Abstract
Epidemiological studies strongly suggest that chronic psychological stress promotes tumorigenesis. However, its direct link in vivo and the underlying mechanisms that cause this remain unclear. This study provides direct evidence that chronic stress promotes tumorigenesis in vivo; chronic restraint, a well-established mouse model to induce chronic stress, greatly promotes ionizing radiation (IR)-induced tumorigenesis in p53+/− mice. The tumor suppressor protein p53 plays a central role in tumor prevention. Loss or attenuation of p53 function contriubutes greatly to tumorigenesis. We found that chronic restraint decreases the levels and function of p53 in mice, and furthermore, promotes the growth of human xenograft tumors in a largely p53-dependent manner. Our results show that glucocorticoids elevated during chronic restraint mediate the effect of chronic restraint on p53 through the induction of serum- and glucocorticoid-induced protein kinase (SGK1), which in turn increases MDM2 activity and decreases p53 function. Taken together, this study demonstrates that chronic stress promotes tumorigenesis in mice, and the attenuation of p53 function is an important part of the underlying mechanism, which can be mediated by glucocortcoids elevated during chronic restraint.
The tumor suppressor p53 plays a crucial role in preventing tumor formation (1, 2). p53 responds to stress signals and transcriptionally regulates its target genes to start various cellular responses, including cell cycle arrest and apoptosis, thus preventing tumor initiation and/or progression. p53 is the most frequently mutated gene in tumors; ∼50% of human tumors harbor inactivating mutations in p53 (3). The p53 protein is under tight regulation in cells. Loss or the attenuation of p53 function (even a twofold modest change) affects cancer risk and tumor progression. For example, loss of one p53 allele in mice (p53+/−) leads to the early development of tumors (4).
Epidemiological studies strongly suggest that chronic psychological stress has negative influences on the onset, progression, and mortality of cancers (5–8). A meta-analysis of 165 longitudinal studies demonstrated that psychosocial factors and stressful life experiences are associated with higher cancer incidence and poorer cancer survival (8). Studies using chronic restraint or social isolation in mice, two well-established chronic stress models, have shown that chronic stress enhances tumor growth and metastasis in xenograft tumor assays (9, 10). However, the direct evidence that chronic stress promotes tumorigenesis in vivo is still lacking, and furthermore, the molecular mechanisms by which chronic stress promotes tumorigenesis remain unclear.
To investigate the impact of chronic stress upon tumorigenesis in vivo, we established a mouse model that combines chronic restraint and ionizing radiation (IR)-induced tumorigenesis. IR induces tumorigenesis in both mice and humans. A single dose of 4 Gy IR results in tumor development in p53+/− mice (11). This report demonstrates that chronic restraint promoted IR-induced tumorigenesis in p53+/− mice, providing direct evidence that chronic stress enhances tumorigenesis in vivo. Furthermore, chronic restraint decreased p53 function and promoted the growth of HCT116 xenograft tumors in a largely p53-dependent manner; chronic restraint enhanced growth of p53+/+ xenograft tumors to a much greater extent than p53−/− tumors. This report further demonstrates that glucocorticoids elevated during chronic restraint decreased p53 function through the induction of serum- and glucocorticoid-induced protein kinase (SGK1), a negative regulator of p53. These data strongly suggest that glucocorticoids mediate the inhibitory effect of chronic restraint on p53 function, which in turn promotes tumorigenesis.
Results
Chronic Restraint Promotes IR-Induced Tumorigenesis in Mice.
