Abstract
Recent studies of Mn2+ transport mutants indicate that manganese is essential for unstressed growth in some bacterial species, but is required primarily for induced stress responses in others. A Bradyrhizobium japonicum mutant defective in the high affinity Mn2+ transporter gene mntH has a severe growth phenotype under manganese limitation, suggesting a requirement for the metal under unstressed growth. Here, we found that activities of superoxide dismutase and the glycolytic enzyme pyruvate kinase were deficient in an mntH strain grown under manganese limitation. We identified pykM as the only pyruvate kinase-encoding gene based on deficiency in activity of a pykM mutant, rescue of the growth phenotype with pyruvate, and pyruvate kinase activity of purified recombinant PykM. PykM is unusual in that it required Mn2+ rather than Mg2+ for high activity, and that neither fructose 1,6-bisphosphate nor AMP was a positive allosteric effector. The mntH-dependent superoxide dismutase is encoded by sodM, the only expressed superoxide dismutase-encoding gene under unstressed growth conditions. An mntH mutant grew more slowly on pyruvate under manganese-limited conditions than did a pykM sodM double mutant, implying additional manganese-dependent processes. The findings implicate roles for manganese in key steps in unstressed oxidative metabolism in B. japonicum.
INTRODUCTION
Manganese is involved in numerous cellular processes (Jakubovics & Jenkinson, 2001), and is an essential nutrient in eukaryotes. In bacteria, manganese is best understood in its roles in protection against oxidative stress as a cofactor of manganese-dependent superoxide dismutase. In addition, manganese substitutes for iron in E. coli ribulose-5-phosphate 3-epimerase, rendering the protein less sensitive to hydrogen peroxide damage (Sobota & Imlay, 2011). This may be a general protective mechanism for mononuclear iron enzymes, which do not require the redox activity of iron for their activity. Manganese is involved in other type of stresses as well. It can substitute for iron in a class I ribonucleotide reductase under conditions where iron is limited and manganese is available (Martin & Imlay, 2011). In addition, it is required for sporulation in Bacillus subtilis (Que & Helmann, 2000) and the stringent response in E. coli (Potrykus & Cashel, 2008).
MntH and MntABC are the two most well represented Mn2+ transport systems in the eubacterial kingdom. MntH is the major high affinity Mn2+ transporter in numerous bacteria (Makui et al., 2000, Que & Helmann, 2000, Hohle & O’Brian, 2009, Domenech et al., 2002, Anderson et al., 2009), and the mntH gene is expressed under manganese limitation to scavenge available metal. Surprisingly, mntH mutants have no or mild growth phenotypes in numerous bacteria under non-stress conditions (Makui et al., 2000, Que & Helmann, 2000, Domenech et al., 2002). Under normal growth, E. coli cells take up and contain little manganese, and manganese-dependent superoxide dismutase is not correctly metallated (Anjem et al., 2009). The lack of a manganese requirement suggests that manganese-dependent processes are not essential under those conditions, that other metals can substitute for manganese in manganese-containing proteins, or that these proteins are rendered non-essential due to compensatory activities. The manganese-dependent superoxide dismutase of E. coli functions under induced oxidative stress, but is not needed under normal aerobic growth due to a different dismutase isoform that uses iron. The class I ribonucleotide reductase of E. coli can use iron or manganese, and there is an iron-dependent reductase as well (Martin & Imlay, 2011).
The Gram-negative bacterium Bradyrhizobium japonicum is a model organism for studying metal metabolism and regulation in the alpha-Proteobacteria, a large and diverse taxonomic group that includes pathogens, symbionts, and bacteria that occupy many other ecological niches (O’Brian & Fabiano, 2010). B. japonicum lives as a free-living organism or as the endosymbiont of soybean, where it fixes atmospheric nitrogen to ammonia within cells of a specialized organ called a root nodule.
