Abstract
The carbonic anhydrases (CAs) in the α class are zinc-dependent metalloenzymes. Previous studies have reported that recombinant forms of carbonic anhydrase IX (CAIX), a membrane-bound form of CA expressed in solid tumors, appear to be activated by low levels of zinc independent of its well-studied role at the catalytic site. In this study, we sought to determine if CAIX is stimulated by zinc in its native environment. MDA-MB-231 breast cancer cells express CAIX in response to hypoxia. We compared CAIX activity associated with membrane ghosts isolated from hypoxic cells with that in intact hypoxic cells. We measured CA activity directly using 18O exchange from 13CO2 into water determined by membrane inlet mass spectrometry. In membrane ghosts, there was little effect of zinc at low concentrations on CAIX activity, although at high concentration zinc was inhibitory. In intact cells, zinc had no significant effect on CAIX activity. This suggests that there is an appreciable decrease in sensitivity to zinc when CAIX is in its natural membrane milieu compared to the purified forms.
Introduction
The carbonic anhydrases (CAs) in the α class are zinc metalloenzymes expressed in higher vertebrates and that catalyze the reversible hydration of carbon dioxide to bicarbonate and a proton: CO2 + H2O ⇋ + H+ + HCO3−. These enzymes play an important role in acid-base balance in organisms and participate in a wide variety of physiological processes [1]. There are 13 active forms of CAs in mammals which differ in their subcellular localization, catalytic activity, and susceptibility to inhibitors [2]. Carbonic anhydrase IX (CAIX) is a unique member of this family. CAIX is a transmembrane glycoprotein whose catalytic domain is oriented toward the extracellular milieu [3]. Its expression is normally limited to the basolateral membrane of gut epithelial cells [4;5]. However, CAIX is overexpressed in many human tumor types [6]. In breast cancer, CAIX expression is a marker for hypoxia [7], and is associated with poor prognosis [8–10] and high-grade, estrogen receptor-negative tumors [8]. Thus, CAIX-specific inhibitors may be useful in the management of hypoxic tumors that do not respond to traditional chemo or radiation therapy (for review see [11]).
The activity of CAII, the most well studied member of the α-CA family, approaches the diffusion-controlled limit [12]. CAII exhibits high affinity and specificity for binding the catalytic zinc which is coordinated by three histidine residues at the bottom of the active site cleft [13;14]. A solvent molecule, water or hydroxide, is bound to zinc, completing a tetrahedral coordination geometry. Substitution of any of the histidine ligands with alanine decreases zinc affinity by 105-fold [15]. These data show that the metal-binding residues and conformation in the catalytic pocket optimize zinc affinity and maintain a very slow rate of zinc dissociation. Several lines of evidence suggest that CAIX has catalytic properties similar to CAII. Wingo, et al. demonstrated that a recombinant form of CAIX, containing only the catalytic domain, exhibits catalytic efficiency for CO2 hydration that is only 2-fold less than that for CAII (5.5 × 107 M−1 s−1 vs 10.0 × 107 M−1s−1, respectively) [16]. Hilvo et al. showed similarly small differences in kinetic parameters between CAII and a recombinant form of the CAIX catalytic domain expressed in an insect cell line (to preserve glycosylation) [17]. Further, Genis et al. have reported that the catalytic efficiency of a CAIX mimic is 5.1 × 107 M−1 s−1 [18]. This mimic was designed by structural alignment of the active sites of CAIX and CAII, and then reconstructing the active site of a recombinant variant of CAII to mimic that in CAIX. Finally, we have shown that the catalytic efficiency of CAIX in its native environment is 6.2 × 107 M−1s−1. Despite these functional similarities, CAIX, but not CAII, exists as a dimer via intermolecular disulfide bonding [17;19;20].
Another apparent difference between CAII and CAIX is the ability of zinc, at near physiological levels, to increase the catalytic activity of CAIX but not CAII [17]. These experiments tested the effect of zinc on CAIX using two soluble recombinant forms of CAIX: one which contained just the catalytic domain and another which contained the catalytic domain and the N-terminal proteoglycan-like domain. In the case of the catalytic domain, zinc increased the catalytic efficiency (kcat/Km) by about 10-fold. In the case of the catalytic domain containing the N-terminal proteoglycan-like domain, zinc increased the catalytic efficiency by 23-fold reaching an activity level even higher than that of CAII. The effect of zinc was not due to insufficient zinc in the catalytic pocket, as constructs of other CA family members did not show activation. The authors proposed that the overall structure was stabilized in the presence of excess zinc by relieving electrostatic repulsions. This phenomenon is intriguing as it suggests that activity in the tumor setting, where CAIX is overexpressed, might be sensitive to zinc concentration. We thus measured the effect of zinc on the activity of CAIX in MDA-MB-231 breast cancer cells and membrane ghosts derived from these cells. In neither setting did we reveal any significant elevation of CAIX activity. We conclude that in a native environment, zinc plays little role in regulating CAIX activity aside from its well-known role in catalysis.
