Abstract
Recent research suggests that an attentional bias towards threat may play a causal role in obsessive-compulsive disorder (OCD) with contamination concerns. However, the attentional components involved in this bias, as well as its behavioral correlates, remain unclear. In the present study, eye movements were recorded in individuals high and low in contamination fear (HCF, LCF) during 30 s exposures to stimulus arrays containing contamination threat, general threat, pleasant, and neutral images. HCF individuals oriented gaze towards contamination threat more often than LCF individuals in initial fixations, and this bias mediated group differences in responding to a behavioral challenge in a public restroom. No group differences were found in the maintenance of gaze on contamination threat, both in terms of initial gaze encounters as well as gaze duration over time. However, the HCF group made shorter fixations on contamination threat relative to other image types. The implications of these findings for further delineating the nature and function of attentional biases in contamination-based OCD are discussed.
Keywords: Eye movements, Contamination, OCD, Bias, Emotion
Contamination fear is the most common theme in obsessive-compulsive disorder (OCD), present in roughly 50% of OCD patients (Rasmussen & Eisen, 1992). The washing rituals associated with contamination obsessions often cause considerable functional impairment, highlighting the importance of gaining insight into underlying etiological processes (Rachman, 2004). A potential mechanism in all anxiety disorders is attentional bias towards threat (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). However, some have argued that this mechanism is less relevant to OCD, because OC-related behaviors (e.g., ordering rituals) often function to reduce tension rather than to prevent harm (Summerfeldt & Endler, 1998). Indeed, many OCD patients do not report anticipating a specific threat (American Psychological Association [DSM-IV-TR], 2000), in which case there would be no target for an attentional bias. In contamination-based OCD, however, washing rituals are typically performed in response to an identifiable threat––contaminants or contaminated objects––that may inspire vigilance (Summerfeldt & Endler, 1998). Accordingly, attentional bias for threat could play a more significant role in contamination-based OCD compared to other subtypes of the disorder.
Although attentional bias for threat is often treated as a unitary construct, contamination-based OCD may be associated with multiple attentional biases that depend on the time point of stimulus exposure (Cisler & Koster, 2010). For example, by varying stimulus onset asynchrony, recent iterations of the modified dot probe and emotional spatial cueing task have revealed distinct effects of threat on the orienting and maintenance of attention in anxiety (Cisler, Bacon, & Williams, 2009). In addition, eye tracking technology, which allows continuous measurement of saccadic eye movements, has revealed novel attentional biases beyond the early window ofvisual processing (e.g., 0-500 ms) typically assessed by reaction time measures. On this larger time scale (e.g., 0-30 s), qualitatively different attentional biases related to anxiety may emerge, including reduced (Rinck & Becker, 2006) or fragmented viewing of threat (Beevers, Lee, Wells, Ellis, & Telch, in press).
While eye tracking research in anxiety disorders is expanding rapidly, only one study has applied the methodology to understanding attentional biases related to OCD. Armstrong, Olatunji, Sarawgi, and Simmons (2010) found that individuals high versus low in contamination concerns were more likely to orient gaze to a fearful expression at the beginning of a trial, and maintained gaze longer on both disgusted and fearful expression throughout 3 s trials (as opposed to exhibiting avoidance). However, the implications of these findings were not entirely clear, due to the ambiguous relations between facial stimuli and contamination concerns. Indeed, the gaze biases observed by Armstrong et al. (2010) did not show the predicted specificity to disgusted expressions, which should be more likely than fearful expressions to elicit contamination concerns (e.g., Lawrence et al., 2007). In addition, the 3 s trials may have been too short to capture biases that emerge over prolonged stimulus exposure. Additional eye tracking research employing images of actual contaminants, presented for longer durations, would enhance understanding of overt attentional biases associated with contamination concerns.