B. japonicum has a single functional mntH gene, and no obvious mntABC gene homologs are present in the genome (Hohle & O’Brian, 2009). A B. japonicum mntH mutant is almost completely defective in high affinity Mn2+ uptake activity. Moreover, the mntH strain has a severe growth phenotype under normal growth conditions (Hohle & O’Brian, 2009), suggesting a greater reliance on manganese compared to E. coli and perhaps other organisms as well. In further support of this, B. japonicum also contains a specific outer membrane channel for Mn2+ translocation into the periplasm (Hohle et al., 2011). MnoP is the only known example of a selective outer membrane channel for an uncomplexed divalent metal. Manganese as an essential nutrient may be a general feature of the alpha-Proteobacteria because manganese transport mutants of Brucella abortus (Anderson et al., 2009) and Sinorhizobium meliloti (Platero et al., 2003, Davies & Walker, 2007) also have a growth phenotypes, and also a defect in manganese-dependent superoxide dismutase. Moreover, the global bacterial iron regulator Fur has been co-opted to respond to Mn2+ rather than Fe2+ in B. japonicum and other alpha-Proteobacteria (Hohle & O’Brian, 2009, Hohle & O’Brian, 2010, Chao et al., 2004, Diaz-Mireles et al., 2005, Platero et al., 2007, Anderson et al., 2009), and has been renamed Mur. Mur transcriptionally regulates mntH and mnoP, resulting in expression under manganese limitation (Hohle et al., 2011, Hohle & O’Brian, 2009). Finally, manganese controls iron homeostasis in B. japonicum by affecting stability of the transcriptional regulator Irr (Puri et al., 2010, Qi & O’Brian, 2002).
We are interested in identifying manganese-dependent processes in B. japonicum towards understanding why it is an essential nutrient. In the present study, we show that manganese is required for a key step in glycolytic metabolism and for the detoxification of endogenously-derived superoxide.
RESULTS
Pyruvate kinase activity is deficient in an mntH mutant
We found previously that a B. japonicum mntH mutant cannot grow in low manganese media supplemented with glycerol as a carbon source, but can grow, albeit less well, when supplemented with pyruvate (Hohle & O’Brian, 2009, Puri et al., 2010). Thus, we can characterize the mntH mutant under manganese deficiency. Here, we wanted to address whether glycerol utilization requires a manganese-dependent step that is bypassed by pyruvate. Dihydroxyacetone phosphate is synthesized from glycerol by enzymatic phosphorylation and oxidation, and then enters the glycolytic pathway and metabolized to pyruvate by six additional enzymatic steps. The last step is catalyzed by pyruvate kinase, which converts phosphoenolpyruvate to pyruvate. Pyruvate kinase is a Mg2+-dependent enzyme in animals, but can use other divalent metal ions in vitro, including Mn2+ (Ainsworth & Macfarlane, 1975, Larsen et al., 1998). Mg2+ is used in assays of bacterial pyruvate kinase, and the coordinating glutamate and aspartate residues of the animal enzyme is conserved in bacteria, but the physiological metal for the bacterial enzyme has not been addressed.
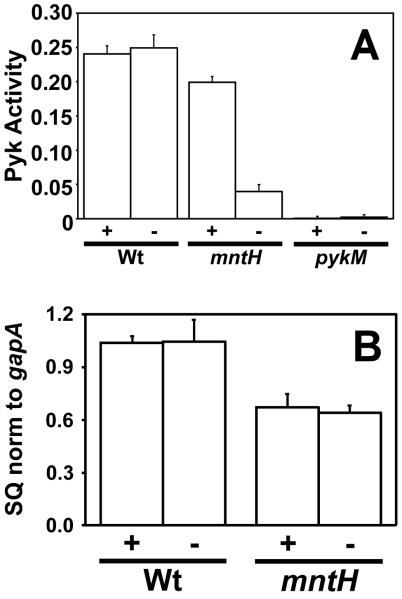
We measured pyruvate kinase activity in extracts from cells of the parent strain or the mntH mutant grown in low or high manganese media (Fig. 1A). Activity in the parent strain was unaffected by the manganese status in the medium, but it was much lower in the mntH mutant grown under manganese limitation. Addition of MnCl2 to cell extracts up to 1 mM did not restore activity in the mntH strain grown under manganese limitation or enhance activity of the wild type (data not shown). These observations show that pyruvate kinase activity requires MntH under manganese limitation, and suggest that it is manganese-dependent.
Figure 1.
Pyruvate kinase activity and pykM mRNA levels in B. japonicum.
A. Pyruvate kinase (Pyk) activity in B. japonicum parent strain and mntH and pykM mutant strains measured in whole cell extracts from cells grown in the presence (+) or absence (−) of 50 μM MnCl2 as described. Activity is expressed as nmol NADH oxidized/min/ μg protein ± the standard deviation of triplicate samples.
B. Steady state transcript levels of pykM in the parent strain and mntH mutant. mRNAs were analyzed by quantitative real-time PCR from cells grown in the presence (+) or absence (−) of 50 μM MnCl2. The data are expressed as the relative starting quantity (SQ) normalized to the housekeeping gene gapA, and presented as triplicate samples ± the standard deviation.