Materials and Methods
Cell culture
The MDA-MB-231 breast cancer cell line was provided by Dr. Kevin Brown (University of Florida). Cells were plated at a density of 10,000 cells/mL Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (Atlanta Biologicals, s11450). At 75% confluence (day three, post-plating), cells were exposed to hypoxia in Modulator Incubator Chambers (MIC-101) from Billups-Rothenberg Inc. (1% O2, 5% CO2, and balanced N2) for 16 hours at 37°C. For cell counting, cells were released from the plates using enzyme-free cell dissociation buffer (GIBCO) and quantified using a Coulter Counter® ZM (Beckman Coulter).
Lysate preparation
Cells were washed 3 times with ice cold PBS and then extracted in lysis buffer [1% Triton X-100, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 25 mM NaF] supplemented with protease inhibitors for 15 minutes on ice. Lysates were collected and clarified by centrifugation at 16,300 × g for 15 minutes at 4°C. Clarified supernatants were collected and aliquots were stored at −20°C. Protein concentration was determined using the Markwell modification of the Lowry procedure [21].
SDS-PAGE and Western blotting
One-dimensional SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting were performed as previously described [22]. For the detection of CAIX, the M75 monoclonal antibody was used at a dilution of 1:1000. This antibody was originally made by Pastorekova et al. [23] and was a gift from Dr. Egbert Oosterwijk (Department of Urology, University Hospital Nijmegen, Nijmegen, The Netherlands). For CAII detection, we used a rabbit polyclonal antibody made against the entire protein (Novus Biologicals). Enhanced chemiluminescence (ECL) was used according to manufacturer’s directions [GE Healthcare (#RPN2106)].
Membrane ghost preparation
Hypoxic cells were washed 3 times with cold PBS (2.7 mM KCl, 10 mM phosphate salts, 120 mM NaCl, pH 7.4) and then exposed to hypotonic buffer (1 mL/plate of a solution containing 2.7 mM KCl, 10 mM phosphate salts, pH 7.4) in the presence of protease inhibitors (Roche Diagnostics) for 15 minutes at 4°C. Cells were scraped from plates and collected by centrifugation at 10,000 × g for 15 minutes at 4°C. Membrane ghosts were collected and washed 4 times with hypotonic buffer. After the final wash, the ghosts were resuspended in hypotonic buffer and immediately assayed for CA activity (see below). Aliquots were stored at −20°C for protein analysis.
Catalysis by carbonic anhydrase
Catalysis was measured by the exchange of 18O from species of CO2 into water [24] determined by membrane inlet mass spectrometry (MIMS), as has been previously described in detail [22]. Hypoxic MDA-MB-231 cells were collected from culture plates using enzyme-free cell dissociation buffer (GIBCO). Cells were extensively washed with bicarbonate-free DMEM buffered containing 25mM Hepes, pH 7.4, and counted. Cells (5 × 105 cells/mL) were added to a reaction vessel containing 2 mL of buffered, bicarbonate-free DMEM at 16°C in which was dissolved 18O-enriched 13CO2/H13CO3− at 25 mM total 13CO2 species. We chose pH 7.4 for our assay conditions so that we could directly compare our data to that of Hilvo et al. [17]. In addition, the catalytic efficiency (kcat/Km) of CAIX for hydration is maximal above pH 7.0 ([16]. We selected 16°C for the reaction temperature (in contrast to the traditional 25°C) for two reasons. First, the lower temperature slows the enzyme-mediated reactions to better contrast the intracellular and extracellular CA activities [22]. Second, we sought to prevent endocytotic events during the course of the experiment which is accomplished at temperatures slightly below the phase transition for lipids in the plasma membrane [25]. A membrane inlet was immersed in the medium in the reaction vessel and used to detect the atom fraction of 18O in extracellular 13CO2. This activity was measured after addition of cells in buffered, bicarbonate-free DMEM in the absence or presence of specific CA inhibitors or zinc at the concentrations indicated. We also assayed CA activity in the medium that overlaid the cells to assess membrane integrity. Assays with membrane ghosts were conducted in hypotonic buffer. For both cell and membrane assays, the pH was controlled at 7.4.
Statistical analysis
Results are expressed as means ± standard error of the means (SE). Data were analyzed by one way ANOVA. Differences of P < 0.05 were considered significant.
