Abstract
Selectivity patterns provide insights into the causes of ancient extinction events. The Late Ordovician mass extinction was related to Gondwanan glaciation; however, it is still unclear whether elevated extinction rates were attributable to record failure, habitat loss, or climatic cooling. We examined Middle Ordovician-Early Silurian North American fossil occurrences within a spatiotemporally explicit stratigraphic framework that allowed us to quantify rock record effects on a per-taxon basis and assay the interplay of macrostratigraphic and macroecological variables in determining extinction risk. Genera that had large proportions of their observed geographic ranges affected by stratigraphic truncation or environmental shifts at the end of the Katian stage were particularly hard hit. The duration of the subsequent sampling gaps had little effect on extinction risk, suggesting that this extinction pulse cannot be entirely attributed to rock record failure; rather, it was caused, in part, by habitat loss. Extinction risk at this time was also strongly influenced by the maximum paleolatitude at which a genus had previously been sampled, a macroecological trait linked to thermal tolerance. A model trained on the relationship between 16 explanatory variables and extinction patterns during the early Katian interval substantially underestimates the extinction of exclusively tropical taxa during the late Katian interval. These results indicate that glacioeustatic sea-level fall and tropical ocean cooling played important roles in the first pulse of the Late Ordovician mass extinction in Laurentia.
Keywords: climate change, stratigraphy, sea level, Hirnantian, marine invertebrates
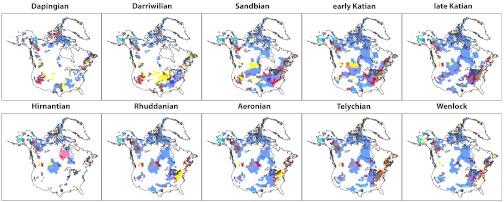
The Late Ordovician Mass Extinction (LOME) was the first of the “Big Five” Phanerozoic mass extinctions, and it eliminated an estimated 61% of marine genera globally (1). The LOME stands out among major mass extinctions in being unambiguously linked to climate change. The primary pulse of extinction near the Katian/Hirnantian stage boundary closely coincided with the rapid growth of south polar ice sheets on Gondwana (1–4). Expansion of continental ice sheets was accompanied by substantial cooling of the tropical oceans (5, 6), a major perturbation of the global carbon cycle (7–9) and a large drop in eustatic sea level (2, 5, 10, 11), which drained the vast cratonic seaways that characterized the Late Ordovician world (12). Extinction rates were particularly high around the tropical paleocontinent of Laurentia (13) where retreat of cratonic seas drove a sharp reduction in the area of preserved sedimentary rock between Katian and Hirnantian time (Fig. 1).
Fig. 1.
Maps of sedimentary rocks deposited across Laurentia from Middle Ordovician (Dapingian) through the Early Silurian (Wenlockian) time. Red points mark PaleoDB collections. Colored polygons indicate sedimentary rock distribution and lithotype. Blue = carbonate, dark blue = mixed carbonate-clastic, gray = fine clastics, tan = mixed clastics, yellow = sand, orange = coarse clastics, blue-green = chert, pink = evaporites, brown = metamorphic indet., dark green = igneous indet. Only the uppermost unit in each column is plotted.
The complex interrelated events surrounding the LOME exemplify a classic problem in paleobiology. Peaks in apparent extinction rate (14) are commonly associated with major gaps in the stratigraphic record or rapid changes in depositional environments. It is not always clear, however, whether these peaks simply reflect the spurious accumulation of last appearances at hiatal surfaces and lithofacies juxtapositions (record bias hypothesis) (15), or if the peaks represent genuine extinction events caused by the action of a shared forcing mechanism on the biota and the sedimentary record (common cause hypothesis) (14). For the LOME, it is useful to split common cause into two hypotheses. The eustatic common cause hypothesis postulates that Gondwanan glaciation drove the extinction by lowering eustatic sea level, thereby reducing the overall area of shallow marine habitats, reorganizing habitat mosaics, and disrupting larval dispersal corridors (16–18). The climatic common cause hypothesis postulates that climate cooling, in addition to being ultimately responsible for sea-level drawdown and attendant habitat losses, had a direct influence on extinction rates by confronting tropical taxa with water temperatures outside of their adaptive range (19–21). These hypotheses are not mutually exclusive. For example, extinctions associated with the draining of cratonic seaways may be most severe when a strong contrast in temperature or seasonality between cratonic and open-shelf waters exists (22). Viewed as end-member models, however, the above provide a useful framework for understanding the relative contributions of different processes to aggregate extinction.