To investigate the impact of chronic stress upon tumorigenesis in vivo, a mouse model that combines chronic restraint and IR-induced tumorigenesis was used. p53 prevents IR-induced tumorigenesis (11). Loss of p53 sensitizes IR-induced tumorigenesis in mice; 4 Gy IR significantly promotes tumor development, mainly lymphomas, in p53+/− mice, but not p53+/+ mice (11). In this study, 7-wk-old p53+/− C57BL6/J male mice were subjected to periodic physical restraint by immobilization in a mouse restraint apparatus (6 h/day) for 1 wk and then treated with 4 Gy IR, followed by periodic restraint for another 3 wk. This treatment induced high corticosterone levels in serum (Fig. 1A), a characteristic of chronic stress. Unrestrained mice were treated with 4 Gy IR as controls. Consistent with previous reports, IR significantly promoted tumor formation in p53+/− mice; the tumor latency was significantly reduced from ∼77 to ∼49 wk of median survival age (P = 0.006, Fig. 1B). Although chronic restraint had no significant effect on the survival of p53+/− mice (P = 0.92), it significantly reduced tumor latency induced by IR (from ∼49 to ∼38 wk of median survival age) (P = 0.004, Fig. 1B). The IR-induced tumor spectrum was similar between mice with and without chronic restraint; both groups of mice developed predominantly lymphomas and sarcomas, suggesting that the reduced tumor latency in mice with chronic restraint is not due to the development of new types of tumors (Fig. 1C). These results demonstrate that chronic restraint stress promotes IR-induced tumorigenesis in mice.
Fig. 1.
Chronic restraint promotes IR-induced tumorigenesis in p53+/− mice. Seven-week-old p53+/− C57BL6/J male mice were subjected to periodic restraint (6 h/day) for 1 wk and then treated with 4 Gy IR followed by periodic restraint for another 3 wk. (A) Chronic restraint increased serum corticosterone levels in mice as measured by ELISAs. (B) Chronic restraint promotes IR-induced tumorigenesis as demonstrated by the Kaplan-Meier curve. IR significantly reduced median survival age of mice. A significantly shorter median survival age was observed in mice with chronic restraint compared with mice without restraint. (C) Similar IR-induced tumor spectrum between mice with and without chronic restraint.
Chronic Restraint Decreases p53 Protein Levels and Function in Response to IR in Mice.
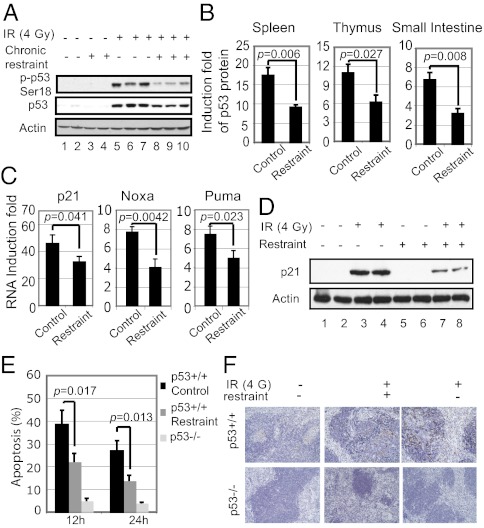
In response to IR, p53 is activated and its protein levels increase, initiating cellular responses to prevent tumorigenesis. Loss of p53 sensitizes IR-induced tumorigenesis (11). The observation that chronic restraint promotes IR-induced tumorigenesis in mice suggested that chronic restraint may reduce p53 function. To test this possibility, 7-wk-old p53+/+ C57BL6/J male mice were subjected to periodic restraint for 1 wk, followed by 4 Gy IR. The phosphorylation of p53 at Ser18 (p-p53 Ser18, equivalent to human p53 Ser15), which is the initial step of p53 activation in response to IR, and the accumulation of p53 protein were measured in the spleen after IR. Spleen is a radiosensitive tissue that displays strong IR-induced p53 responses. The increase of p-p53 Ser18 levels and the accumulation of p53 protein in response to IR were significantly lower in spleen from mice with chronic restraint as determined at different time points after IR (decreased by ∼50% at 6 h after IR, Fig. 2 A and B and Fig. S1A for 12 and 24 h after IR). Similar results were observed in other tissues, including thymus and small intestine (Fig. 2B) and in spleen from p53+/− mice where the promoting effect of chronic restraint on IR-induced tumorigenesis was observed (Fig. S1B). These results demonstrate that chronic restraint decreases the accumulation of p53 protein in response to IR.
Fig. 2.