Results and Discussion
MDA-MB-231 breast cancer cells exhibit a triple negative phenotype and display an aggressive behavior when implanted into nude mice [26]. We have shown that hypoxia induces the expression of CAIX against a background in which no other membrane-associated CA family members can be detected by reverse transcriptase polymerase chain reaction [27]. Thus, we can reasonably assume that membrane-bound CA activity represents CAIX activity, specifically. These characteristics have allowed us to previously determine the kinetic parameters of CAIX in membrane ghosts isolated from hypoxic MDA-MB-231 cells [22]. Here, we use this model to test the effect of zinc on CAIX activity. These data are shown in Figure 1. To be assured that membrane ghosts are free of intracellular CAII, which is abundant in MDA-MB-231 cells [27], we performed western blots of the fractions collected during the isolation process. As expected, CAII is preferentially located in the cytoplasmic fraction, while CAIX is associated with the membranes (Figure 1A). The autoradiographic film selected for this figure is purposefully overexposed but shows only a trace of CAII in the membrane ghost fraction suggesting that CAII will contribute very little to the CA activity measured in membrane ghosts. We proceeded to analyze the effect of zinc concentration CA activity in membrane ghosts by the 18O exchange method (Figures 1B and 1C). In Figure 1B, we show the effect of specific concentrations of zinc sulfate on CA activity when added after the addition of membrane ghosts. While we expected linear kinetics with the addition of membrane ghosts, we noted some curvature immediately after the addition of the membranes (see the control progress curve). This suggests that a small proportion of the CAIX pool is sequestered in a membrane-bound compartment. This may be due to inversion of the some of the membrane ghosts such that the catalytic domain of CAIX faces inward. The majority of the CA activity is associated with the second phase (~100 seconds after addition of the membrane ghosts). This linear phase was used to calculate the control slope and the effect of zinc on that slope (+ Zn progress curve). Compared to the control, low concentrations of zinc (added after membrane ghosts) had little effect on CAIX activity, while higher concentrations were mildly inhibitory. To further explore this effect, we preincubated membranes with 0.1mM zinc for specific times before assaying for activity, as shown in Figure 1C. These data demonstrate that extended preincubation with zinc had no more effect than when added at time zero resulting in a 30% loss of activity (see inset). The same was observed for cells treated with 0.3 mM zinc (progress curves not shown), although inhibition was about 40% (Figure 1C, inset). It is known that metal ions inhibit CAII activity most likely by binding to His64 to block the proton shuttle mechanism [28;29]. A similar effect may be in play with the equivalent histidine (His200) in CAIX. We also contemplated a role for sulfate, as carbonic anhydrases are inhibited by metal complexing anions [30;31]. However, the Ki of sulfate for CAIX is greater than 300 mM [32], excluding this possibility for the zinc-dependent inhibition that we observe in membrane ghosts.
Figure 1.
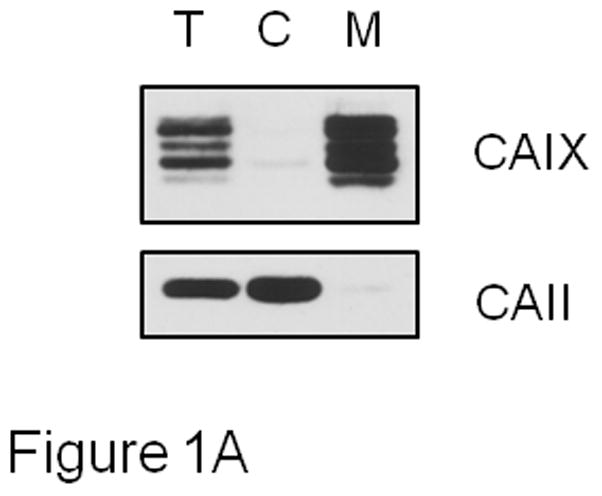
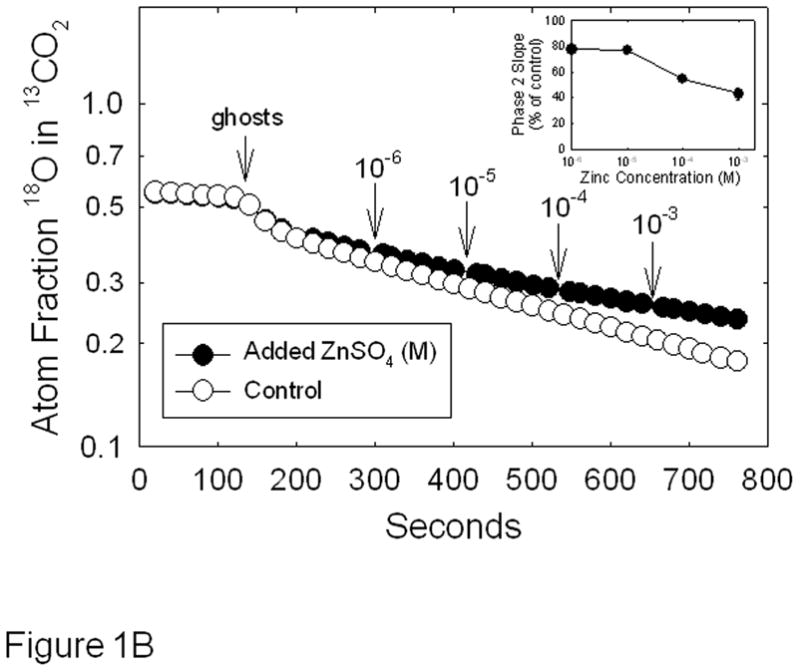
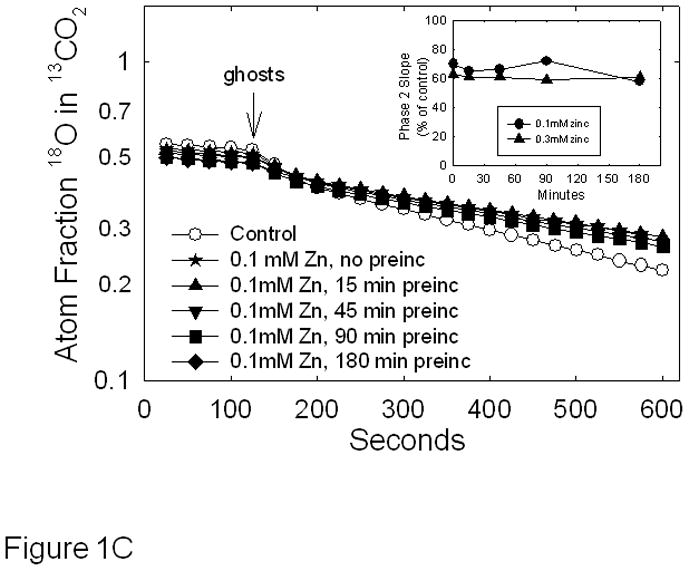