Relating attentional biases associated with contamination concerns to “real-world” outcomes may also enhance current understanding of the function of such biases. In research on addiction, an attentional bias towards substance-related stimuli has been shown to predict drug relapse (e.g., Marissen et al., 2006). Similarly, a bias towards suicide-related stimuli has been found to predict future suicide attempts (Cha, Najmi, Park, Finn, & Nock, 2010). Given the emphasis on attentional biases in theoretical accounts of contamination-based OCD (Summerfeldt & Endler, 1998), an analogous association between attentional bias for threat and an ecologically valid, behavioral outcome would be expected. For example, an attention bias for contamination may predict behavioral avoidance of contamination risks encountered in everyday life. Such a correlation would suggest a common disease mechanism underlying attentional biases and behavioral avoidance, or perhaps a causal relationship, as suggested by recent attention retraining studies finding proximal effects of bias reduction on behavioral outcomes (Najmi & Amir, 2010).
With these considerations in mind, the present study examined the time course and components of overt attentional biases toward threat in contamination fear, and the relation of such biases to behavioral avoidance and distress during a public restroom behavioral avoidance task (BAT). To parse orienting and maintenance of gaze, and to probe later avoidance, eye movements were recorded in individuals high and low in contamination fear (HCF, LCF) during 30 s exposures to stimulus arrays containing contamination threat, general threat, pleasant, and neutral images. It was predicted that compared to the LCF group, the HCF group would show biases toward contamination threat in the location (orienting) and duration (maintenance) of initial fixation. Further, it was predicted that the HCF group would show greater attentional avoidance of contamination threat, compared to the LCF group, over the course of the 30 s trials. Biases were predicted for contamination threat specifically, and not for threat in general, as the HCF group should be distinguished by a bias for content consistent with their symptom concern.
Methods
Participants
Three large undergraduate classes (n = 289) were screened using the Padua Inventory contamination fear subscale (PI; Burns, Keortge, Formea, & Sternberger, 1996), in order to identify students high and low in contamination concerns. Using criteria informed by the PI means of OCD patients and healthy controls (Burns et al., 1996), individuals were recruited to form a high (PI total score > 13) contamination fear group (HCF; n = 19; PI M = 22.21, SD = 6.18; age M = 19.25, SD = 1.02; % female = 68; % Caucasian = 85), and low (PI total score < 6) contamination fear group (LCF; n = 20; PI M = 3.25, SD = 1.71; age M = 19.26, SD = .81; % female = 35; % Caucasian = 79). Similar methods for identifying analogue contamination fear groups have been employed in prior studies (e.g., Olatunji, Lohr, Sawchuk, & Tolin, 2007).
Measures
The Padua Inventory (PI; Burns et al., 1996) contamination fear subscale is a 10-item measure of contamination obsessions and washing compulsions. The PI contamination fear subscale had an alpha coefficient of .96 in the present study.
The Disgust Scale—Revised (DS-R; Olatunji et al., 2007) is a 25-item questionnaire assessing sensitivity to a range of disgust elicitors, including core, animal-reminder, and contamination disgust. The DS-R had an alpha coefficient of .83 in the present study.
The Positive Affect Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) consists of two 10-item scales that assess the extent to which a person experiences positive and negative mood states. The 10-item Negative Affect (NA) scale, which assesses the extent to which a person generally experiences subjective distress such as anxiety, fear, guilt, and shame, was used in the present study. The NA had an alpha coefficient of .88 in the present study.
Public restroom BAT
Participants were led into a nearby public restroom, and were asked to touch surfaces that sampled a spectrum of perceived contamination risk. Participants were asked to touch inside of the sink, inside of the trashcan, on the toilet seat, on the rim below the toilet seat, and inside of the toilet (in that order). After each step, experienced distress was rated verbally on a 0 (no distress) to10 (extreme distress) scale. If participants declined to complete a step, they were asked to imagine completing the step with their eyes closed, and then provide a rating. Number of steps completed and total distress ratings over the 5 steps were recorded. Given the high correlation between these response indices (r = −.66, p < .001), a composite score was computed by 1) reverse scoring steps completed, 2) converting both variables to percentages of the highest possible value, and 3) averaging these two percentages. Previous research (Olatunji & Armstrong, 2009) suggests that behavioral avoidance and distress on this task are reliable indicators of contamination concerns related to OCD.