Chronic restraint decreases p53 protein levels and function in transcriptional activity and apoptosis in response to IR in mice. Seven-week-old p53+/+ C57BL6/J male mice with or without chronic periodic restraint (6 h/day for 1 wk) were treated with 4 Gy IR, and various tissues were collected at different time points after IR (n = 5 per group). (A) Chronic restraint decreased the phosphorylation of p53 Ser18 (p-p53 Ser18) and accumulation of p53 protein in response to IR in the spleen. Levels of p-p53 Ser18 and p53 protein were determined at 6 h after IR by Western blot assays. (B) Chronic restraint decreased the accumulation of p53 protein induced by IR in different tissues. The induction fold of p53 protein in the spleen, thymus, and small intestine was determined at 6 h after IR by ELISAs. (C) Chronic restraint decreased the induction of p53 target genes. mRNA levels of p21, Noxa, and Puma were determined at 6 h after IR by Taqman real-time PCR and normalized with actin. (D) Chronic restraint decreased the protein levels of p21. Protein levels of p21 were determined at 6 h after IR by Western blot assays. (E and F) Chronic restraint decreased p53-mediated apoptosis in the spleen in response to IR. Splenocytes were isolated from the mice at 12 and 24 h after IR, stained with annexin V, and analyzed in a flow cytometer to detect apoptotic cells (E). TUNEL assay was used to detect apoptotic cells in spleen tissues at 12 h after IR (F).
To investigate whether chronic restraint decreases p53 function, p53 transcriptional activity was determined. The mRNA levels of a group of well-known p53 target genes, including p21, Puma, and Noxa, were examined in spleen at 6 h after IR. These genes were induced by IR in a p53-dependent manner; they were induced at high levels in p53+/+ mice (Fig. 2C), but to a significantly lesser extent in p53−/− mice (Fig. S2A). Notably, the induction of these mRNA levels was significantly lower in mice with chronic restraint versus mice without restraint by up to 50% (Fig. 2C). The decrease of p21 induction was confirmed by Western blot assays (Fig. 2D). Similar results were observed in spleen from p53+/− mice (Fig. S2B). These results demonstrate that chronic restraint decreases p53 transcriptional activity.
One major p53 function in tumor suppression is to induce apoptosis in response to stress. To investigate whether p53-mediated apoptosis is reduced by chronic restraint, p53+/+ and p53−/− C57BL6/J mice with or without chronic restraint were irradiated with IR (4 Gy). IR-induced apoptosis was measured in splenocytes using Annexin V staining in a flow cytometer. Splenocytes underwent p53-dependent apoptosis in response to IR in p53+/+ mice without chronic restraint (∼40 and ∼25% apoptotic cells at 12 and 24 h after IR, respectively) (Fig. 2E). As a control, very few splenocytes (<5%) died of apoptosis in p53−/− mice treated similarly. Compared with p53+/+ mice without restraint, IR induced significantly less apoptosis in p53+/+ mice with chronic restraint (∼20 and ∼15% apoptotic cells at 12 and 24 h after IR, respectively) (Fig. 2E). These results were confirmed by TUNEL assays in the spleen (Fig. 2F), demonstrating that chronic restraint reduces p53-dependent apoptosis induced by IR.
Attenuation of p53 Function by Chronic Restraint Promotes Tumorigenesis in Xenograft Tumors.
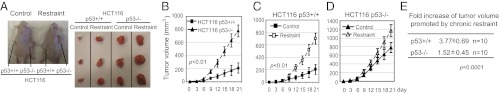
Considering the role of p53 in tumor prevention, the attenuation of p53 function could be an important mechanism by which chronic restraint promotes tumorigenesis in response to IR. In p53−/− mice, due to rapid development of spontaneous tumors (at ∼4 mo of age), the same 4-Gy IR treatment does not accelerate tumor development (11). Therefore, it is difficult to compare the impact of chronic restraint upon IR-induced tumorigenesis between mice with or without p53. Here, the xenograft tumor assays were used to investigate whether the attenuation of p53 contributes to the promoting effect of chronic restraint on tumorigenesis. A pair of isogenic human colorectal cancer cell lines with or without p53 (HCT116 p53+/+ and HCT116 p53−/−) were used to measure the impact of chronic stress upon the maintenance and propagation of tumor growth. BALB/c athymic nude mice with periodic restraint for 1 wk were s.c. inoculated with HCT116 p53+/+ or HCT116 p53−/− cells, followed by periodic restraint for another 3 wk. Consistent with previous studies showing that p53 deficiency promotes xenograft tumor growth, the growth rate of HCT116 p53−/− tumors was faster than that of HCT116 p53+/+ tumors in mice without restraint (Fig. 3 A and B). Notably, chronic restraint significantly promoted the growth rate of HCT116 p53+/+ tumors in mice; the average tumor size was increased by 3.77-fold in mice with chronic restraint versus mice without restraint (Fig. 3 A and C), which demonstrates that chronic restraint promotes the maintenance and propagation of xenograft tumors. Furthermore, the promoting effect of chronic restraint on the growth of HCT116 p53−/− tumors was significantly less pronounced (increased by 1.52-fold) compared with HCT116 p53+/+ tumors (P < 0.0001) (Fig. 3 A and C–E). These results demonstrate that chronic restraint promotes xenograft tumor growth in a largely p53-dependent manner, suggesting that attenuation of p53 function is an important mechanism by which chronic stress promotes tumorigenesis. This experiment also demonstrates that the psychological state and its physiological consequence upon the host can affect tumor growth and that p53 plays a critical role in mediating this effect.