These hypotheses can be evaluated by examining patterns of differential survivorship (i.e., extinction selectivity) through the LOME (Fig. S1). Eustatic common cause posits (1) that changes in sedimentary rock area were correlated with changes in habitat availability, and (2) that habitat loss was an important extinction mechanism. Consequently, this hypothesis predicts that taxa that had large proportions of their ranges affected by stratigraphic truncation (e.g., many of the sites that they occupied in late Katian time were characterized by hiatuses during Hirnantian time) should have experienced higher extinction rates than those that did not. A similar relationship is expected under the record bias hypothesis because taxa that were strongly affected by stratigraphic truncation would have been less likely to be preserved in the following interval, even if they remained extant. A critical distinction can be made between these hypotheses, however, because the record bias hypothesis also predicts that apparent extinction risk should depend on the duration of stratigraphic gaps-long gaps increase the probability that a taxon would have gone extinct during the unsampled interval and, therefore, will appear to have gone extinct near the initiation of the gap. No similar prediction is made by the eustatic common cause hypothesis, which posits that extinction risk is influenced by the extent of gaps in space but not time. Finally, the climatic common cause hypothesis predicts that exclusively tropical taxa should have experienced higher extinction rates than taxa with broader meridional distributions-a pattern expected from the relationship between meridional range and thermal tolerance range in modern marine species (23, 24) and observed during the onset of Carboniferous (25) and Cenozoic (26, 27) glaciations.
We integrated paleontological (28) and macrostratigraphic (29) databases for Late Ordovician-Early Silurian strata of Laurentia and matched fossil occurrence records to spatiotemporally explicit, gap-bound stratigraphic packages following the procedures of Heim and Peters (30–32), albeit at a higher temporal resolution (Dataset S1). This data structure provided a framework within which relevant macrostratigraphic, macroecological, and macroevolutionary parameters could be quantified (Fig. S2). The paleoenvironmental information contained within the data structure, though only coarsely constrained by lithotype, allowed us to untangle two factors convolved in the record bias hypothesis: stratigraphic truncations and environmental truncations (“habitat bias” sensu ref. 15). We calculated the first and last appearance of each genus in Laurentia from the merged dataset with analyses focused on Laurentian extinction (e.g., extirpation) rather than global extinction. Laurentian extirpation does not always coincide with global extinction; however, global stratigraphic ranges in the PaleoDB may be too poorly resolved to differentiate these scenarios across the Katian-Hirnantian boundary, and we do not have sufficient macrostratigraphic data for other paleocontinents. The processes underlying continental extirpation and global extinction are probably similar but the former case is complicated by the potential for reinvasion from other paleocontinents and terranes (33).
For each genus sampled in a given time interval, we calculated four potential determinants of extinction risk relevant to evaluating the eustatic common cause and record bias hypotheses: percent truncation (i.e., the percentage of sites occupied by a genus that experienced stratigraphic truncation in that interval), percent environmental truncation (i.e., the percentage of occupied sites that experienced a major shift in depositional environment as measured by sedimentary lithology), median stratigraphic gap duration (i.e., median time apportioned to the local hiatus for all occupied sites that experienced truncation), and median environmental gap duration (i.e., median time to recurrence of a given lithofacies for all occupied sites that experienced environmental truncation). Percent truncation and median stratigraphic gap duration test the proposition that preservation probability and extinction risk depend only on the distribution in time and space of preserved sedimentary rock, whereas percent environmental truncation and median environmental gap duration acknowledge the potential importance of habitat/substrate preference. Employing alternative measures of the distribution of gap durations (mean, maximum, and minimum) did not substantially change the results of analyses. To control for geographic range size, a major correlate of extinction risk in many Phanerozoic intervals (34), we measured Laurentian occupancy (percent of potential sites where we sampled the genus) and great-circle distance.
As an indirect measure of thermal tolerance, we determined the highest-paleolatitude occurrence (irrespective of hemisphere) of each genus in the PaleoDB during, or prior to, the interval in question. Genera previously sampled above 40° paleolatitude were scored as one and those restricted to paleolatitudes < 40° were scored as zero. We chose this value to reflect the approximate boundary between tropical and temperate/polar waters indicated by Late Ordovician general circulation models (35) and zooplankton biotopes (6). Because our analysis was limited to the low-latitude paleocontinent of Laurentia, all of the genera in the dataset have demonstrated ability to maintain viable populations in relatively warm, low-latitude settings. Maximum paleolatitude provides a measure of their ability to also tolerate cooler, more seasonably variable seawater temperatures. Genera with a record of temperate or high-latitude occurrences should be less sensitive to environmental cooling than exclusively tropical genera. These genera also tend to be older, wider ranging, more speciose, and to have broader habitat ranges than genera that were limited to low latitudes (32, 36), all of which may reduce their susceptibility to extinction (34, 37). To control for the covariance of these factors, we quantified genus age (time since first appearance), global geographic range (great circle distance), Laurentian and global species richness, and substrate preference (proportion of occurrences in carbonate vs. clastic units) for each genus in each interval. Finally, we used the PaleoDB to assign a number of static variables that we assumed were invariant throughout a genus’ duration, including taxonomic class, trophic group, motility, life habit, and Laurentian endemicity.