Effect of zinc on CAIX activity in MDA-MB-231 membrane ghosts - Cells were grown for three days at which point they were exposed to hypoxic conditions for 16h. Cells were then washed with PBS and incubated in hypotonic buffer. The resulting membrane ghosts were collected by centrifugation. Panel A: CAIX and CAII were evaluated by Western blotting of fractions collected after exposure to hypotonic buffer. These fractions included the total cell fraction (T), the cytoplasmic fraction (C), and the membrane ghost fraction (M). These data represent two independent experiments. Panel B: CAIX activity was measured in the membrane ghost fraction using the 18O exchange assay. Control activity was compared to activity measured in the presence of increasing concentrations of zinc sulfate, added at intervals. Data are shown at 20 sec intervals. These data are representative of two independent experiments. The inset shows the dose response curve in which the average slope data ± SE are presented as percent of control. Panel C: Membrane ghosts were pre-incubated for specific times in the absence or presence of zinc at 0.1 mM before the 18O assay. Data are shown at 25 sec intervals. Inset: Slopes from Panel B are presented as percent of control. These data are representative of two independent experiments.
In intact MDA-MB-231 cells, we are able to measure cytoplasmic CAII activity independently from CAIX [22;33]. However, it is important to demonstrate that the tools that we use to release cells from cell culture plates for the 18O exchange assay, do not also impact CAIX content. As shown in Figure 2A, trypsin treatment resulted in a complete loss of CAIX from the cell surface compared to cells exposed to enzyme-free dissociation buffer. Only cells treated with cell dissociation buffer exhibit CAIX activity. Note that CAII levels did not change in trypsin-treated cells compared to cells exposed to cell dissociation buffer indicating that the trypsin-treated cells were intact despite the loss of CAIX at the cell surface. Figure 2B illustrates the depletion of 18O from 13CO2 caused by catalysis by carbonic anhydrase after addition of hypoxic MDA-MB-231 cells, or the cell-free medium (supernatant) which overlays the cells. As we have shown previously, the time course of 18O depletion in the presence of MDA-MB-231 cells is biphasic [22;33]. The first phase, which occurs in the initial 20–40 s after addition of cells, is dominated by the rapid diffusion of CO2 into cells where the hydration-dehydration cycles (accelerated by CAII) deplete 18O followed by efflux of CO2 from the cells. The second phase, which in Figure 2 begins 100 s after addition of cells, is dominated by the CA hydration-dehydration activity outside of the cells (i.e., catalyzed by CAIX). This is confirmed by addition of the classical CA inhibitor, acetazolamide, which is impermeant over the time course of the experiment [33]. It can be seen in Figure 2B that the slope of the second phase in the presence of the inhibitor is decreased relative to its absence. Based on four independent experiments, the slope of the second phase is higher by a factor of 3.2 ± 0.3 in hypoxic cells in the absence compared to the presence of acetazolamide. As might be expected, this value is similar to the activity ratio comparing hypoxic versus normoxic cells in which the expression of CAIX is low and exofacial CA activity is close to background [22]. Note that acetazolamide had no effect on phase 1 which is evidence that it did not enter the cells to inhibit CAII. The experiment in which only medium (which overlays the cells) is added to the reaction chamber demonstrates that no CAII is released from the cells and additionally represents the uncatalyzed exchange (background) between 18O and CO2 as do the data collected before addition of intact cells.
Figure 2.