Identification of the B. japonicum pyruvate kinase gene pykM, and demonstration that PykM is a manganese-dependent enzyme
B. japonicum is predicted to have a single pyruvate kinase encoded by blr7138 based on genome sequence information. A mutant strain with a deletion in the blr7138 gene was constructed, and found to be essentially completely defective in pyruvate kinase activity (Fig. 1A), confirming that B. japonicum has one pyruvate kinase-encoding gene. This gene was designated pykM to denote manganese-dependence and other unique features as described below. pykM mRNA was diminished by about 35% in an mntH mutant compared to the wild type as determined by quantitative real time PCR (qPCR) (Fig. 1B), but transcript levels were not manganese-dependent, and thus cannot explain the low pyruvate kinase activity in the mntH strain under manganese limitation.
The pykM open reading frame was isolated, over expressed in E. coli and purified (Fig. 2A). This yielded a single band 53 kDa in size, which is in good agreement with its predicted size of 51.3 kDa. The purified recombinant protein had negligible activity when assayed in the absence of metal, but showed good activity in the presence of manganese (MnSO4) (Fig. 2B). By contrast, the sulfate salts of Mg2+, Ni2+, Zn2+, Fe2+ or Co2+ conferred very little activity on the recombinant pyruvate kinase. Manganese was also shown to affect secondary structure of purified PykM as determined by the change in the circular dichroism (CD) spectrum compared to the unmetallated protein (Fig. 2C). However, magnesium did not alter the CD spectrum, consistent with the very low activity in the presence of that divalent ion. The specificity of the pure protein, along with the low activity in an mntH mutant (Fig. 1A), strongly suggests that B. japonicum pyruvate kinase uses manganese under physiological conditions. The ability of manganese to confer activity on purified pyruvate kinase but not on cell extracts of the mntH mutant grown under manganese limitation suggests the protein is absent or in an inactive form that cannot be reconstituted by metal.
Figure 2.
Purification of pyruvate kinases and and properties of B. japonicum PykM.
A. Pyruvate kinases from B. japonicum (PykM) and E. coli (PykF and PykA) were overexpressed and purified as described in the text. 20 pmol of purified proteins were run on 12% SDS-PAGE. Molecular weight standards are in the right lane.
B. Metal-dependent activity of B. japonicum PykM. Pyruvate kinase activity of purified PykM was measured as described in the presence or absence of 100 μM of the noted metal. Each metal was supplied as the SO 2-4 salt. Sodium ascorbate (1 mM) was added to the reaction containing Fe2+ to prevent oxidation. Activity is expressed as nmol NADH oxidized/min/ μg protein ± the standard deviation of triplicate samples.
C. Effect of Mn2+ and Mg2+ on the circular dichroism spectrum of purified recombinant PykM. Far UV spectra of PykM (4.5 μM ) were recorded in the absence (solid line) or presence of 1 mM MnSO4 (dashed line) or 1 mM MgSO4 (dotted line). [θ] = Mean Residual Ellipticity.
D. Activator-independent activity of B. japonicum PykM. Pyruvate kinase activity of purified PykM was measured as described in the presence or absence of 100 μM MnSO4 or 100 μM MgSO4 and the presence or absence of 1mM fructose 1,6-bisphosphate (FBP) or 1mM adenosine 5’-monophosphate (AMP). Activity is expressed as nmol NADH oxidized/min/ μg protein ± the standard deviation of triplicate samples.
B. japonicum pyruvate kinase does not require a fructose 1, 6 bisphosphate or AMP effector for activity
Fructose 1,6 bisphosphate (FBP) is a positive allosteric effector of pyruvate kinases in eukaryotes and prokaryotes. FBP is a key intermediate in glycolysis, and its positive effect on pyruvate kinase coordinates enzyme activity with flux through the glycolytic pathway. We found that Mn2+-dependent pyruvate kinase activity of PykM was unaffected by FBP in the reaction (Fig. 2D). Pyruvate kinases from other organisms are routinely measured in the presence of Mg2+, and thus we addressed whether the very low activity of PykM as shown in Fig. 2B was due to the lack of a cofactor. Addition of FBP failed to stimulate activity in the presence of Mg2+ or Mn2+. In agreement with this, the lysine-382 residue of E. coli pyruvate kinase PykF implicated in FBP binding (Valentini et al., 2000) is missing in B. japonicum PykM, and contains threonine in the corresponding position.
In addition to the FBP-dependent pyruvate kinase PykF, some bacteria contain an additional isozyme, PykA, that is dependent on AMP (Muñoz & Ponce, 2003). We found that PykM was not affected by AMP in the presence of Mn2+ or Mg2+ (Fig. 2D). Our findings show that B. japonicum pyruvate kinase does not require the cofactors previously identified for the other bacterial enzymes. Thus, the designations pykF and pykA are not appropriate for the B. japonicum pyruvate kinase gene, and it was named pykM to denote this fact as well as its dependence on manganese.