We used random forest classification models (38) to evaluate the relative importance of each variable for determining extinction risk in ten Late Ordovician and Early Silurian time slices. Although random forest models have attractive properties for evaluating overall variable importance and constructing predictive models with a high degree of accuracy (38), they do not provide easily interpreted measures of effect sign, strength, or statistical significance. To complement the random forest approach, we used multiple logistic regression (39) to examine the subset of the variables most commonly implicated in determining extinction risk throughout the time series. Finally, we used preLOME (early Katian) extinction patterns to train a random forest model for predicting which late Katian genera would have been expected to go extinct if the LOME simply represented a continuation of “background” extinction processes. Differences between the predicted and observed selectivity patterns highlight specific changes in extinction regime that accompanied the first pulse of the LOME.
Results and Discussion
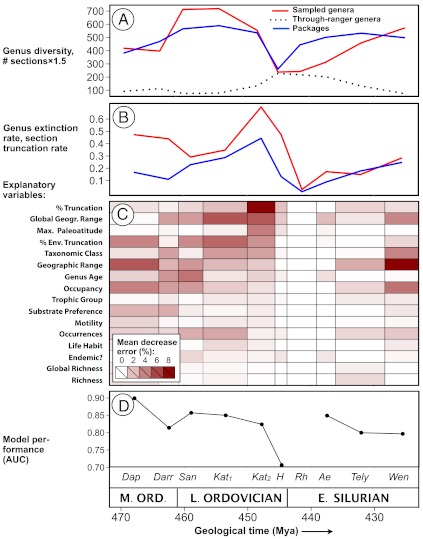
Laurentian sampled genus diversity and total number of sedimentary packages (i.e., local sections) display similar trends (Fig. 2A): a Middle Ordovician to Late Ordovician rise followed by a Hirnantian drop and Early Silurian recovery. Notably, the number of Early Silurian packages rebounded much faster than sampled diversity, likely due to delayed immigration from other paleocontinents and terranes (33, 40), lag between the creation of new habitat via cratonic flooding and evolutionary response in genus origination (33, 41), and, possibly, to under-sampling of Rhuddanian-Aeronian strata (42). The per-capita extinction rate (43) of sampled genera and the package truncation rate peaked in the second half of the Katian stage; however, extinction rates remained slightly elevated in the Hirnantian stage despite a sharp decline in the rate of package truncations (Fig. 2B).
Fig. 2.
(A) Time series of sampled genus diversity, number of through-ranger genera, and number of stratigraphic packages (i.e., local sections) in Laurentia from Middle Ordovician (Dapingian) through Early Silurian (Wenlockian) time. (B) Genus extinction rate (43) and package truncation rate (14) within each interval. Diversity and extinction rates are based only on the genera sampled in a given interval and exclude unobserved “through-ranger” genera. (C) Heatmap showing the importance (measured as OOB error) of each predictor in classifying genera as extinct or surviving from random forest models of each interval. Variables are ranked from top to bottom by their importance in the late Katian interval. (D) Area under receiver operating characteristic curve (AUC), a measure of model sensitivity, for each interval. Perfect classification would be indicated by an AUC of 1.0, a model that is no better than random would have an AUC of approximately 0.5. The Rhuddanian model fails to predict any extinctions and hence AUC is undefined for this interval. Dap = Dapingian, Darr = Dariwillian, San = Sandbian, K1 = early Katian, K2 = late Katian, H = Hirnantian, Rh = Rhuddanian, Ae = Aeronian, Tely = Telychian, Wen = Wenlockian.
The number of through-rangers (genera that are sampled at some point before and after the analyzed interval but not within it) increases sharply in the Hirnantian stage and remains relatively high until the late Early Silurian period (Fig. 2A). This pattern is in accord with previous studies that found that, although preservation probability decreased through this interval, the decrease could not fully explain the late Katian extinction pulse (42, 44, 45). We excluded through-ranger genera from selectivity analyses for both practical and theoretical reasons. Practically, it is impossible to measure aspects of the geographic or environmental ranges of unsampled genera. In addition, through-rangers represent a mixture of genera that were present in Laurentia, but were not preserved, and genera that were extirpated in Laurentia but subsequently reinvaded from other paleocontinents or terranes (33, 40). Even if the aforementioned practical constraints could be overcome, it would be inappropriate to include the latter group when analyzing intervals where they were not present in Laurentia. Further restricting our analyses to genera that are sampled in every interval within their stratigraphic range, which are more likely to record true first and last occurrences (46), does not substantively alter observed selectivity patterns in most intervals (Fig. S3).
The relative importance of the variables in our analysis for determining extinction risk; i.e., accurately classifying genera as extinct or surviving, varies substantially from interval to interval (Fig. 2C). Variables that measure aspects of geographic and environmental range were the most consistent and important predictors of extinction risk, a pattern observed in other analyses (34, 47). Taxonomic class membership was also a consistent and important determinant of extinction risk, reflecting variation in intrinsic turnover rate among major taxa (48). Autecological variables such as trophic level and life habit had little independent influence on extinction risk during most intervals, perhaps because they are closely tied to class membership.