Fig. 3.
Chronic restraint promotes the growth of HCT116 xenograft tumors in a largely p53-dependent manner. Seven-week-old BALB/c athymic nude mice with or without chronic restraint were inoculated (s.c.) with HCT116 p53+/+ or HCT116 p53−/− cells (n = 10 per group). (A) Representative images of mice at day 21 after inoculation of tumor cells (Left) and tumors harvested from these mice (Right) are presented. (B–D) Tumor volume is presented as mean ± SD. (B) Loss of p53 promotes HCT116 tumor growth in mice without restraint; growth rate of HCT116 p53−/− tumors was much faster than HCT116 p53+/+ tumors in mice without restraint. (C and D) Chronic restraint preferentially promoted the growth of HCT116 p53+/+ xenograft tumors (C) compared with HCT116 p53−/− tumors (D). (E) Fold increase of tumor volume promoted by chronic restraint at day 21 after inoculation of tumor cells was much higher in HCT116 p53+/+ xenograft tumors than HCT116 p53−/− tumors.
Glucocorticoids Decrease p53 Protein Levels and Function in Cultured Cells.
Glucocorticoids are among the hormones elevated during chronic stress and affect the systemic physiology of the host (12). Prolonged elevation of glucocorticoids could have a direct impact upon tumorigenesis. It has been reported that glucocorticoids promote survival and proliferation of tumor cells and the chemotherapeutic resistance of tumor cells (13). However, the underlying mechanisms are largely unknown. To investigate whether the elevated glucocorticoids reduce p53 function, HCT116 p53+/+ cells were treated with cortisol for 12 h, a major glucocorticoid elevated during chronic stress in humans, followed by 4 Gy IR. Cortisol greatly decreased the levels of p-p53 Ser15 and p53 protein at 6 h after IR in cells (Fig. 4A). Glucocorticoid receptor (GR), which is ubiquitously expressed in many types of cells, mediates most of the effects of glucocorticoids (14). RU486, a GR antagonist, was used to determine whether cortisol decreases p53 protein levels through GR signaling. The inhibitory effect of cortisol on p53 protein accumulation was abolished in cells treated with RU486 (Fig. 4B). Consistently, knockdown of GR by siRNA abolished the inhibitory effect of cortisol on p53 accumulation in response to IR in HCT116 p53+/+ cells (Fig. 4C). Similar results were observed for two different siRNA oligos against GR. These results suggest that cortisol down-regulates p53 levels through GR signaling. In addition to IR, cortisol greatly decreased the levels of p-p53 Ser15 and p53 protein accumulation induced by etoposide, a chemotherapeutic agent, in HCT116 p53+/+ cells (Fig. S3A). Similar results were observed in other human cells, such as breast cancer cell MCF7 (Fig. S3B). Similarly, corticosterone, a major glucocorticoid synthesized in mice, greatly decreased the levels of p-p53 Ser18 and the accumulation of p53 protein in response to IR in mouse embryonic fibroblasts (MEFs) (Fig. 4D), which can be abolished in RU486-treated cells (Fig. 4E).
Fig. 4.