Evidence that pyruvate kinase activity in E. coli does not require manganese
An mntH mutant of E. coli grows well aerobically on glucose in the absence of oxidative stress (Anjem et al., 2009), suggesting that manganese is not essential for growth under those conditions. We examined growth of the E. coli mntH mutant on glycerol because oxidation of that carbon source cannot bypass pyruvate kinase. We found that, unlike B. japonicum, an mntH mutant of E. coli grew on glycerol, albeit with a longer lag phase and to a lower final cell density (Fig. 3A). This suggests that the conversion of glycerol to pyruvate does not require manganese in E. coli. The mutant also showed a longer lag when grown on glucose (Fig.3B). E. coli contains two pyruvate kinase genes encoded by pykF and pykA. We overexpressed each gene purified the respective proteins (Fig. 2A) to determine their metal requirement (Fig. 3C, D).
Figure 3.
Pyruvate kinase activity of purified E. coli PykF and PykA.
A. Glycerol-dependent growth of E. coli parent strain and mntH mutant in low manganese media. M9 minimal media supplemented with 2% (v:v) glycerol as carbon source with no added manganese source was inoculated with 5 × 105 cells ml−1 of parent strain (closed squares) or mntH mutant (open squares). Aliquots were taken at the indicated time points and the optical density was measured at 600 nm (OD600).
B. Glucose-dependent growth of E. coli parent strain and mntH mutant in low manganese media. M9 minimal media supplemented with 2% (v:v) glucose as carbon source with no added manganese source was inoculated with 5 × 105 cells ml−1 of parent strain (closed squares) or mntH mutant (open squares). Aliquots were taken at the indicated time points and the optical density was measured at 600 nm (OD600).
C. Activator and metal dependent activity of E. coli PykF. Pyruvate kinase activity of purified PykF was measured as described in the presence or absence of 1 mM MnSO4 or 1 mM MgSO4 and the presence or absence of 1mM fructose-1,6-bisphosphate (FBP) or 1mM adenosine 5’-monophosphate (AMP). Activity is expressed as nmol NADH oxidized/min/ μg protein ± the standard deviation of triplicate samples.
D. Activator and metal dependent activity of E. coli PykA. Pyruvate kinase activity of purified PykA was measured as described in the presence or absence of 1 mM MnSO4 or 1 mM MgSO4 and the presence or absence of 1mM fructose-1,6-bisphosphate (FBP) or 1mM adenosine 5’-monophosphate (AMP). Activity is expressed as nmol NADH oxidized/min/ μg protein ± the standard deviation of triplicate samples.
PykF is the major pyruvate kinase in E. coli, accounting for over 90% of the activity in cells (Ponce et al., 1995). In the presence of Mg2+, PykF was found to be strictly dependent on FBP (Fig. 3C), in agreement with previous studies (Malcovati & Valentini, 1982). PykF was also active in the presence of Mn2+, but it was no longer dependent on FBP. Because FBP is well established as a positive allosteric effector of pyruvate kinases from both eukaryotes and prokaryotes, and Mn2+ is not taken up in unstressed E.coli cells (Anjem et al., 2009), it is very likely that the Mg2+-dependent activity observed here is physiologically relevant. This suggests that pyruvate kinase activity in E. coli does not require manganese.
Purified recombinant PykA activity was also metal-dependent, showing low activity in the presence of Mg2+, and much higher activity in the presence of Mn2+ (Fig. 3D). The activity with either metal was modestly stimulated in the presence of AMP. Thus, the metal requirement for PykA in vivo is less clear, but it accounts for only a small fraction of the pyruvate kinase activity in E. coli cells (Ponce et al., 1995).
Superoxide dismutase activity is defective in an mntH mutant
A manganese-dependent superoxide dismutase is common in many bacteria, but deficiency in this enzyme often does not necessarily have a strong growth phenotype under normal aerobic growth due to a second iron-dependent isozyme that serves to detoxify superoxide arising from respiration (Carlioz & Touati, 1986, Hassett et al., 1995). B. japonicum has three putative superoxide dismutase-encoding genes, but none of them have been characterized previously. We wanted to address the manganese-dependence on cellular superoxide dismutase activity by examining its status in an mntH mutant.