When compared to preceding and succeeding intervals, selectivity patterns associated with the first pulse of the LOME show both similarities and striking differences. In the late Katian interval, percent truncation and percent environmental truncation were important determinants of extinction risk. Genera that experienced stratigraphic hiatuses or lithofacies shifts over large proportions of their range were more likely to go extinct than those that did not. Percent truncation was particularly important, reflecting the peak in section truncations (Fig. 1 and Fig. 2B). Notably, percent truncation was also a moderately important determinant of extinction risk during the early Katian interval. This pattern could reflect genuine extinctions due to sea level fall and habitat loss accompanying initial growth of midsized ice sheets in late Katian time (5, 49). Alternatively, the pattern could be attributable to Signor-Lipps backsmearing of late Katian extinctions combined with removal of late Katian strata by erosion following the much larger end-Katian glacioeustatic regression. The moderate importance of percent truncation during the latest Ordovician Hirnantian stage is intriguing given the relatively low truncation rate within this interval (Fig. 2B); however, the exceptionally low sensitivity (true positive rate vs. false positive rate) of the Hirnantian model (Fig. 2D) suggests that selectivity patterns associated with this interval should be viewed with caution.
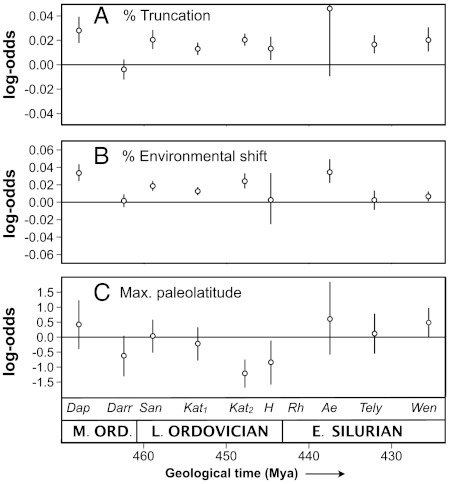
Log-odds* associated with percent truncation in multiple logistic regression models are strikingly similar throughout most of the study interval, including the late Katian interval (Fig. 3A). This interval, therefore, is unusual only in that a very large number of genera were affected by truncation: the form of the relationship between percent truncation and extinction risk did not change substantially. With respect to the effect of stratigraphic truncation on extinction rate, the first pulse of the LOME appears to represent an intensification of “background” patterns more than the initiation of a new extinction regime.
Fig. 3.
The results of multiple logistic regressions of extinction risk on (A) percent truncation, (B) percent environmental shift/truncation, and (C) maximum paleolatitude for each interval. Positive log-odds indicate that extinction risk increases as the variable in question increases and vice versa. In addition to these three variables, regressions controlled for genus age, occupancy, substrate preference, and Laurentian and global geographic range. Dap = Dapingian, Darr = Dariwillian, San = Sandbian, K1 = early Katian, K2 = late Katian, H = Hirnantian, Rh = Rhuddanian, Ae = Aeronian, Tely = Telychian, Wen = Wenlockian.
Whereas percent truncation was most important in the late Katian interval, percent environmental truncation was similarly important in most Late Ordovician intervals (Fig. 2C). This pattern can be attributed to the frequency of environmental truncations compared to hiatal truncations. Log-odds associated with percent environmental truncation are similar in most intervals (Fig. 3B) but are somewhat higher in the late Katian than in most preceding intervals. This pattern may indicate that environmental shifts at this time were generally more abrupt and severe in their effects on the biota than environmental shifts in the preceding approximately ten million years. Many stratigraphic sections record high-frequency relative sea-level oscillations during the late Katian-Hirnantian transition (49, 50), and nearly all continuous sections record dramatic shallowing and/or changes in environmental conditions (51).
From these patterns alone, it is not clear if the association between percent stratigraphic truncation, percent environmental truncation, and extinction risk should be attributed to record bias or to eustatic common cause. The relationship between gap duration and extinction risk provides a test to distinguish these hypotheses. We used the subset of genera that experienced at least some truncation in each interval and standard maximum likelihood model selection criteria to determine if including median gap duration in logistic regression models resulted in a significant improvement in model fit (Table S1). Accounting for gap duration significantly improved fits for some preLOME Middle and Late Ordovician intervals, raising the possibility that extinction rates in these intervals are inflated by sampling gaps. Including gap duration did not, however, significantly improve the fit of the late Katian model. In contrast, adding percent truncation to a late Katian model that already included median gap duration significantly improved its fit (Table S1). While it is not possible to completely rule out a role for record bias (15) or habitat bias, given the coarse lithological resolution of Macrostrat), our analysis suggested that the relationship between percent truncation and extinction risk during the LOME was primarily due to habitat loss rather than record failure.
