Many vital epithelial organs develop in the embryo from simple tubes that branch and ramify into complex, treelike structures. This tubular morphology greatly increases the total cellular area available to the body for metabolic processes, such as nutrient and gas exchange, while reducing the distances over which substances have to travel (1). Epithelial organs such as kidney and mammary gland can be viewed as a complex network of networks of tubes of many kinds. Proper functioning of the body requires perfect matching and coordination between these networks, which keep changing during life.
Tubulogenesis is an essential process in embryonic development, giving rise to many types of tubules. Despite decades of much-improved understanding of tubulogenesis, we are still baffled by questions regarding the fundamental mechanisms by which cells can organize into precise networks of tubular structures (2). Evidently, new ideas are needed to resolve this critical puzzle. In PNAS, Guo et al. (3) discover unique cellular mechanisms that govern the branching morphogenesis process. These mechanisms are found to be derived and induced by mechanical forces that include crosstalk between acini as detailed further below. To place the unique findings in a broader context we describe additional mechanisms that are involved in epithelial tubulogenesis and discuss their relevance for tumor development.
What Supervises the Tubulogenesis Master Plan?
The organization of epithelial cells into tubular structures is a complex task involving self-propelled cell rearrangements that require control of both cell adhesion and migration followed by formation of branched hollow tubules lined by polarized cells (4). This process has to be conducted in a precise, supervised yet flexible manner. The different cellular building blocks self-assemble to form an intricate structure while they move, change shape, proliferate, and differentiate (some also die). All cells have the same blueprint encoded by their genome. This blueprint is differentially transcribed and translated in each cell, generating the ability to perform specific cellular functions in coordination with other cells. It is as if a team of constructors, each with their own blueprint, are trying to build a high-rise without a master plan or supervisors. The molecular, physical, and cellular mechanisms by which individual cells interact to coordinate their positioning over long spatial scales and the effects of the microenvironment on this morphogenesis process are not fully understood. The emerging picture is that each cell is a specialized unit with a unique function, a self-propelled constructor, and a supervisor in the master plan.
Supervision and Cues from Mechanical Forces.
The mechanisms for tubulogenesis described by Guo et al. (3) are derived and induced by mechanical forces that include crosstalk between acini. The authors study the effects of environmental cues provided by collagen density on the mechanical forces that induce mammary cell tubulogenesis, using a 3D normal mammary cellular model. They demonstrate that epithelial cells develop various morphological patterns in response to minute changes of collagen percentage in the ECM. These patterns are formed and maintained by traction forces generated by cells rather than by cell-secreted diffusible growth factors. Collagen-dependent transmission of force in the ECM leads to interactions between distant cells located up to 600 μm apart. Branching morphogenesis was discovered to be dependent on a mechanical feedback effect: Cells apply traction forces to induce motion; and moving cells change collagen distribution and orientations, which in turn induce traction forces. This feedback leads to a bistable state in the formation of linear, tubule-like patterns: either globular aggregates or linear tubular structures. Using micropatterning techniques, the authors demonstrate that the stability of tubule-like patterns depends on the tubule length. Another important finding is that tubule formation can be achieved by cell migration between two interacting acini and not only by repositioning of cells from a single acinus.
Soluble Growth Factors as Part of the Master Regulation Scheme.
Hepatocyte growth factor/scatter factor (HGF/SF)-Met signaling is known to induce tubulogenesis in an in vitro tubulogenesis model system of Madin-Darby canine kidney (MDCK) epithelial cells (5). HGF/SF stimulation induces membrane protrusions of individual MDCK cells in the cyst that extend into the extracellular matrix. Each acinus develops chains of cells that are connected to the cyst. Next, HGF/SF induces the protrusions to form cords that are two to three cells thick and develop discontinuous lumens. Finally, the discontinuous lumens grow and coalesce to become continuous with the lumen of the cyst (Fig. 1) (6). HGF/SF has been shown to induce epithelial cell tubulogenesis in collagen and matrigel in many types of epithelial cells and in the development of mammary tubular structures in vivo (7). Tubulogenesis is influenced by both tubulogenesis-facilitating growth factors (such as HGF/SF, epidermal growth factor receptor ligands, and insulin-like growth factors) and inhibitory growth factors (such as transforming growth factor-β family members). The balance between these two groups of growth factors is assumed to play a central role in branching morphogenesis regulation (8). A tubule is induced from one cyst and contains a lumen. Growth factors induce epithelial cell proliferation and migration and modulate the expression of a variety of proteins. On the basis of these models tubulogenesis consists of four distinct stages: (i) extensions, (ii) chains, (iii) cords, and (iv) tubules. In conclusion, growth factors play a major role in the development of epithelial tubules of many organs.