Glucocorticoids decrease p53 levels and function in cultured cells. (A) Cortisol decreased p-p53 Ser15 levels, p53 accumulation, and induction of p21 protein in response to IR. HCT116 p53+/+ cells were treated with cortisol for 12 h, followed by IR, and analyzed at 6 h after IR. (B) RU486, a GR antagonist, blocked the effect of cortisol on p-p53 Ser15 levels, p53 accumulation, induction of p21 protein in response to IR in HCT116 p53+/+ cells, as determined at 6 h after IR. (C) Knockdown of GR by siRNA blocked the effect of cortisol on IR-induced p53 accumulation in HCT116 p53+/+ cells (Left). Knockdown of GR mRNA levels was confirmed by Taqman real-time PCR and normalized with actin (Right). (D) Corticosterone (CORT) decreased p-p53 Ser18 levels and p53 accumulation induced by IR in p53+/+ MEFs. Cells were treated with corticosterone for 12 h, followed by IR, and analyzed at 6 h after IR. (E) RU486 blocked the effect of corticosterone on IR-induced p53 accumulation in MEFs. (F) Cortisol (200 nM for 12 h) decreased p53 transcriptional induction of p21 and Puma in response to IR, which can be blocked by RU486 in HCT116 p53+/+ cells. mRNA levels were determined at 6 h after IR by Taqman real-time PCR and normalized with actin. (G) Cortisol (200 nM for 12 h) decreased p53-mediated apoptosis induced by IR (4 Gy) in HCT116 p53+/+ cells, which can be blocked by RU486, as determined at 36 h after IR by annexin V staining. HCT116 p53−/− cells were used as negative controls in F and G. Con, control.
The impact of cortisol upon p53-mediated transcriptional activity and apoptosis was determined in HCT116 p53+/+ cells with or without cortisol treatment followed by IR (4 Gy). Cortisol significantly decreased the induction of p53 target genes, including p21 and Puma (Fig. 4F). The decrease of p21 induction was confirmed at the protein level (Fig. 4A). Similar results were also observed in MEFs treated with corticosterone (Fig. S4). Cortisol clearly decreased p53-mediated apoptosis induced by IR (Fig. 4G). The inhibitory effect of cortisol on the p53-mediated transcriptional activity and apoptosis was abolished in RU486-treated cells (Fig. 4 F and G). These results demonstrate that glucocorticoids reduce p53 protein levels and functions, including transcriptional activity and apoptosis.
Glucocorticoids Decrease p53 Protein Levels and Function in Vivo.
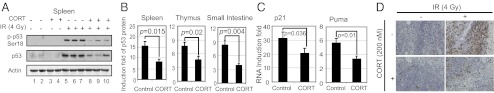
Repeated s.c. injection of corticosterone can mimic stress-induced elevation of corticosterone levels and induces depression in animal models (15). To investigate the effect of glucocorticoids on p53 in vivo, p53+/+ C57BL6/J mice were injected with corticosterone (20 mg/kg) for 1 wk followed by IR (4 Gy). Corticosterone decreased p53 protein accumulation in response to IR in the spleen (Fig. 5 A and B), thymus, and small intestine (Fig. 5B). Furthermore, corticosterone injection decreased p53 function in transcriptional induction of p21 and Puma by IR as demonstrated in the spleen (Fig. 5C). Corticosterone injection also reduced IR-induced apoptosis as determined by the TUNEL assays in the spleen (Fig. 5D) and small intestine (Fig. S5).
Fig. 5.
Corticosterone decreases p53 protein levels and function in vivo. p53+/+ C57BL6/J male mice were s.c. injected with corticosterone (CORT; 20 mg/kg) daily for 1 wk. Mice with or without corticosterone injection were exposed to IR, and various tissues (spleen, thymus, and small intestine) were collected at different time points after IR (n = 5 per group). (A and B) Corticosterone injection decreased the accumulation of p53 protein in response to IR in vivo. Levels of p53 protein in various tissues were determined at 6 h after IR by Western blot (A) and ELISA (B) assays, respectively. (C) Corticosterone injection decreased the transcriptional induction of p53 targets in response to IR in vivo. mRNA levels of p21 and Puma were determined at 6 h after IR in the spleen by Taqman real-time PCR and normalized with actin. (D) Corticosterone injection greatly decreased p53-mediated apoptosis in spleen in response to IR. TUNEL assay was used to detect apoptotic cells in the spleen at 12 h after IR.