The remaining variable that has a strong effect on extinction risk during the LOME is maximum paleolatitude (Fig. 2C, Fig. 3C, Fig. S4). This pattern is not driven by outliers; rather, it represents a well defined step function in the relationship between extinction risk and maximum paleolatitude (Fig. S4). The importance of maximum paleolatitude even when controlling for covariates such as geographic range, richness, genus age, and substrate preference, suggests that thermal tolerance range played an important role in determining extinction vs. survival during the LOME. These observations support the climatic common cause hypothesis. Although we did not include graptolites in our analysis because of taphonomic complications, it is notable that they exhibit a similar pattern of preferential extinction of low-latitude taxa through this interval (52).
Maximum paleolatitude stands out as the only important determinant of late Katian extinction risk that did not have a strong effect during the early Katian interval. This pattern is consistent with results from classical oxygen isotope paleothermometry (7, 53, 54) and clumped isotope paleothermometry (5), which indicate that despite the existence of at least moderate-sized Gondwanan ice sheets from mid-late Katian time. Shallow tropical seas did not cool substantially until latest Katian-Hirnantian time. Weaker but significant inverse associations between maximum latitude and extinction risk are also apparent for the late Middle Ordovician Darriwilian stage (Figs. 2C and 3C), during which there is evidence of an earlier tropical cooling step (6, 54–56), and the latest Ordovician Hirnantian stage.
Our results implicate cooling and habitat loss as important extinction drivers in the first pulse of the LOME in Laurentia. To further assay the changes in extinction regime associated with this event, we used a random forest model trained on early Katian extinction patterns (and hence incorporating interactions among variables), to predict which genera would be expected to go extinct and which genera would be expected to survive if the “background” selectivity regime of early Katian time were superimposed on the macroecological and macrostratigraphic milieu of the late Katian interval. This model predicts a 31% extinction rate for late Katian genera, which is substantially lower than the 47% observed. Most of the “excess” (e.g., unpredicted) extinction occurs among exclusively low-paleolatitude genera, especially those genera that experienced relatively minor (< 50%) stratigraphic truncation (Table S2). Previous studies have shown that endemic genera tend to exhibit higher extinction risk than cosmopolitan genera (32), and all of the Laurentian endemics in our dataset have exclusively low-paleolatitude distributions. In addition, because they tend to have smaller ranges even in Laurentia (32), endemic genera may be more strongly affected by stratigraphic truncation (Table S2). However, the unexpectedly high extinction rate of low-paleolatitude genera was not due to high rates of endemicity as the extinction rates of endemic genera are relatively well predicted by the background model (Table S2). The biggest mismatch between the model prediction and late Katian observations is, rather, underprediction of the extinction rate of nonendemic, exclusively low-paleolatitude genera (Table S2).
We have focused our discussion on the first pulse of the LOME because it offers an attractive test case for evaluating the common cause hypothesis. The causes behind the second, smaller extinction pulse during the latest Ordovician Hirnantian stage are more elusive for several reasons. Globally, the end-Hirnantian extinction pulse largely reflects extinction of the “Hirnantia fauna”, an informal grouping of cold-adapted taxa–many derived from high-latitude regions–that flourished and expanded their ranges during peak glaciation (47). The Hirnantia fauna was a relatively minor presence in most parts of Laurentia, however, and most taxa were either Katian holdovers, invaders from other low-latitude paleocontinents and terranes, or endemics that evolved after the first extinction pulse (3, 33). These taxa experienced only modest extinction at the Ordovician-Silurian boundary (3, 44, 57, 58). The macrostratigraphic and macroecological variables in our analysis do a much poorer job of predicting extinction risk during the Hirnantian stage than during other intervals (Fig. 2D), implying that the drivers of extinction differed substantively from those involved in either the first pulse of the LOME or in the “background” extinctions of the Middle-Late Ordovician. It is possible that these extinctions were related to changes in water mass characteristics (59) or other environmental parameters not currently captured by our dataset. Finally, the large number of through-ranger genera during the Hirnantian-Aeronian interval (Fig. 2A) poses a challenge to our approach of limiting selectivity analyses to sampled-in-interval genera. Excluding through-ranger genera is appropriate if they were not present in Laurentia during the analyzed interval, but is potentially problematic if they were present but unsampled: ignoring a large set of genera that were extant and by definition survived into subsequent intervals could bias selectivity patterns if the unsampled genera were nonrandomly distributed with respect to relevant risk factors.
Mass extinctions are complex events that involve interactions among multiple processes and their associated risk factors. The resulting fossil occurrence patterns are distorted by the incompleteness of the stratigraphic record. Our integration of macrostratigraphic and paleobiological datasets provides a framework within which key variables related to taxon/record interactions can be quantified. This approach allows us to characterize the selective fingerprint of the LOME in Laurentia. Our analysis provides support for both eustatic and climatic common cause mechanisms, with Late Ordovician southern hemisphere glaciation driving eustatic habitat losses in shallow seas and a drop in tropical seawater temperatures, both of which served as important determinants of extinction risk in the first pulse of the LOME. More broadly, by helping to define the biotic response to a major global environmental change, these results sharpen our understanding of how physical processes manifest as common causes—affecting both the evolutionary histories of the biota and the sedimentary rocks from which these histories are read.