Materials and Apparatus
Stimuli were primarily selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005), with additional images selected from publically available online sources. Fifty-two images were used in the experiment, consisting of 13 images in each of the following categories: contamination threat (e.g., man walking through landfill, stool sample), general threat (e.g., gun, masked man with knife), pleasant (e.g., children playing, cute animals), and neutral images (e.g., buildings, landscapes). One image from each category was used in a practice trial. Images were rated by an independent sample (n = 16, age: M = 22.88, SD = 2.70; % female = 63; % Caucasian = 75) in terms of how pleasant-unpleasant, aroused, afraid, and disgusted they made the participant feel, using a 0-6 Likert scale (0 = “extremely unpleasant,” 6 = “extremely pleasant” for pleasant-unpleasant ratings; 0 = “not at all,” 6 = “extremely” for all other ratings). Of most relevance, contamination threat elicited more disgust than all other image types (ps < .001); general threat elicited more fear (ps < .001) and arousal (ps < .02) than all other image types; and pleasant images elicited more pleasant affect (ps < .001) than all other image types (ps < .02).
Images were sized to fit 13.35° × 7.85° frames, and presented in 2 × 2 arrays consisting of one image from each category. The images were presented against a black background, with their innermost corners 3.15° from the central fixation point, and their centers 10.85° from the central fixation point. Stimuli were presented using E-Prime version 1.0 software on a 17-in. widescreen monitor (1280 × 1024 resolution, 60 Hz). Eye movements were recorded with the iView X RED-III system from SensoMotoric Instruments (SMI), a video-based eye tracker with a dark pupil tracking method. This system has a sampling rate of 60 Hz, and a spatial resolution of .5°-1°. Participants’ heads were stabilized with a chinrest at a viewing distance of 60.5 cm.
Procedure
Following completion of measures, participants read instructions explaining the eye tracking task. To conceal the recording of gaze, the eye tracking cameras were said to measure pupil dilation during the task, and participants were asked to respond to the fixation target (“x” or “o”) by pressing keys with corresponding labels (Caseras, Garner, Bradley, & Mogg, 2007). The fixation image offset after participants responded, or after 700 ms, depending on which occurred first. A stimulus array was then presented for 30 s, followed by an inter-trial interval of 3000 ms. Participants were instructed to fixate on the central target prior to stimulus onset. During stimulus presentation, participants were asked to view the images as they pleased, and not to look away from the monitor. There was one 30 s practice trial, after which a 9-point calibration procedure was completed. There were 12 experimental trials, presented in pseudorandom order and divided into 4 blocks of 3. Images were only shown once, and location was balanced across image types. After each block prior to the last, participants were given a brief resting period before the calibration procedure was repeated. Participants then completed the BAT.
Eye movement data reduction
Eye movement events (saccades, fixations, blinks) were defined using BeGaze 1.0 software from SensoMotoric Industries (SMI). Gaze direction was sampled every 16.7 ms, with a fixation classified as 80 ms or more in which gaze was stable within 1.5° of visual angle. Areas of interest were defined as rectangles containing each image, as well as a circle with 1.5° radius at the location of the fixation target (central region). Trials were excluded from initial fixation analyses if gaze was not directed at the central region for at least 80 ms following stimulus onset. After removing blocks of trials with poor system calibration (3.21% of trials), invalid first fixations occurred on 11.1% of trials. Independent samples t-tests revealed that the amount of missing trials did not significantly differ between groups [t (38) = .64, p > .05].