SGK1 Mediates the Inhibitory Effect of Glucocorticoids on p53 Protein.
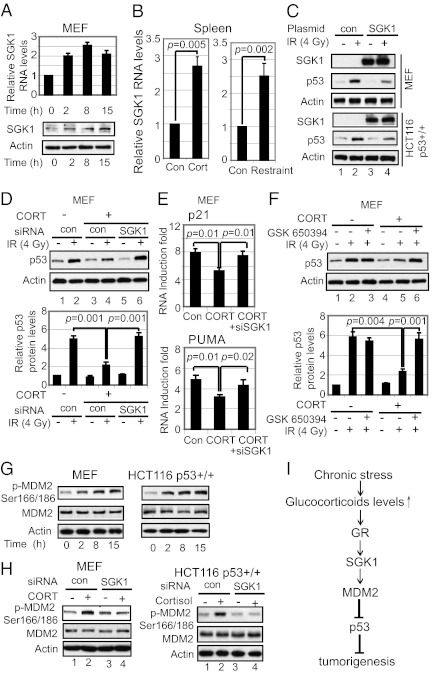
Glucocorticoids exert most of their functions through binding to GR, which functions as a transcription factor to regulate a group of genes (16). SGK1, a ubiquitously expressed serine-threonine kinase, can be transcriptionally induced by glucocorticoids (17). Consistent with the previous report (17), corticosterone clearly increased the expression of SGK1 at both RNA and protein levels in MEFs (Fig. 6A), whereas IR had no obvious effect on the expression of SGK1 (Fig. S6A). Furthermore, both chronic restraint and corticosterone injection clearly induced the expression levels of SGK1 in mice (Fig. 6B). SGK1 shares high sequence homology with AKT (∼50% through their catalytic domain) and a similar consensus phosphorylation site RXRXXS/T with AKT (17), which negatively regulates p53 function through the activation of MDM2. A recent study reported that SGK1 negatively regulates p53 in several human cancer cell lines through the activation of MDM2 (18). This raises the possibility that the induction of SGK1 under chronic restraint may mediate the effect of glucocorticoids on p53. Indeed, SGK1 overexpression in both MEFs and HCT116 p53+/+ cells by SGK1 expression plasmid decreased p53 accumulation in response to IR (Fig. 6C), which clearly demonstrates that SGK1 negatively regulates p53 function. Furthermore, knockdown of SGK1 by siRNA (Fig. S6B) abolished the inhibitory effect of glucocorticoids on p53 accumulation (Fig. 6D) and transcriptional activity (Fig. 6E) in response to IR in MEFs. Similar results were observed for two different siRNA oligos against SGK1. These results were confirmed by blocking the function of SGK1 with a SGK1-specific inhibitor, GSK650394 (19), in MEFs (Fig. 6F). Similarly, blocking SGK1 function by siRNA or GSK650394 abolished the inhibitory effect of cortisol on p53 accumulation in response to IR in HCT116 p53+/+ cells (Fig. S7). These results indicate that SGK1 mediates the inhibitory effect of glucocorticoids on p53 function.
Fig. 6.