Methods
We matched US and Canadian fossil occurrence records from the Paleobiology Database (PaleoDB) (60) to sedimentary units in the Macrostrat Database (41), using the criteria outlined by Heim and Peters (30–32) (Dataset S1). Dataset S2 provides a full list of the publications from which the PaleoDB collections were derived. The 43,993 Dapingian-Wenlockian occurrences from the United States, Canada, and Greenland described in the PaleoDB, which included stratigraphic information as of 15 January 2012, 39,331 (89.4%) occurrences could be matched to units in the Macrostrat database. Calculated diversity trends were similar regardless all PaleoDB occurrences or only matched occurrences, indicating that the subset of matched occurrences is unbiased with respect to diversity patterns. The matched dataset includes occurrences of 1,983 genera from 318 published sources (Dataset S2). Sedimentary units in the Macrostrat database were assigned to stratigraphic packages bounded by temporal gaps (41) and environmental packages bounded by shifts in lithofacies.
Analyses of extinction selectivity typically focus on one or a few explanatory variables because of the difficulty of quantifying potentially important variables from fossil record data and the statistical effects of adding explanatory variables to models. We used a Random Forest classification model technique (38) to address the latter limitation. Random forests average across large numbers of decision trees based on subsamples of the observations and explanatory variables, and they perform well for “low Nhigh P” problems, where the number of observations is relatively small and the number of potential variables is high (38). Random forest models have additional advantages that are useful for examining extinction patterns. First, the decision trees in random forests are nonparametric, making no assumption of linearity. Second, interactions among predictors are automatically incorporated into the model and into variable importance estimates (38) that quantify the increase in classification error that occurs when a given predictor variable is randomly permuted. We used conditional inference trees as base learners because they provide unbiased variable selection (61). We quantified the overall success of each model for correctly classifying genera as extinctions or survivors using the area under the receiver operating characteristic curve (AUC), which is sensitive to type I and type II error. Analyses were performed using the R programming environment (62) and the “party” package (61). SQL code for downloading data and R code for processing and analyzing data are available from the authors upon request.
Supplementary Material
Acknowledgments.
This manuscript benefitted greatly from reviews by Steve Holland and an anonymous reviewer. We thank other workers who contributed relevant data to the Paleobiology Database, especially S. Holland, M Patzkowsky, K. Layou, A. Stigall, W. Kiessling, M. Hopkins, A.I. Miller, M. Foote and J. Alroy. This work was supported by Agouron Institute and National Science Foundation (EAR-1053523) awards to WWF. This is Paleobiology Database contribution #154.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.K.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117039109/-/DCSupplemental.
*A statistic analogous to the slope in a linear regression model.
References
- 1.Brenchley PJ, Marshall JD, Underwood CJ. Do all mass extinctions represent an ecological crisis? Evidence from the Late Ordovician. Geol J. 2001;36:329–340. [Google Scholar]
- 2.Kaljo D, Hints L, Mannick P, Nolvak J. The succession of Hirnantian events based on data from Baltica: brachiopods, chitinozoans, conodonts, and carbon isotopes. Est J Earth Sci. 2008;57:197–218. [Google Scholar]
- 3.Sheehan PM. The Late Ordovician mass extinction. Annu Rev Earth Pl Sc. 2001;29:331–364. [Google Scholar]
- 4.Brenchley PJ, et al. Bathymetric and isotopic evidence for a short-lived Late Ordovician glaciation in a greenhouse period. Geology. 1994;22:295–298. [Google Scholar]
- 5.Finnegan S, et al. The magnitude and duration of Late Ordovician-Early Silurian glaciation. Science. 2011;331:903–906. doi: 10.1126/science.1200803. [DOI] [PubMed] [Google Scholar]
- 6.Vandenbroucke TRA, et al. Polar front shift and atmospheric CO2 during the glacial maximum of the Early Paleozoic Icehouse. Proc Nat'l Acad Sci USA. 2010;107:14983–14986. doi: 10.1073/pnas.1003220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley PJ, et al. High-resolution stable isotope stratigraphy of Upper Ordovician sequences: Constraints on the timing of bioevents and environmental changes associated with mass extinction and glaciation. Geol Soc Am Bull. 2003;115:89–104. [Google Scholar]