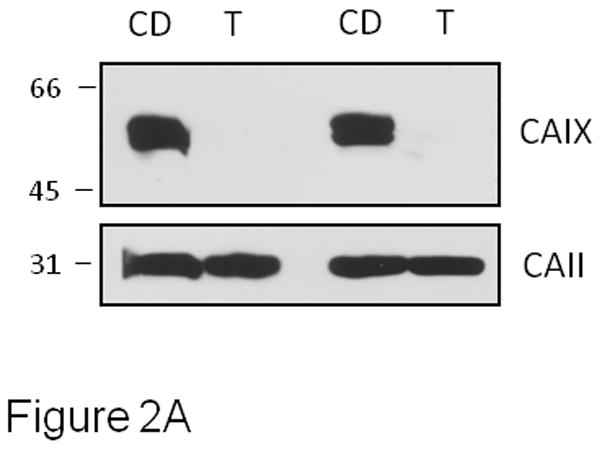
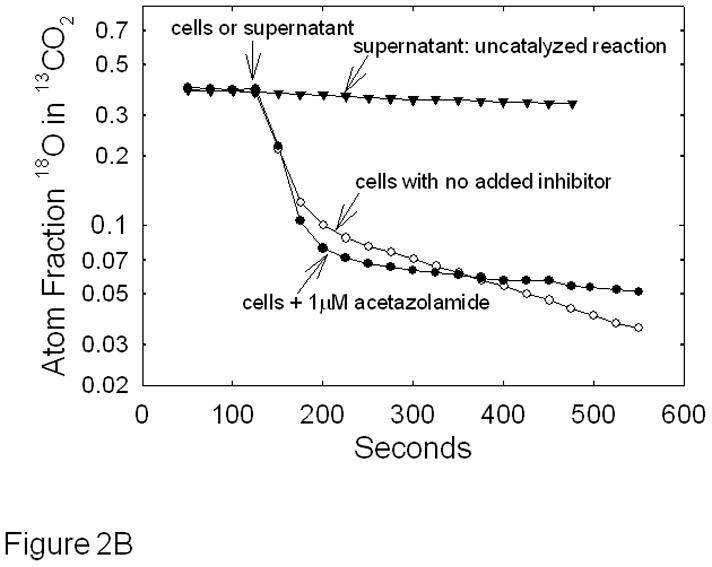
Effect of acetazolamide on CAIX activity in intact MDA-MB-231 cells - MDA-MB-231 cells were grown and exposed to hypoxia as described in Figure 1. Panel A: Cells were harvested using either trypsin (T) treatment (0.25%, 3 min at 37°C) or enzyme-free cell dissociation (CD) buffer (5 min at 37°C). Cells were collected and lysed. Western blotting was performed to detect the presence of CAIX and CAII. Two independent experiments are shown. Panel B: Cells harvested using Cell Release Buffer were assayed for carbonic anhydrase activity by 18O exchange in the absence or presence of 1 μM acetazolamide at pH 7.4, 16°C. Each progress curve was generated with 5 × 105 cells/mL. We also allowed the cells to settle to test for activity in the medium that overlaid the cells (supernatant: uncatalyzed reaction). Atom fractions of 18O in CO2 were continuously collected. For ease of illustration, data points at 25 second intervals are shown. Data are representative of at least three independent experiments.
To determine the effect of zinc on CAIX activity, we utilized the 18O exchange assay in which zinc was added to the reaction chamber after the addition of cells or preincubated with the cells prior to assay (Figure 3). Figure 3A illustrates the effect of zinc added after the addition of hypoxic cells. The slope of 2nd phase after each addition of zinc changed very little in comparison to control (see inset). We next exposed cells to zinc for various times prior to assaying for activity. These data are shown in Figure 3B. Here, we report the corrected slopes (catalyzed – uncatalyzed) of the 2nd phase for cells exposed to either 100 or 300 μM zinc over 3 hours of pre-incubation. Again, little change was noted in CAIX activity.
Figure 3.
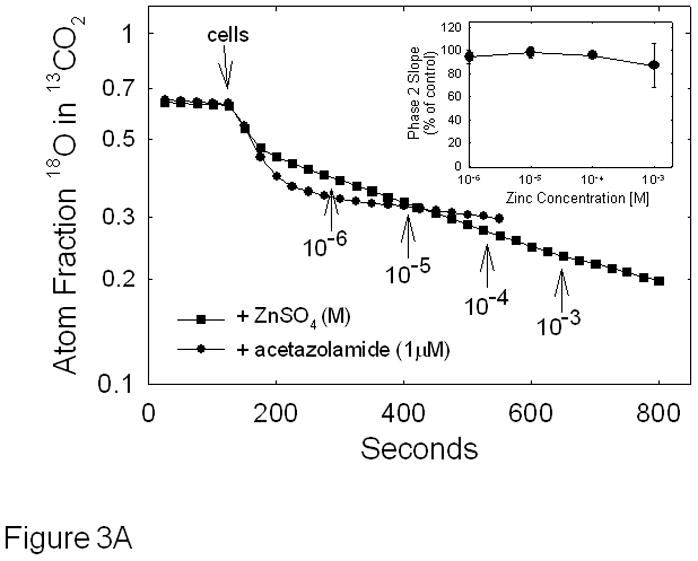
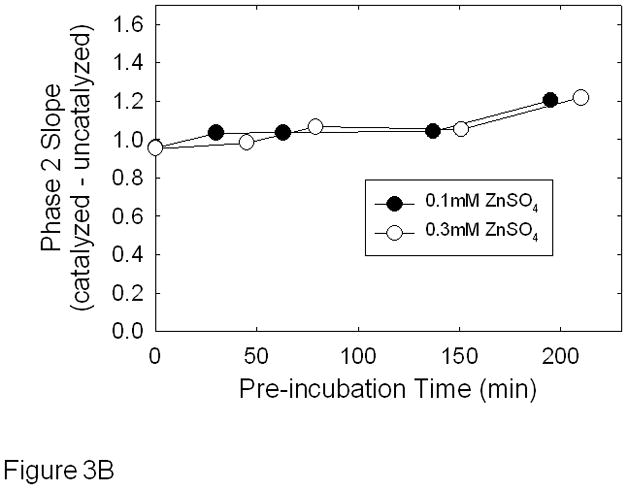
Effect of zinc on CAIX activity in hypoxic MDA-MB-231 cells - Cells were grown and harvested as described in Figure 1. Panel A: Cells were assayed for carbonic anhydrase activity using the 18O exchange assay and the effect of zinc was determined after initiating the activity assay. The time and concentration of zinc additions are indicated on the figure. These data are representative of two independent experiments. The inset shows the dose response curve in which the average slope data ± SE are presented as percent of control. Panel B: Cells were exposed to either 0.1 mM or 0.3 mM zinc for up to 3 hours before initiating the 18O exchange assay. The rates of the uncatalyzed reactions were subtracted and the slopes of each corrected phase 2 are plotted against time. Data are representative of three independent experiments.