SGK1 mediates the inhibitory effect of glucocorticoids on p53. (A and B) Corticosterone induced SGK1 in cultured cells and in vivo. (A) mRNA and protein levels of SGK1 were determined in p53+/+ MEFs treated with corticosterone (200 nM). (B) SGK1 mRNA levels were determined in the spleen of mice with corticosterone (CORT) injection (Left) or chronic restraint (Right). Con, control. (C) SGK1 overexpression by expression plasmid (pCMV-SGK1) decreased IR-induced p53 protein accumulation in MEFs (Upper) and HCT116 p53+/+ cells (Lower) as determined at 6 h after IR. (D–F) Blocking SGK1 function abolished the inhibitory effect of corticosterone on p53. Blocking SGK1 by SGK1 siRNA (siSGK1) (D and E) or a SGK1 inhibitor, GSK650394 (200 nM) (F) abolished the effect of corticosterone (CORT; 200 nM) on p53 accumulation (D and F), and p53 transcriptional activity (E) in response to IR in MEFs. Bar graphs in D and F represent the quantification of p53 levels in Western blot assays. (G) Glucocorticoids increased phosphorylation of MDM2 on Ser166/186 (p-MDM2 Ser166/186) in MEFs treated with corticosterone (200 nM) (Left) and HCT116 p53+/+ cells treated with cortisol (200 nM) (Right) for different hours. (H) Knockdown of SGK1 by siRNA blocked the increase of p-MDM2 Ser166/186 in MEFs treated with corticosterone (CORT; 200 nM) (Left) and HCT116 p53+/+ cells treated with cortisol (200 nM) (Right). (I) Schematic model depicting the negative regulation of p53 by chronic stress, which could be an important mechanism that contributes to tumor promoting effect of chronic stress. Positive and negative regulatory interactions are indicated.
AKT can negatively regulate p53 through phosphorylation of MDM2, a key negative regulator of p53, on Ser166/186 to increase MDM2 activity (20). Glucocorticoid treatment increased the phosphorylation of MDM2 on Ser166/186 in both MEFs and HCT116 p53+/+ cells (Fig. 6G), which raised the possibility that SGK1 mediates the inhibitory effect of glucocorticoids on p53 through increasing MDM2 activity. Indeed, the increase of MDM2 phosphorylation by glucocorticoids largely disappeared in cells when SGK1 was knocked down by siRNA (Fig. 6H). These results demonstrate that the induction of SGK1 by glucocorticoids mediates the inhibitory effect of glucocorticoids on p53 through increasing MDM2 activity.
Discussion
Epidemiological studies have strongly suggested that chronic psychological stress promotes tumor development. However, the role of chronic stress in tumorigenesis remains controversial and has not been well established in animal models. Chronic restraint and social isolation in mice are two well-established chronic stress models that mimic stress-induced neuroendocrine responses in humans and induce depression- and anxiety-like states in mice. Most studies used xenograft tumor assays to study the effect of chronic restraint or social isolation on tumor growth (9, 10, 21, 22). However, in xenograft tumor assays, the tumor growth could be greatly affected by the properties of the injected cancer cells in addition to the condition of the host. Here, the impact of chronic restraint upon IR-induced tumorigenesis was directly investigated in vivo. In p53+/− mice, chronic restraint greatly promoted IR-induced tumorigenesis. These results provide direct evidence that chronic stress promotes tumorigenesis in vivo.
The mechanisms by which chronic psychological stress promotes tumorigenesis remain unknown. Many previous studies focused upon the down-regulation of the immune system. For instance, it has been reported that chronic stress decreases immune functions, including natural killer (NK) cell cytotoxicity and T-cell responses to mitogen stimulation (23, 24). Previous studies (10) and this study show that the promoting effect of chronic stress on the growth of xenograft tumors can be observed in immune-deficient nude mice with defective T-cell systems but intact NK cell activity, suggesting that additional mechanisms contribute to tumor promoting effect of chronic stress. Indeed, this study demonstrates that the attenuation of p53 could be an important part of the mechanism by which chronic stress promotes tumorigenesis. Our results demonstrated that chronic stress decreases p53 protein levels and function in mice. Furthermore, chronic restraint of the host promotes the growth of a xenograft tumor in a largely p53-dependent manner. Considering the critical role of p53 in preventing the initiation and/or progression of cancer, the attenuation of p53 function by chronic stress could lead to the promoting effect of chronic stress on the initiation and/or progression of cancer. This study further demonstrates that glucocortioids released during chronic stress decrease p53 protein levels and function through induction of SGK1, which in turn increases MDM2 activity to down-regulate p53 (Fig. 6I). These results provide a mechanism by which chronic restraint decreases p53 function. This study expands our understanding of the biological pathways by which chronic stress regulates cancer pathogenesis and demonstrates that the systemic communication between the circulation of neurohormones and the molecular pathways within the cells enables chronic psychological stress to promote the initiation and propagation of tumorigenesis. These results suggest that the reactivation and/or restoration of p53 functions may prevent tumor initiation, maintenance, and progression during or after chronic stress.