Cells of the parent strain and mntH mutant were grown in medium supplemented with 50 μM MnCl2 or no manganese added (0.4 μM Mn as determined by atomic absorption spectroscopy). The mntH gene is only expressed under manganese limitation, and it is this condition in which an mntH mutant has a phenotype (Hohle et al., 2011, Hohle & O’Brian, 2009). Superoxide dismutase activity was measured in extracts prepared from each cell type and condition (Fig. 4A). Activity in the wild type grown in manganese-replete medium was similar to that found in cells grown in low manganese media. However, superoxide dismutase activity was severely diminished in the mntH mutant grown under manganese limitation compared with those cells grown in the presence of manganese or in the wild type cells. Thus, activity was dependent on mntH and on manganese transport into the cytoplasm. As a control, we measured catalase activity (Fig. 4B) because catalases are oxidative stress response proteins, and B. japonicum KatG and other catalases uses heme rather than manganese as a cofactor. KatG is the primary hydrogen peroxide detoxifier in B. japonicum, and a katG mutant has almost no catalase activity (Panek & O’Brian, 2004)(Fig. 4B). The mntH mutant showed similar catalase activity when grown under manganese limitation compared with cells grown in in media supplemented with the metal, and was also comparable to activity in wild type cells. Thus, catalase activity is not mntH-dependent.
Figure 4.
Superoxide dismutase and catalase activities of B. japonicum parent and mutant strains.
A. Superoxide dismutase (SOD) activity. SOD activity in the B. japonicum parent strain and mntH, sodM, and katG mutants was measured using 10 μg cell extracts grown in the presence (+) or absence (−) of 50 μM MnCl2 as described. The data are expressed as ΔAbs (550 nm)/ min /μg protein, and presented as triplicate samples ± the standard deviation.
B. Catalase activity. Catalase activity in the B. japonicum parent strain and mntH, sodM, and katG mutants was measured using 10 μg whole cell extracts grown in the presence (+) or absence (−) of 50 μM MnCl2 as described. The data are expressed as μM H2O2 consumed per min per μg protein, and presented as triplicate samples ± the standard deviation.
Superoxide dismutase activity was also measured in proteins from the wild type and mntH separated by non-denaturing gel electrophoresis using an activity stain (Fig. 5A). A single band was observed in each cell type grown under low or high manganese conditions. However, the band intensity was diminished in the mntH mutant grown under manganese limitation. These findings suggest a single superoxide dismutase that is manganese-dependent.
Figure 5.
Detection of superoxide dismutase activity B. japonicum parent and mutant strains on non-denaturing gels.
A. Extracts of cells were electrophoresed through a nondenaturing PAGE. SOD activity was visualized by the inhibition of O2− scavenging by nitroblue tetrazolium in the presence of the O2− generator riboflavin by superoxide dismutase. The presence of SOD appeared as a colorless band against a purple background. 25 μg of protein was loaded in each lane.
B. Steady state transcript levels of sodM in the parent strain and mntH mutant. mRNAs were analyzed by quantitative real time PCR from cells grown in the presence (+) or absence (−) of 50 μM MnCl2. The data are expressed as the relative starting quantity (SQ) normalized to the housekeeping gene gapA, and presented as triplicate samples ± the standard deviation.
Identification of Bll7774 (SodM) as the major superoxide dismutase in B. japonicum under unstressed growth
Although B. japonicum appears to express a single superoxide dismutase under the conditions examined herein, the B. japonicum USDA110 sequenced and annotated genome contains three genes, bll7559, bll7774 and bll8073, that encode putative superoxide dismutases of the SodM/SodF type (Kaneko et al., 2002). In addition, blr5051 is annotated as a superoxide dismutase, but has no homology to the known enzymes, and it was not considered further. Annotation of the metal cofactor of the products of uncharacterized genes is unreliable because the manganese- and iron-dependent superoxide dismutases are similar to each other in their primary amino acid sequences.
To determine which genes are expressed, steady state mRNA levels of bll7559, bll7774 and bll8073 were measured in wild type cells by quantitative real time PCR (Fig. 6). Only bll7774 transcripts were detectable, and the levels were similar in cells grown in low or high manganese media. This gene was designated sodM to denote manganese-dependence as described below. A mutant strain was constructed that is missing the sodM gene (see Experimental procedures) and analyzed further. Superoxide dismutase activity in extracts from the sodM mutant was low in the liquid assay (Fig. 4A) regardless of the manganese status, but retained catalase activity (Fig. 4B). Moreover, the single superoxide dismutase activity band observed in the wild type was absent in the sodM mutant (Fig. 5A). sodM mRNA levels were similar in the wild type and mntH mutant (Fig. 5B). The findings show that SodM is the major functional superoxide dismutase under the conditions examined, and this activity is manganese-dependent.
Figure 6.