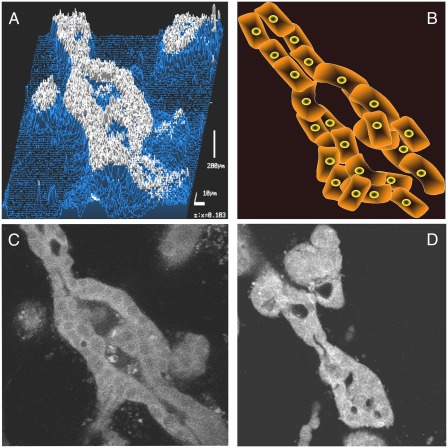
Fig. 1.
(A–D) MDCK cells (C) and DA3 mouse mammary tumor cells expressing dominant negative (DN) Met (D) were grown in collagen gels in the presence of 80 ng HGF/SF for 7 d, fixed, stained for pan-cytokeratin expression, and analyzed by confocal laser scanning microscopy. (A) Topography analysis of cytokeratin expression. (B) Schematic representation of tubule formation. (C and D) Tubulogenesis in (C) MDCK and (D) DA3 DN-Met cells.
The Supervising Role of the Extracellular Matrix.
The ECM is a key component in a cell’s microenvironment and is responsible for directing cell fate and maintaining tissue specificity. Bidirectional crosstalk exists between the nucleus and the chromatin of a cell and its surrounding ECM (“dynamic reciprocity”), where the ECM influences gene expression and the cell, in turn, remodels the ECM, which then further acts on the cell, creating a feedback loop (9). The ability of cells to interact with the matrix environment is an important determinant of tubulogenesis and branching morphogenesis. Among the candidate molecules likely to be important in matrix modulation are extracellular proteases, extracellular matrix proteins, and integrins (8). Growth factors serve as soluble cues that synergize with insoluble cues from the ECM to dictate the morphogenesis of the ductal tree. Mammary tubule growth and remodeling is controlled by the ECM, especially by its degrading enzymes (matrix metalloproteinases) and their inhibitors (tissue inhibitors of metalloproteinases) that play
Guo et al. discover unique cellular mechanisms that govern the branching morphogenesis process.
significant roles in this regard (10). In summary it has been demonstrated that the ECM plays multiple roles in epithelial tubule formation.
2D Cues.
We have previously shown that HGF/SF induces formation of lumen-like structures of human epithelial carcinoma cell lines on 2D surfaces (11). It was recently shown that large (2 mm) functional human kidney tubules can be generated in vitro on 2D without the use of 3D ECM. Tubulogenesis on 2D surfaces involves interactions between epithelial and mesenchymal cells. The process is induced by transforming growth factor-β (1) and enhanced by a 3D substrate architecture. However, after triggering the process, the formation of tubules is independent from the substrate architecture (12). These results further demonstrate that there are different mechanisms to generate a tubule.
Tubules Form from a Single Acinus or Crosstalk Between Acini.
Guo et al. (3) elegantly show that the acinus–acinus interaction can form tubular structures. This interaction is mediated by mechanical forces induced and modulated by collagen. It was demonstrated in many papers that tubules can arise from a single acinus and that tubulogenesis can be induced by soluble growth factors. When the tubule is formed by a single acinus and is induced by growth factors/morphogens, the mechanical crosstalk between the cells is less crucial and the dependence on mechanical force through collagen is no longer necessary.
Looking Ahead.