For each participant, orienting of gaze was assessed by computing the number of trials in which the image type of interest captured the initial fixation, divided by the total number of valid trials1. For trials in which the image type of interest captured the initial fixation, subsequent maintenance of gaze was assessed by summing the durations of consecutive fixations within the image (Garner et al., 2006). Attentional avoidance was assessed by examining maintenance of gaze as a function of time. Time spent fixating the image type of interest was computed for 6 time intervals: 0–5 s, 5 s–10 s, 10 s–15 s, 15 s–20 s, 20 s–25 s, 25 s–30 s ms. Total number of fixations and average length of fixations were also computed for each image type. For these latter measures, fixations were assigned to the time interval in which they began, and fixations terminated by trial offset were excluded when computing average fixation length. Kolmogorov-Smirnov tests were conducted to identify eye movement variables with distributions that differed from normality. Due to positive skew in the individual means for initial gaze duration, these variables were log transformed in order to impose a normal distribution. No other variables were found to have non-normal distributions.
Data analytic overview
To assess hypotheses regarding the orienting and maintenance of initial fixations, planned comparisons were used. Planned contrasts provide a more appropriate test of a priori predictions, compared to analyses of variance, which include all possible main effects and interactions (Wilkinson, 1999). However, analyses of variance were necessary for examining attentional avoidance, as the a priori prediction involved the comparison of linear trends revealed in interaction effects, as opposed to individual means.
Results
Group characteristics
Compared to the LCF group, the HCF group reported significantly higher levels of disgust sensitivity [M = 56.11 (SD = 10.98) versus M = 39.95 (SD = 12.23), t (37) = 4.33, p < .001], and negative affect [M = 22.63 (SD = 7.11) versus M = 17.70 (SD = 5.17), t (37) = 2.49, p < .05. During the public restroom BAT, the HCF group completed fewer steps and reported higher levels of distress compared to the LCF group [composite score: M = .53 (SD = .22) versus M = .24 (SD = .20), t (37) = 4.20, p < .001]. Groups did not significantly differ in age (t < 1, p >. 05), gender (χ2 = 4.36, p > .05), or ethnicity (χ2 = 3.10, p > .05).
Orienting of gaze
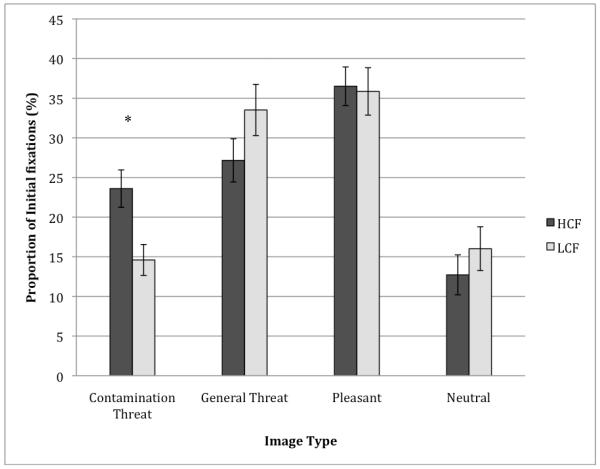
Orienting bias scores were compared to test the hypothesis that the HCF group would orient gaze toward the contamination threat image more often than the LCF group. As predicted, independent samples t-tests revealed that the HCF group oriented gaze toward contamination threat more often than the LCF group [M = 23.60% (SD = 10.28) versus M = 14.60% (SD = 8.74), t (37) = 2.95, p < .01] 2. As revealed in Figure 1, the HCF group did not significantly differ from the LCF group in orienting bias scores for the other image types [general threat: M = 27.16% (SD = 11.85) versus M = 33.52% (SD = 14.42); pleasant: M = 36.52% (SD = 10.58) versus M = 35.86% (SD = 13.38); neutral: M = 12.72% (SD = 11.00) versus M = 16.02% (SD = 12.34); all ts < 1.50, ps > .05].
Figure 1.
Group differences in orienting bias for each image type. Error bars represent standard error.
Maintenance of gaze during initial fixation
To test the hypothesis that HCF individuals would show increased initial maintenance of attention on contamination threat relative to the LCF group, average duration of initial gaze on contamination threat was compared. The HCF group did not significantly differ from the LCF group in maintenance of initial gaze on contamination threat [M = 764 ms (SD = 598) versus M = 1271 ms (SD = 1306); t (35) = 0.73, p > .05, after log transformation] or other image types [general threat: M = 747 ms (SD = 704) versus M = 766 ms (SD = 619); pleasant: M = 1018 ms (SD = 1305) versus M = 747 ms (SD = 531); neutral: M = 656 ms (SD = 926) versus M = 333 ms (SD = 388); all ts ≤ 1.00, p > .05, after log transformation].