We undertook this study for several reasons. First, Hilvo et al. have observed significantly elevated CAIX activity in the presence of 50 μM zinc for soluble recombinant CAIX with and without the proteoglycan domain [17]. These authors suggested that the interaction may occur within the catalytic domain but is enhanced by the presence of the proteoglycan domain. While this concentration is a little above the normal physiological level of serum zinc (which ranges from 14 to 23 μM [34]), their data show that recombinant CAIX activity is particularly sensitive to zinc. This observation might be pertinent as the catalytic domain of CAIX faces the extracellular milieu. Because the expression of CAIX is associated with a variety of solid tumors, it is possible that zinc-induced activation of CAIX might impact tumor progression and prognosis. The data in our report, however, show that CAIX in its natural membrane environment, either in intact MDA-MB-231 cells or in membrane ghosts, is not sensitive to this range of zinc concentration. This is an intriguing result which suggests that there is an appreciable decrease in sensitivity to zinc when CAIX is in its natural membrane milieu. The nature of this effect of the membrane environment is not known, but may be due to different conformational states of the proteoglycan domain with respect to the catalytic domain in soluble versus membrane-bound forms of CAIX. Another possibility is that CAIX is more constrained by the dimeric structure adopted in the membrane [19] versus that in solution and thus cannot respond to zinc.
Many different types of cells, including the MDA-MB-231 cells which we used in this study, are grown in Dulbecco’s modified Eagle medium (DMEM) whose formulation contains no added zinc. Thus, the only source of zinc is the serum that supplements the growth medium. It has been reported that the concentration of zinc in fetal bovine serum (FBS) is about 17 μM (BioAssay Systems). Because DMEM is supplemented with only 10% FBS, this means that the cells are exposed to only 1.7 μM zinc which could impact the activity not only of CAIX, but other zinc-dependent proteins. That said, we did not observe significant changes in CAIX activity in intact cells or membrane ghosts when exposed over time to a range of zinc concentrations. Further, we did not observe changes in CAIX expression when exposed to DMEM containing physiological levels of zinc (data not shown). The concentration of CAIX in our assays is very low. In the membrane ghost experiments, we estimate that only 0.03% of the total protein (~ 1.6 mg/assay) is actually CAIX based on ethoxzolamide titration (data not shown). Thus, the concentration of CAIX in our reaction vessel is about 3 nM, varying some with each membrane ghost preparation. This suggests that the demand for zinc is low, at least for CAIX. It has been suggested that zinc-bound CAII folds more rapidly during biosynthesis than does apo-CAII [35;36] and that once folded loses zinc slowly, with a half life of 95 days [37]. The half life of CAIX is about 40 hours [38] which indicates that CAIX is degraded considerably faster than zinc is lost from the binding site, assuming that CAIX has the same zinc affinity as displayed by CAII. Given these insights, our data suggest that CAIX sequesters sufficient zinc during biosynthesis for folding, targeting, and gain of function. Further, our data show that CAIX in its native environment cannot be further activated by additional zinc.
Highlights (for review).
CAIX activity in membranes is not affected by zinc at physiological concentrations
CAIX activity associated with hypoxic MDA-MB-231 cells is not affected by zinc
Data contrast to recombinant forms of CAIX which are activated by zinc
Natural membrane milieu reduces sensitivity of CAIX to zinc
Acknowledgments
The authors would like to thank Dr. Egbert Oosterwijk for his generous gift of the M75 antibody. They would also like to acknowledge Ms Xiaowei Gu for her excellent cell culture work. The work described was supported in part by grants to SCF from the Department of Defense (BC073020) and NIH (DK45035) and to DNS from NIH (GM25154).