Loss of normal tubular and glandular structures is one of the primary characteristics of breast tumors. Rather than forming organized tubular structures, tumor cells proliferate and grow out of the ducts. A poor degree of differentiation or tubular organization correlates with a poor prognosis in invasive ductal carcinoma. We have previously shown that down-regulation of HGF/SF signaling in aggressive breast cancer cells, using a dominant negative form of the receptor, results in increased tubulogenesis. Thus, alterations in HGF/SF-Met signaling can shift the balance between differentiation and invasiveness, changing the cells’ fate from nondifferentiated invasive cells to gland-forming cells (13) (Fig. 1). By improving our understanding of epithelial cell tubulogenesis (Fig. 2) in vitro in general and studying the role of mechanical force and the interaction of acini as performed by Guo et al. in particular (3), we would be able to better understand the development of normal breast ducts and the abrogation of the normal tubular structures in breast cancer. Down-regulating the growth factor signaling in combination with increasing the trajectory forces mediating signaling could be the basis of a unique anti-breast cancer therapeutic modality.
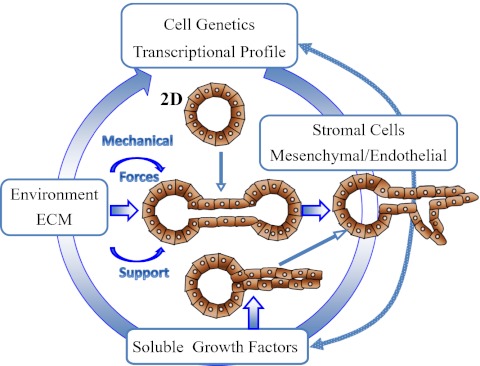
Fig. 2.
Schematic representation of the different factors effecting tubulogenesis and the different cellular mechanisms of tubule formation.
Acknowledgments
We thank Dr. Rom Altstock for providing data. Funding for this work was provided in part by the Breast Cancer Research Foundation, the Binational Science Foundation, the Tauber Family Foundation, and the University of Tel Aviv.
Footnotes
The authors declare no conflict of interest.
See companion article on page 5576 of issue 15 in volume 109.
References
- 1.Hogan BL, Kolodziej PA. Organogenesis: Molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Fraticelli AE, Gálvez-Santisteban M, Martín-Belmonte F. Divide and polarize: Recent advances in the molecular mechanism regulating epithelial tubulogenesis. Curr Opin Cell Biol. 2011;23:638–646. doi: 10.1016/j.ceb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Guo C-L, et al. Long-range mechanical force enables self-assembly of epithelial tubular patterns. Proc Natl Acad Sci USA. 2012;109:5576–5582. doi: 10.1073/pnas.1114781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosário M, Birchmeier W. How to make tubes: Signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003;13:328–335. doi: 10.1016/s0962-8924(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 5.Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 6.Pollack AL, Runyan RB, Mostov KE. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- 7.Pepper MS, et al. Modulation of hepatocyte growth factor and c-met in the rat mammary gland during pregnancy, lactation, and involution. Exp Cell Res. 1995;219:204–210. doi: 10.1006/excr.1995.1220. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai H, Nigam SK. In vitro branching tubulogenesis: Implications for developmental and cystic disorders, nephron number, renal repair, and nephron engineering. Kidney Int. 1998;54:14–26. doi: 10.1046/j.1523-1755.1998.00969.x. [DOI] [PubMed] [Google Scholar]
- 9.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: Insights from imaging. Histochem Cell Biol. 2008;130:1105–1118. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsarfaty I, et al. The met proto-oncogene receptor and lumen formation. Science. 1992;257:1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, et al. Generation of easily accessible human kidney tubules on two-dimensional surfaces in vitro. J Cell Mol Med. 2011;15:1287–1298. doi: 10.1111/j.1582-4934.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firon M, et al. Dominant negative Met reduces tumorigenicity-metastasis and increases tubule formation in mammary cells. Oncogene. 2000;19:2386–2397. doi: 10.1038/sj.onc.1203557. [DOI] [PubMed] [Google Scholar]