dysfunction associated with diabetes, obesity, and cardiovascular diseases. J Biomed Res. 2010;24:6–15. doi: 10.1016/S1674-8301(10)60003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institutes of Health . Guide for the Care and Use of Laboratory Animals. Bethesda, MD: National Institutes of Health; 1985. publication no. 86–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.