Steady state transcript levels of putative SOD homologues in the B. japonicum parent strain. mRNAs were analyzed by quantitative real-time PCR from cells grown in the presence (+) or absence (−) of 50 μM MnCl2. The data are expressed as the relative starting quantity (SQ) normalized to the housekeeping gene gapA, and presented as triplicate samples ± the standard deviation. The level detection is about 0.003, and the readings for bll7559 and bll8073 fall below the level of detection.
Growth phenotypes of mutants suggest a role for manganese in addition to PykM and SodM
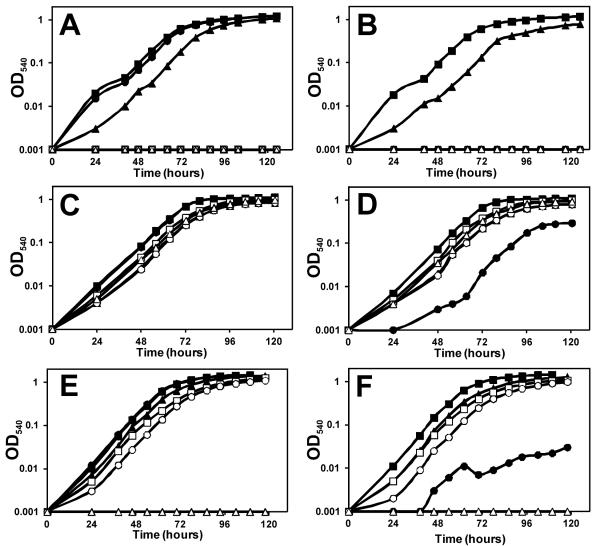
An mntH mutant of B. japonicum has almost no growth phenotype on glycerol in manganese replete media, but it grows very little on that carbon source when manganese is limiting (Hohle & O’Brian, 2009) (Fig. 7A, B). The pyruvate kinase mutant pykM showed almost no growth on glycerol regardless of the manganese status, consistent with the conclusion that PykM is the only pyruvate kinase. The pykM strain grew fairly well on pyruvate (Fig. 7C, D) as expected because pyruvate is the product of PykM. Pyruvate also partially rescued growth of the mntH strain under manganese limitation, but it grew less well under those conditions than did the pykM mutant.
Figure 7.
Dependence of manganese supplementation and carbon source for growth of B. japonicum parent strain and mutant derivatives. Growth media were inoculated with 5 × 105 cells/ml of parent strain (closed squares), mntH mutant (closed circles), pykM mutant (open squares), sodM mutant (closed triangles), pykM sodM double mutant (open circles), and katG mutant (open triangle) and grown in media containing (A) 50 μM MnCl2 and glycerol as carbon source, (B) no exogenous manganese and glycerol as carbon source, (C) 50 μM MnCl2 and pyruvate as carbon source, or (D) no exogenous manganese and pyruvate as carbon source. (E) 50 μM MnCl2 and gluconate as carbon source, or (F) no exogenous manganese and gluconate as carbon source. Unsupplemented media contains 0.4 μM manganese. Aliquots were taken at the indicated time points and the optical density was measured at 540 nm (OD540).
Unlike the mntH or pykM strains, the sodM mutant grew on glycerol (Fig. 7A, B). However, sodM did not grow as well as the wild type, consistent with the generation of superoxide during respiration. The lag phase of growth of the sodM strain was similar to what is observed in a Pseudomonas aeruginosa mutant defective in both cytoplasmic superoxide dismutases (Hassett et al., 1995). Like the pykM strain, the sodM mutant grew better on pyruvate than did the mntH strain under manganese limitation (Fig. 7C, D). A pykM sodM double mutant was constructed to address whether deficiencies in pyruvate kinase and superoxide dismutase activities were sufficient to explain the growth defect of the mntH strain. The double mutant barely grew on glycerol, similar to the pykM single mutant, and grew similarly to the sodM mutant on pyruvate. The double mutant also grew better on pyruvate than did the mntH strain under manganese limitation. These findings suggest that defective PykM and SodM activities likely contribute to the mntH phenotype, but additional manganese-dependent processes occur in cells.
Pyruvate has been shown to detoxify hydrogen peroxide (Nath et al., 1995), and mntH is required for Mn2+ uptake for H2O2-induced stress responses in E. coli (Anjem et al., 2009, Sobota & Imlay, 2011). The catalase-peroxidase KatG accounts for most of the H2O2 consumption in B. japonicum cells (Panek & O’Brian, 2004), thus, we compared the growth phenotypes of mntH and katG strains. The katG strain grew very poorly aerobically on glycerol, which was not rescued by manganese supplementation to the medium (Fig. 7A,B). It did, however grow fairly well on pyruvate (Fig. 7C, D), supporting the idea that pyruvate detoxifies H2O2. Moreover, the katG mutant grew better on pyruvate than did the mntH strain even though the katG strain likely needs to detoxify much more H2O2. These findings suggest that the growth phenotype of the mntH strain cannot be fully explained by impaired protection from H2O2 produced from aerobic metabolism.