Footnotes
We declare that there are no potential conflicts of interest that are relevant to the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sly WS, Hu PY. Human Carbonic Anhydrases and Carbonic Anhydrase Deficiencies. Ann Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 2.Neri D, Supuran CT. Interfering with pH Regulation in Tumours as a Therapeutic Strategy. Nature Reviews:Drug Discovery. 2011;10:767–777. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 3.Opavsky R, Pastoreková S, Zelník V, Gibadulinová A, Stanbridge EJ, Závada J, Kettmann R, Pastorek J. Human MN/CA9 Gene, A Novel Member of the Carbonic Anhydrase Family: Structure and Exon to Protein Domain Relationships. Genomics. 1996;33:480–487. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 4.Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, Pastoreková S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS. Immunohistochemistry of Carbonic Anhydrase Isozyme IX (MN/CA IX) in Human Gut Reveals Polarized Expression in Epithelial Cells with the Highest Proliferative Capacity. J Histochem Cytochem. 1998;46:497–504. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- 5.Pastorekova S, Parkkila S, Parkkila A, Opavsky R, Zelnik V, Saarnio J, Pastorek J. Carbonic Anhydrase IX, MN/CAIX: Analysis of Stomach Complementary DNA Sequence and Expression in Human and Rat Alimentary Tracts. Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of Hypoxia-inducible Cell-surface Transmembrane Carbonic Anhydrases in Human Cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wykoff CC, Beasley NJP, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. Hypoxia-inducible Expression of Tumor-associated Carbonic Anhydrase. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 8.Span PM, Bussink J, Manders P, Beex LVAM, Sweep CGJ. Carbonic Anhydrase-9 Expression Levels and Prognosis in Human Breast Cancer: Association with Treatment Outcome. Br J Cancer. 2003;89:271–276. doi: 10.1038/sj.bjc.6601122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Generali D, Fox SB, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield SM, Bruzzi P, Bersiga A, Allevi G, Milani M, Aguggini S, Dogliotti L, Bottini A, Harris AL. Role of Carbonic Anhydrase IX Expression in Prediction of the Efficacy and Outcome of Primary Epirubicin/Tamoxifen Therapy for Breast Cancer. Endocrine-Related Cancer. 2006;13:921–930. doi: 10.1677/erc.1.01216. [DOI] [PubMed] [Google Scholar]
- 10.Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, Gatter KC, Ratcliffe P, Harris AL. Prognostic Significance of a Novel Hypoxia-Regulated Marker, Carbonic Anhydrase IX, in Invasive Breast Cancer. J Clin Oncol. 2005;19:3660–3668. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- 11.Supuran CT. Carbonic Anhydrase: Novel Therapeutic Applications for Inhibitors and Activators. Nature Reviews:Drug Discovery. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 12.Silverman DN, Lindskog S. The Catalytic Mechanism of Carbonic Anhydrase - Implication of a Rate Limiting Protolysis of Water. Accounts of Chemical Research. 1988;21:30–36. [Google Scholar]
- 13.Hakansson K, Carlsson M, Svensson LA, Liljas A. Structure of Native and Apo Carbonic Anhydrase II and Structure of Some of Its Anion-Ligand Complexes. J Mol Biol. 1992;227:1192–1204. doi: 10.1016/0022-2836(92)90531-n. [DOI] [PubMed] [Google Scholar]
- 14.Christianson SW, Fierke S. Carbonic Anhydrase: Evolution of the Zinc Binding Site by Nature and by Design. Accounts of Chemical Research. 1996;29:331–339. [Google Scholar]
- 15.Kiefer LL, Fierke CA. Functional Characterization of Human Carbonic Anhydrase II Variants with Altered Zinc Binding Sites. Biochem. 1994;33:15233–15240. doi: 10.1021/bi00255a003. [DOI] [PubMed] [Google Scholar]
- 16.Wingo T, Tu C, Laipis PJ, Silverman DN. The Catalytic Properties of Human Carbonic Anhydrase IX. Biochem Biophys Res Comm. 2001;288:666–669. doi: 10.1006/bbrc.2001.5824. [DOI] [PubMed] [Google Scholar]
- 17.Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A, Scozzafava A, Monti SM, Di Flore A, De Simone G, Lindfors M, Janis J, Valjakka J, Pastorekova S, Pastorek J, Kulomaa MS, Mordlund HR, Supuran CT, Parkkila S. Biochemical Characterization of CA IX, One of the Most Active Carbonic Anhydrase Isozymes. J Biol Chem. 2008;283:27799–27809. doi: 10.1074/jbc.M800938200. [DOI] [PubMed] [Google Scholar]