Materials and Methods
Mouse strains used in this study include the p53+/+, p53+/−, and p53−/− C57BL6/J mice, BALB/c nu/nu athymic nude mice. Mouse restraint system (flat bottom restrainers; Kent Scientific) was used for chronic periodic restraint. Details for mouse strains, chronic restraint, IR and corticosterone administration, Western blot and ELISAs, Taqman real-time PCR, apoptosis analysis, xenograft tumorigencity analysis, cell culture and treatments, and statistic analysis are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
W.H. is supported by National Institutes of Health (NIH) Grant 1P30CA147892-01, Department of Defense Grant W81XWH-10-1-0435, CINJ new investigator award, and the Ellison Foundation. Z.F. is supported by NIH Grant 1R01CA143204-01 and the New Jersey Commission on Cancer Research (NJCCR). C.Z. is supported by a postdoctoral grant from NJCCR.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203930109/-/DCSupplemental.
References
- 1.Levine AJ, Hu W, Feng Z. The P53 pathway: What questions remain to be explored? Cell Death Differ. 2006;13:1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 2.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 3.Olivier M, Hussain SP, Caron de Fromentel C, Hainaut P, Harris CC. TP53 mutation spectra and load: A tool for generating hypotheses on the etiology of cancer. IARC Sci Publ. 2004;(157):247–270. [PubMed] [Google Scholar]
- 4.Donehower LA, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 5.Lillberg K, et al. Stressful life events and risk of breast cancer in 10,808 women: A cohort study. Am J Epidemiol. 2003;157:415–423. doi: 10.1093/aje/kwg002. [DOI] [PubMed] [Google Scholar]
- 6.Buccheri G. Depressive reactions to lung cancer are common and often followed by a poor outcome. Eur Respir J. 1998;11:173–178. doi: 10.1183/09031936.98.11010173. [DOI] [PubMed] [Google Scholar]
- 7.Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94:2719–2727. doi: 10.1002/cncr.10533. [DOI] [PubMed] [Google Scholar]
- 8.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 9.Villano Bonamin L, Barbuto JA, Malucelli BE. Effects of social isolation on ehrlich tumor growth and tumor leukocyte infiltration in mice: Evidence of participation of the submaxillary salivary gland. Neuroimmunomodulation. 2001;9:313–318. doi: 10.1159/000059388. [DOI] [PubMed] [Google Scholar]
- 10.Thaker PH, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 11.Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet. 1994;8:66–69. doi: 10.1038/ng0994-66. [DOI] [PubMed] [Google Scholar]
- 12.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, et al. Corticosteroid co-treatment induces resistance to chemotherapy in surgical resections, xenografts and established cell lines of pancreatic cancer. BMC Cancer. 2006;6:61. doi: 10.1186/1471-2407-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, et al. A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol. 2008;581:113–120. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Revollo JR, Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann N Y Acad Sci. 2009;1179:167–178. doi: 10.1111/j.1749-6632.2009.04986.x. [DOI] [PubMed] [Google Scholar]
- 17.Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amato R, et al. Sgk1 activates MDM2-dependent p53 degradation and affects cell proliferation, survival, and differentiation. J Mol Med (Berl) 2009;87:1221–1239. doi: 10.1007/s00109-009-0525-5. [DOI] [PubMed] [Google Scholar]
- 19.Sherk AB, et al. Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res. 2008;68:7475–7483. doi: 10.1158/0008-5472.CAN-08-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou BP, et al. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 21.Mazur-Kolecka B, et al. Effect of immobilization stress on tumor growth in mice. Neoplasma. 1994;41:183–186. [PubMed] [Google Scholar]
- 22.Hasegawa H, Saiki I. Psychosocial stress augments tumor development through β-adrenergic activation in mice. Jpn J Cancer Res. 2002;93:729–735. doi: 10.1111/j.1349-7006.2002.tb01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser R, et al. Evidence for a shift in the Th-1 to Th-2 cytokine response associated with chronic stress and aging. J Gerontol A Biol Sci Med Sci. 2001;56:M477–M482. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- 24.Lutgendorf SK, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.