Gaze patterns over 30 s
A series of 2 (group: HCF, LCF) X 4 (image type: contamination threat, general threat, pleasant, neutral) X 6 (time interval: 0-5, 5-10, 10-15, 15-20, 20-25, 25-30 s) mixed-factor Analyses of Variance (ANOVA) were conducted on total fixation duration, total number of fixations, and average fixation length, in order to test the hypothesis that HCF individuals would avoid visual contact with contamination threat more than LCF individuals. For terms involving repeated-measures factors, violations of sphericity were addressed with Greenhouse-Geisser adjustment of degrees of freedom. In the analyses of total fixation duration and total number of fixations, none of the predicted effects involving group were significant. However, group differences in gaze patterns were found in the analysis of average fixation lengths. Of most relevance to the present hypotheses, there was a significant group by image type interaction [F (3, 111) = 2.76, p < .05, pη2 = .07], which was not qualified by time interval [F (7.78, 287.88) = 1.14, p > .05, pη2 = .03].
To examine the interaction between group and image type, planned complex comparisons were used to determine if HCF participants made shorter fixations to contamination threat stimuli, compared to other stimulus types. In both groups, the average length of fixations on contamination threat was compared to the average length of fixations on other image types. In the HCF group, fixations on contamination threat (M = 186 ms, SD = 35) were shorter than fixations on other image types [M = 195 ms, SD = 37; t (18) = 2.65, p = .016]; in the LCF group, there was no difference between the length of fixation on contamination threat (M = 188 ms, SD = 34) and other image types (M = 184 ms, SD = 32; t (19) = 1.27, p > .05).
Orienting bias for contamination threat as a mediator of behavioral avoidance
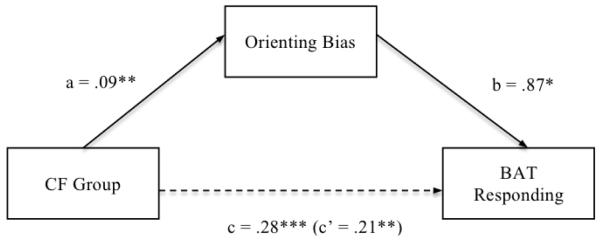
In light of recent findings suggesting that attentional biases may have proximal effects on both behavioral and affective responding to a stressor (Amir, Weber, Beard, Bomyea, & Taylor, 2008), we examined the possibility that group differences in responding to the public restroom BAT were partially mediated by group differences in orienting bias towards contamination threat. A test of mediation was conducted with Preacher and Hayes (2008) bootstrapping procedure, which does not impose distributional assumptions often violated in smaller samples. Of most relevance to the present hypotheses, the direct path from orienting bias for contamination threat to BAT responding was significant [B = .87, SE = .34, t (2, 35) = 2.55, p = .015], indicating that the bias uniquely predicted behavioral avoidance and distress, and the total indirect path from CF group to BAT responding via orienting bias for contamination threat was significant (p < .05), as indicated by the 95% confidence interval not containing zero (lower limit = .01, upper limit = .17; B = .08, SE = 0.04). Hence, significant mediation was demonstrated (see Figure 2).
Figure 2.
Mediational model of the relations between group, orienting bias for contamination threat, and BAT responding.
Note: All coefficients are unstandardized, CF = contamination fear, BAT = behavioral avoidance task, * p < .05, ** p < .01, *** p < .001
Discussion
The present study is the first, to our knowledge, to record eye movements in individuals with contamination fear during extended exposure to contamination threat. Inline with predictions, the HCF group oriented gaze towards contamination threat more frequently relative to the LCF group. This finding is consistent with prior research in contamination-based OCD that has observed vigilance for threat using other cognitive tasks such as the modified dot probe (Tata, Liebowitz, Prunty, Cameron, & Pickering, 1996). Contrary to predictions, the HCF group did not maintain initial fixations longer on contamination threat, or avoid viewing contamination threat on a larger time scale, compared to the LCF group. However, the HCF group was characterized by a fragmented viewing style for contamination threat relative to other images, as indicated by shorter individual fixations.