- 18.Genis C, Sippel KH, Case N, Cao W, Avvaru BS, Tartaglia LJ, Govindasamy L, Tu C, Agbandje-McKenna M, Silverman DN, Rosser CJ, McKenna R. Design of A Carbonic Anhydrase IX Active-Site Mimic to Screen Inhibitors for Possible Anticancer Properties. Biochem. 2009;48:1322–1331. doi: 10.1021/bi802035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Wang H, Tu C, Shiverick KT, Silverman DN, Frost SC. Role of Hypoxia and EGF on Expression, Activity, Localization, and Phosphorylation of Carbonic Anhydrase IX in MDA-MB-231 Breast Cancer Cells. Biochim Biophys Acta. 2011;1813:159–167. doi: 10.1016/j.bbamcr.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alterio V, Hilvo M, Di Fiore A, Supuran CT, Pan P, Parkkila S, Scaloni A, Pastorek J, Pastorekova S, Pedone C, Scozzafava A, Monti SM, De Simone G. Crystal Structure of the Catalytic Domain of the Tumor-associated Human Carbonic Anhydrase IX. Proc Natl Acad Sci USA. 2009;106:16233–16238. doi: 10.1073/pnas.0908301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markwell MAK, Haas SM, Lieber LL, Tolbert NE. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Tu C, Wang H, Silverman DN, Frost SC. Catalysis and pH Control by Membrane-associated Carbonic Anhydrase IX in MDA-MB-231 Breast Cancer Cells. J Biol Chem. 2011;286:15789–15796. doi: 10.1074/jbc.M110.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastorekova S, Zavadova Z, Kostal M, Babusikova O, Zavada J. A Novel Quasi-viral Agent, MaTu, is a Two-Component System. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-z. [DOI] [PubMed] [Google Scholar]
- 24.Silverman DN. Carbonic Anhydrase: Oxygen-18 Exchange Catalyzed by an Enzyme with Rate-contributing Proton-transfer Steps. Meth Enzymol. 1982;87:732–752. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- 25.Weigel PA, Oka JA. Temperature Dependence of Endocytosis Mediated by Asialyglycoprotein Receptor in Isolated hepatocytes: Evidence for Two Potentially rate-limiting Steps. J Biol Chem. 1981;256:2615–2617. [PubMed] [Google Scholar]
- 26.Zhang RD, Fidler IJ, Price JE. Relative Malignant Potential of Human Breast Cancer Cell Lines Established from Pleural Effusions and a Brain Metastasis. Invasion Metastasis. 1991;11:204–215. [PubMed] [Google Scholar]
- 27.Li Y, Wang H, Oosterwijk E, Tu C, Shiverick KT, Silverman DN, Frost SC. Expression and Activity of Carbonic Anhydrase IX Is Associated with Metabolic Dysfunction in MDA-MB-231 Breast Cancer Cells. Cancer Investigation. 2009;27:613–623. doi: 10.1080/07357900802653464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu CK, Wynns GC, Silverman DN. Inhibition by Cupric Ions of 18O Exchange Catalyzed by Humanc Carbonic Anhydrase II: Relation to the Interaction between Carbonic Anhydrase and Hemoglobin. J Biol Chem. 1981;256:9466–9470. [PubMed] [Google Scholar]
- 29.Eriksson EA, Jones TA, Liljas A. Crystallographic Studies of Human Carbonic Anhydrase II. In: Gray H, Bertini I, editors. Zinc Enzymes. Birkhauser; 1986. pp. 317–28. [Google Scholar]
- 30.Maren TH, Sanyal G. The Activity of Sulfonamides and Anions against the Carbonic-Anhydrases of Animals, Plants, and Bacteria. Annual Review of Pharmacology and Toxicology. 1983;23:439–459. doi: 10.1146/annurev.pa.23.040183.002255. [DOI] [PubMed] [Google Scholar]
- 31.Simmonson I, Lindskog S. The Interaction of Sulfate with Carbonic-Anhydrase. Eur J Biochem. 1982;123:29–36. doi: 10.1111/j.1432-1033.1982.tb06494.x. [DOI] [PubMed] [Google Scholar]
- 32.Innocenti A, Vullo D, Pastorek J, Scozzafava A, Pastorekova S, Nishimori I, Supuran CT. Carbonic Ahnydrase Inhibitors. Inhibition of Transmembrane Isozymes XII (Cancer-associated) and XIV with Anions. Bioorganic and Medicinal Chemistry Letters. 2007;17:1532–1537. doi: 10.1016/j.bmcl.2006.12.113. [DOI] [PubMed] [Google Scholar]
- 33.Delacruz J, Mikulski R, Tu C, Li Y, Wang H, Shiverick KT, Frost SC, Horenstein NA, Silverman DN. Detecting Extracellular Carbonic Anhydrase Activity Using Membrane Inlet Mass Spectrometry. Anal Biochem. 2010;403:74–78. doi: 10.1016/j.ab.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauberlich HE. Laboratory Tests for Assessment of Nutritional Status. CRC Press; 1998. [DOI] [PubMed] [Google Scholar]
- 35.Yazgan A, Henkens RW. Role of Zn(II) in the Refolding of Guanidine Hydrochloride Denatured Bovine Carbonic Anhydrase. Biochem. 1972;11:1314–1318. doi: 10.1021/bi00757a031. [DOI] [PubMed] [Google Scholar]
- 36.Wong KP, Hamlin LM. The Role of Zn(II) on the Folding of Bovine Carbonic Anhydrase B. Arch Biochem Biophys. 1975;170:12–22. doi: 10.1016/0003-9861(75)90093-4. [DOI] [PubMed] [Google Scholar]
- 37.Hunt JA, Fierke CA. Selection of Carbonic Anhydrase Variants Displayed on Phage: Aromatic Residues in Zinc Binding Site Enhance Metal Affinity and Equilibration. J Biol Chem. 1997;272:20364–20372. doi: 10.1074/jbc.272.33.20364. [DOI] [PubMed] [Google Scholar]
- 38.Rafajova M, Zatovicova M, Kettmann R, Pastorek J, Pastorekova S. Induction by Hypoxia Combined with Low Glucose or Low Bicarbonate and High Posttranslational Stability upon Reoxygenation Contribute to Carbonic Anhydrase IX Expression in Cancer Cells. Int J Oncol. 2004;24:995–1004. [PubMed] [Google Scholar]