We wanted to grow cells on a carbon source that bypasses the need for pyruvate kinase, but that did not scavenge H2O2, and then test the growth phenotype of the mntH mutant on that carbon source. The B. japonicum genome putatively has the three genes needed to synthesize pyruvate from gluconate, namely gluconate kinase (blr6762), phosphogluconate dehydratase (bll6092) and 2-keto-3-deoxylguconate -6-phosphate aldolase (blr3923). In agreement with this, the parent strain and pykM mutant grew on gluconate regardless of the manganese status (Fig. 7 E, F). The katG mutant did not grow on gluconate, confirming that it cannot detoxify H2O2. The mntH mutant grew only very slightly better on gluconate under low manganese conditions (Fig. 7F) than it did on glycerol (Fig. 7B). These observations further support the conclusion that pyruvate kinase requires MntH, but that there are additional MntH- and manganese-dependent processes.
DISCUSSION
Previous studies of mutants defective in the Mn2+ transporter MntH indicate that manganese is required for unstressed aerobic growth in B. japonicum (Hohle & O’Brian, 2009), but in E. coli the metal is needed primarily in response to H2O2-induced stress (Anjem et al., 2009, Sobota & Imlay, 2011). In the present study, we identified two manganese-dependent enzymes that function under normal, unstressed growth conditions, and B. japonicum appears unable to compensate for deficiencies in either enzyme with a manganese-independent activity. Thus, B. japonicum appears to be more reliant on manganese than has been described in E. coli. Our findings do not rule out an additional role for manganese in H2O2 stress response, but it is unlikely to be a primary role under the conditions used in the present study.
Unlike eukaryotic pyruvate kinases, B. japonicum PykM uses Mn2+ rather than Mg2+ for activity. Mn2+ can substitute for other metals, particularly Mg2+, in enzymatic reactions in vitro and therefore it is often difficult to establish the physiological role for Mn2+ in cellular processes. However, the deficient activity in the Mn2+ transport mutant and the clear preference for Mn2+ by the purified recombinant enzyme strongly suggests that Mn2+ is the physiological metal for B. japonicum pyruvate kinase. PykM (Blr7138) is the only pyruvate kinase in B. japonicum based on the lack of activity in a pykM mutant, as well as the growth defect on glycerol. Thus, our findings strongly implicate manganese in normal glycolytic function. The glycolytic enzyme phosphoglycerate mutase from Bacillus stereothermophilus is Mn2+-dependent (Jedrzejas et al., 2000), but that isozyme is phylogenetically-restricted and no structural homolog of it was found in the B. japonicum genome. The putative phosphoglycerate mutase genes of B. japonicum (blr0686 and blr2630) are well-represented in eubacteria, and no metal requirement has been described for the proteins they encode.
Pyruvate shuttles into numerous biosynthetic and energy-generating pathways, placing pyruvate kinase, hence manganese, at a crucial metabolic intersection. In this light, it is not surprising that B. japonicum has not only a high affinity inner membrane transporter for Mn2+, but also the specific outer membrane channel MnoP for uptake of the metal (Hohle et al., 2011). This Mn2+ transport system of B. japonicum can acquire manganese from the environment down to nanomolar levels (Hohle et al., 2011, Hohle & O’Brian, 2009).
An E.coli mntH mutant grows well on glucose (Anjem et al., 2009), but that substrate renders pyruvate kinase not essential because pyruvate can be generated from PEP by the phosphotransferase system (Pertierra & Cooper, 1977, Ponce et al., 1995). Moreover, there are two pyruvate kinases, suggesting that no single enzyme is essential. Here, we found that the E. coli mntH mutant grows on glycerol, albeit less well than the wild type, a condition which requires pyruvate kinase. Our findings suggest that the major pyruvate kinase PykF can use Mg2+ as the metal cofactor, similar to what is found for the animal enzyme.
Fructose 1,6-bisphosphate (FBP) is a key regulator of glycolysis in eukaryotes and prokaryotes. However, we found that PykM is not responsive to FBP, and a similar result was found for the Staphylococcus aureus pyruvate kinase (Zoraghi et al., 2010). Interestingly, B. japonicum can metabolize glucose via the Entner-Doudoroff pathway, which does not yield a FBP intermediate, and some strains favor that route (Mulongoy & Elkan, 1977). Further studies are needed to assess allosteric control of B. japonicum pyruvate kinase.