- 8.Jones DS, et al. Terminal Ordovician carbon isotope stratigraphy and glacioeustatic sea-level change across Anticosti Island (Quebec, Canada) Geol Soc Am Bull. 2011;123:1645–1664. [Google Scholar]
- 9.Kump LR, et al. A weathering hypothesis for glaciation at high atmospheric pCO(2) during the Late Ordovician. Palaeogeogr Palaeocl. 1999;152:173–187. [Google Scholar]
- 10.Sheehan PM. Late Ordovician events and the terminal Ordovician extinction. New Mexico Bureau of Mines and Mineral Resources Memoirs. 1988;44:405–415. [Google Scholar]
- 11.Ghienne J-F, Le Heron DP, Moreau J, Denis M, Deynoux M. Glacial Sedimentary Processes and Products. Oxford, United Kingdom: Blackwell Publishing Ltd; 2009. The Late Ordovician glacial sedimentary system of the North Gondwana Platform; pp. 295–319. [Google Scholar]
- 12.Berry WBN, Boucot AJ. Glacio-eustatic control of Late Ordovician-Early Silurian platform sedimentation and faunal changes. Geol Soc Am Bull. 1973;84:275–284. [Google Scholar]
- 13.Rasmussen CMò, Harper DAT. Interrogation of distributional data for the End Ordovician crisis interval: where did disaster strike? Geol J. 2011;46:478–500. [Google Scholar]
- 14.Peters SE. Genus extinction, origination, and the durations of sedimentary hiatuses. Paleobiology. 2006;32:387–407. [Google Scholar]
- 15.Holland SM, Patzkowsky ME. Models for simulating the fossil record. Geology. 1999;27:491–494. [Google Scholar]
- 16.Johnson JG. Extinction of perched faunas. Geology. 1974;2:479–482. [Google Scholar]
- 17.Newell ND. Revolutions in the history of life. Geological Society of America Special Publication. 1967;89:63–91. [Google Scholar]
- 18.Simberloff D. Permo-Triassic extinctions: effects of area on biotic equilibrium. J Geol. 1974;82:267–274. [Google Scholar]
- 19.Stanley SM. Climatic cooling and mass extinction of Paleozoic reef communities. Palaios. 1988;3:228–232. [Google Scholar]
- 20.Stanley SM. Paleozoic mass extinctions-shared patterns suggest global cooling as a common cause. Am J Sci. 1988;288:334–352. [Google Scholar]
- 21.Stanley SM. Temperature and biotic crises in the marine realm. Geology. 1984;12:205–208. [Google Scholar]
- 22.Stanley SM. Thermal barriers and the fate of perched faunas. Geology. 2010;38:31–34. [Google Scholar]
- 23.Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. P Roy Soc B: Biol Sci. 2011;278:1823–1830. doi: 10.1098/rspb.2010.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Compton TJ, Rijkenberg MJA, Drent J, Piersma T. Thermal tolerance ranges and climate variability: A comparison between bivalves from differing climates. J Exp Mar Biol Ecol. 2007;352:200–211. [Google Scholar]
- 25.Powell MG. Timing and selectivity of the Late Mississippian mass extinction of brachiopod genera from the Central Appalachian Basin. Palaios. 2008;23:525–534. [Google Scholar]
- 26.Hansen TA. Extinction of late Eocene to Oligocene mollusks: relationship to shelf area, temperature changes, and impact events. Palaios. 1987;2:69–76. [Google Scholar]
- 27.Stanley SM. Anatomy of a regional mass extinction: Plio-Pleistocene decimation of the western Atlantic bivalve fauna. Palaios. 1987;1:17–36. [Google Scholar]
- 28.Paleobiology Database contributors. 2011. The Paleobiology Database, http://paleodb.org/
- 29.Peters SE. Macrostrat. 2011. http://macrostrat.org/
- 30.Peters SE, Heim NA. The geological completeness of paleontological sampling in North America. Paleobiology. 2010;36:61–79. [Google Scholar]
- 31.Heim NA, Peters SE. Covariation in macrostratigraphic and macroevolutionary patterns in the marine record of North America. Geol Soc Am Bull. 2011;123:620–630. [Google Scholar]
- 32.Heim NA, Peters SE. Regional environmental breadth predicts geographic range and Llongevity in fossil marine genera. PLoS ONE. 2011;6:e18946. doi: 10.1371/journal.pone.0018946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasmussen CMò, Harper DAT. Did the amalgamation of continents drive the end Ordovician mass extinctions? Palaeogeogr Palaeocl. 2011;311:48–62. [Google Scholar]
- 34.Payne JL, Finnegan S. The effect of geographic range on extinction risk during background and mass extinction. Proc Nat'l Acad Sci USA. 2007;104:10506–10511. doi: 10.1073/pnas.0701257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrmann AD, Haupt BJ, Patzkowsky ME, Seidov D, Slingerland RL. Response of Late Ordovician paleoceanography to changes in sea level, continental drift, and atmospheric pCO (2); potential causes for long-term cooling and glaciation. Palaeogeogr Palaeocl. 2004;210:385–401. [Google Scholar]