Spatial orienting towards contamination threat was also found to predict responding to potential contamination risk in a public restroom. In fact, group differences in spatial orienting bias for contamination threat were found to mediate group differences in BAT responding, suggesting that an orienting bias for contamination threat is one mechanism through which contamination fear increases behavioral avoidance and distress in response to everyday contamination threats. Although this finding is correlational in nature, it is corroborated by a recent study that manipulated attentional biases in HCF individuals. Najmi and Amir (2010) found that attention retraining for HCF individuals reduced behavioral avoidance on BATs, and that the proximal mechanism mediating the effect was reductions in an early attentional bias towards threat. A goal for future research is to delineate precise causal relations between attentional biases and behavior in contamination-based OCD. Whereas some effects of biases on behavior may be highly proximal—for example, biases may increase the detection of stimuli that cue avoidance (e.g., a smudge on a doorknob)—other effects may be more distal and operate on a larger time scale; for example, increased detection of contaminants may lead to an exaggerated sense of vulnerability to germs and disease, which then lowers thresholds for behavioral avoidance across a variety of contexts.
Although it was predicted that the HCF group would exhibit attentional avoidance of contamination threat in addition to behavioral avoidance, no group difference emerged in overall fixation duration or total number of fixations on contamination threat. In contrast, other eye tracking studies have found that anxious (Rohner, 2002) or spider fearful individuals (Hermans et al., 1999; Rinck & Becker, 2006) avoid viewing threat compared to controls. Although HCF individuals did not show reduced viewing of contamination threat overall, their fixations on contamination threat were shorter, relative to their fixations on other image types––a pattern of gaze not found in the LCF group. This fragmented style of viewing threatening stimuli was observed to have clinical significance in a recent study. Beevers et al. (in press) found that shorter fixations on fearful faces––but not reduced viewing of fearful faces overall ––increased vulnerability to PTSD in response to combat stress. These authors suggest that fragmented viewing may interfere with habituation and extinction processes. However, future research is needed to clarify the underlying mechanisms and proximal effects of this gaze pattern in anxiety disorders.
Although the present findings have important implications for delineating the nature and function of attentional biases in contamination-based OCD, limitations of the study deserve consideration. First, the passive viewing task contained relatively few trials, which may have limited the study’s ability to reveal biases in the maintenance of initial fixation. Second, while the HCF group reported contamination fear well above clinical cutoffs, these findings would be strengthened by replication in a community sample of patients meeting diagnostic criteria for OCD. Despite these limitations, the present study extends previous findings of an attentional bias toward threat in contamination-based OCD by clarifying its overt components, as well as its behavioral correlates.
Acknowledgments
Preparation of this manuscript was supported in part by grant 5F31MH087018-02 awarded by the National Institute of Mental Health to Thomas Armstrong.
Footnotes
The results of analyses related to orienting biases did not differ after applying the appropriate arcsine transformation to these proportional data. For simplicity, statistical values based on the untransformed data are presented below.