- 36.Jablonski D, Roy K, Valentine JW. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314:102–106. doi: 10.1126/science.1130880. [DOI] [PubMed] [Google Scholar]
- 37.Finnegan S, Payne JL, Wang SC. The Red Queen revisited: reevaluating the age selectivity of Phanerozoic marine genus extinctions. Paleobiology. 2008;34:318–341. [Google Scholar]
- 38.Breiman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 39.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley; p. 375. [Google Scholar]
- 40.Jin J, Zhan R. Late Ordovician orthide and billingsellide brachiopods from Anticosti Island, Eastern Canada; diversity change through mass extinction. Ottowa: NRC Research Press; 2008. p. 159. [Google Scholar]
- 41.Peters SE. Geologic constraints on the macroevolutionary history of marine animals. Proc Nat’l Acad Sci USA. 2005;102:12326–12331. doi: 10.1073/pnas.0502616102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krug AZ, Patzkowsky ME. Geographic variation in turnover and recovery from the Late Ordovician mass extinction. Paleobiology. 2007;33:435–454. [Google Scholar]
- 43.Foote M. Origination and extinction components of taxonomic diversity: Paleozoic and post-Paleozoic dynamics. Paleobiology. 2000;26:578–605. [Google Scholar]
- 44.Peters SE, Ausich WI. A sampling-adjusted macroevolutionary history for Ordovician-Early Silurian crinoids. Paleobiology. 2008;43:104–116. [Google Scholar]
- 45.Foote M. Inferring temporal patterns of preservation, origination, and extinction from taxonomic survivorship analysis. Paleobiology. 2001;27:602–630. [Google Scholar]
- 46.Marshall CR. Confidence-intervals on stratigraphic ranges. Paleobiology. 1990;16:1–10. [Google Scholar]
- 47.Sheehan PM, Coorough PJ. Brachiopod zoogeography across the Ordovician-Silurian extinction event. In: McKerrow WS, Scotese CR, editors. Palaeozoic Palaeogeography and Biogeography. Vol. 12. London: Geological Society of London Memoir; 1990. pp. 181–187. Series. [Google Scholar]
- 48.Wang SC, Bush AM. Adjusting global extinction rates to account for taxonomic susceptibility. Paleobiology. 2008;34:434–455. [Google Scholar]
- 49.Holland SM, Patzkowsky ME. The stratigraphic distibution of fossils in a tropical carbonate sucession: Ordovician Bighorn Dolomite, Wyoming, USA. Palaios. 2009;24:303–317. [Google Scholar]
- 50.Desrochers A, Farley C, Achab A, Asselin E, Riva JF. A far-field record of the end Ordovician glaciation: The Ellis Bay Formation, Anticosti Island, Eastern Canada. Palaeogeogr, Palaeocl. 2010;296:248–263. [Google Scholar]
- 51.Brenchley PJ, Carden GAF, Marshall JD, Brenchley P. Environmental changes associated with the “first strike” of the Late Ordovician mass extinction. Modern Geology. 1995;20:69–82. [Google Scholar]
- 52.Xu C, Melchin MJ, Sheets HD, Mitchell C, Jun-Xuan FAN. Patterns and processes of latest Ordovician graptolite extinction and recovery based on data from South China. J Paleontol. 2005;79:842–861. [Google Scholar]
- 53.Marshall JD, Middleton PD. Changes in marine isotopic composition and the Late Ordovician glaciation. Journal of the Geological Society of London. 1990;147:1–4. [Google Scholar]
- 54.Trotter JA, Williams IS, Barnes CR, Lecuyer C, Nicoll RS. Did cooling oceans trigger Ordovician biodiversification? Evidence from conodont thermometry. Science. 2008;321:550–554. doi: 10.1126/science.1155814. [DOI] [PubMed] [Google Scholar]
- 55.Veizer J, et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem Geol. 1999;161:59–88. [Google Scholar]
- 56.Ainsaar L, et al. Middle and Upper Ordovician carbon isotope chemostratigraphy in Baltoscandia: a correlation standard and clues to environmental history. Palaeogeogr, Palaeocl. 2010;294:189–201. [Google Scholar]
- 57.Tuckey ME, Anstey RL. Late Ordovician extinctions of bryozoans. Lethaia. 1992;25:111–117. [Google Scholar]
- 58.Elias RJ, Young GA. Coral diversity, ecology and provincial structure during a time of crisis: the latest Ordovician and earliest Silurian Edgewood Province in Laurentia. Lethaia. 1998;13:98–112. [Google Scholar]
- 59.Zhang T, Shen Y, Zhan R, Shen S, Chen X. Large perturbations of the carbon and sulfur cycle associated with the Late Ordovician mass extinction in South China. Geology. 2009;37:299–302. [Google Scholar]
- 60.Alroy J, et al. Phanerozoic trends in the global diversity of marine invertebrates. Science. 2008;321:97–100. doi: 10.1126/science.1156963. [DOI] [PubMed] [Google Scholar]
- 61.Strobl C, Boulesteix A-L, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.R Core Development Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.