Due to the higher percentage of female participants in the HCF group, orienting bias for contamination threat was regressed on PI group while covarying for gender. PI group continued to predict orienting bias toward contamination threat (β = .33, p = .035) in this model.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Revised 4th ed Author; Washington, DC: 2000. [Google Scholar]
- Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. Journal of Abnormal Psychology. 2008;117:860–868. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, Olatunji BO, Sarawgi S, Simmons C. Orienting and maintenance of gaze in contamination-based OCD: Biases for disgust and fear cues. Behaviour Research and Therapy. 2010;48:402–408. doi: 10.1016/j.brat.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Lee HJ, Wells TT, Ellis AJ, Telch MJ. Eye gaze bias for emotion stimuli prospectively predicts PTSD and depression symptoms among soldiers deployed in Iraq. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2011.10091309. (in press) [DOI] [PubMed] [Google Scholar]
- Burns GL, Keortge SG, Formea GM, Sternberger LG. Revision of the Padua Inventory of obsessive-compulsive disorder symptoms: Distinctiveness between worry, obsessions, and compulsions. Behaviour Research and Therapy. 1996;34:163–173. doi: 10.1016/0005-7967(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Caseras X, Garner M, Bradley BP, Mogg K. Biases in visual orienting to negative and positive scenes in dysphoria: An eye movement study. Journal of Abnormal Psychology. 2007;116:491–497. doi: 10.1037/0021-843X.116.3.491. [DOI] [PubMed] [Google Scholar]
- Cha CB, Najmi S, Park JM, Finn CT, Nock MK. Attentional bias toward suicide-related stimuli predicts suicidal behavior. Journal of Abnormal Psychology. 2010;119:616–622. doi: 10.1037/a0019710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115:760–770. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: A critical review. Cognitive Therapy and Research. 2009;33:221–234. doi: 10.1007/s10608-007-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Vansteenwegen D, Eelen P. Eye movement registration as a continuous index of attention deployment: Data from a group of spider anxious students. Cognition and Emotion. 1999;13:419–434. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. University of Florida, the Center for Research in Psychophysiology; Gainesville, FL: 2001. (Tech. Rep. No. A-5) [Google Scholar]
- Lawrence NS, An SK, Mataix-Cols D, Ruths F, Speckens A, Phillips ML. Neural responses to facial expression of disgust but not fear are modulated by washing symptoms in OCD. Biological Psychiatry. 2007;61:1072–1080. doi: 10.1016/j.biopsych.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- Najmi S, Amir N. The effect of attention training on a behavioral test of contamination fears in individuals with subclinical obsessive-compulsive symptoms. Journal of Abnormal Psychology. 2010;119:136–142. doi: 10.1037/a0017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji BO, Armstrong T. Contamination fear and effects of disgust on distress in a public restroom. Emotion. 2009;9:592–597. doi: 10.1037/a0016109. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Lohr JM, Sawchuk CN, Tolin DF. Multimodal assessment of disgust in contamination-related obsessive–compulsive disorder. Behaviour Research and Therapy. 2007;45:263–276. doi: 10.1016/j.brat.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Williams NL, Tolin DF, Sawchuk CN, Abramowitz JS, Lohr JM, et al. The Disgust Scale: Item analysis, factor structure, and suggestions for refinement. Psychological Assessment. 2007;19:281–297. doi: 10.1037/1040-3590.19.3.281. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rachman S. Fear of contamination. Behavior Research and Therapy. 2004;42:1227–1255. doi: 10.1016/j.brat.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive-compulsive disorder. Psychiatric Clinics of North America. 1992;15:743–758. [PubMed] [Google Scholar]
- Rinck M, Becker ES. Spider fearful individuals attend to threat, then quickly avoid it: Evidence from eye movements. Journal of Abnormal Psychology. 2006;115:231–238. doi: 10.1037/0021-843X.115.2.231. [DOI] [PubMed] [Google Scholar]
- Rohner JC. The time-course of visual threat processing: High trait anxious individuals eventually avert their gaze from angry faces. Cognition and Emotion. 2002;16:837–844. [Google Scholar]
- Summerfeldt LJ, Endler NS. Examining the evidence for anxiety related cognitive biases in obsessive–compulsive disorder. Journal of Anxiety Disorders. 1998;12:579–598. doi: 10.1016/s0887-6185(98)00035-8. [DOI] [PubMed] [Google Scholar]
- Tata PR, Leibowitz JA, Prunty MJ, Cameron M, Pickering AD. Attentional bias in obsessional compulsive disorder. Behaviour Research and Therapy. 1996;34:53–60. doi: 10.1016/0005-7967(95)00041-u. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilkinson L. Statistical methods in psychology journals: Guidelines and explanations. American Psychologist. 1999;54:594–604. [Google Scholar]