Oxygen detoxifying enzymes are involved in normal aerobic metabolism even in the absence of an external source of reactive oxygen species (ROS) because respiration generates ROS through the incomplete reduction of O2. In E. coli and Pseudomonas aeruginosa, an iron-dependent superoxide dismutase serves in basal oxidative defense, whereas the manganese-dependent enzyme detoxifies superoxide generated from an induced stress (Hassett et al., 1995, Carlioz & Touati, 1986). The iron-dependent enzyme may contribute to a decreased dependence on manganese under unstressed conditions. In the current study, we identified SodM as the only functional superoxide dismutase expressed under normal aerobic growth even though there are three putative genes encoding this enzyme in the genome. Moreover, SodM is manganese-dependent and thus, unlike E. coli, manganese is involved in detoxification of superoxide in B. japonicum cells that are not under induced stress. It remains to be determined whether other organisms with a single manganese superoxide dismutase are generally more dependent on manganese.
Studies of the rhizobia and Brucella suggest common features in manganese metabolism, including phenotypes of Mn2+ transport mutants, the Mur transcriptional regulator, and manganese-dependent superoxide dismutase. These bacteria can reside within eukaryotic cells, albeit with a different outcome from the host perspective. It is plausible that manganese is advantageous for infection or intracellular maintenance of the bacterium. Host cells respond to infection with an oxidative burst in both the pathogenic and symbiotic interactions (Santos et al., 2001), and manganese may be protective. We note, however, that the Mur repressor and the iron response regulator Irr typify the alpha-Proteobacteria as a whole, and this group collectively occupies many ecological niches. Thus, it may not be simple to identify common themes to account for similar modes of metalloregulation. Additional work with other members of the alpha-Proteobacteria should bring this question into sharper focus.
EXPERIMENTAL PROCEDURES
Strains and media
Bradyrhizobium japonicum strain USDA110 is the parent strains used in this study. Strains mntHΔΩ (Hohle & O’Brian, 2009) and katGΔΩ (Panek & O’Brian, 2004) were described previously. pykMΔΩ, and sodMΔΩ are mutant derivatives of USDA110 in which the respective gene is replaced by an Ω-cassette (Prentki & Krisch, 1984). The pykMΔΩ mutant was constructed by deleting nucleotides 40 through 1371 of the open reading frame of the pykM gene, and replacing it with a BamHI-cut Ω-cassette into BglII sites engineered into the primers used for the deletion. The sodMΔΩ mutant was constructed by deleting nucleotides 62 through 589 within sodM opening reading frame, and replacing it with a SmaI-cut Ω-cassette into EcoRV sites engineered into the primers used for the deletion. Strain pykMΔΩsodMΔ was constructed by mutating pykMΔΩ by removal of a portion of the sodM open reading frame as described above, resulting in an in-frame deletion of sodM. B. japonicum strains were routinely grown at 29°C in glycerol-salts-yeast (GSY) medium as previously described (Frustaci et al., 1991). For low manganese conditions, modified GSY was used, containing 0.5 g l−1 yeast extract instead of 1 g/l, with no exogenous manganese added. The actual concentration of manganese in unsupplemented media was 0.4 μM as determined by atomic absorption using a Perkin-Elmer model 1100B atomic absorption spectrometer. High manganese media was supplemented with 50 μM MnCl2. The concentration of carbon source in the modified media was 54.3 mM.
E.coli strain K12 BW25113 is the parent strain used in this study. Strain JW2388-1 is a mutant derivative of BW25113 in which the mntH gene is replaced by a kanamycin resistance cassette. Both E. coli strains were obtained from the Coli Genetic Stock Center at Yale (http://cgsc2.biology.yale.edu/index.php). E. coli strains were routinely grown at 37°C in LB medium.
Preparation of cellular extracts
Cellular extracts of the parent strain, mntHΔΩ, pykΔΩ sodMΔΩ, and katGΔΩ were prepared from 100 ml cultures grown in 500 ml flasks to O.D. (540 nm) to 0.5. Cells were harvested by centrifugation and washed 2 times in lysis buffer (50 mM Tris Buffer, pH 8.0) before being resuspended in 3 ml lysis buffer. Cells were disrupted by passage through a French pressure cell at 900 p.s.i. and clarified by centrifugation at 13000 x g for 5 minutes. Extracts were quantified using the Bradford Assay.
Analysis of RNA
100 ml cultures were grown with shaking in 500 ml flasks to O.D. (540 nm) to 0.5. Expression levels of selected genes were determined by qPCR with iQ™ SYBR green supermix (Bio-Rad) using iCycler thermal cycler (Bio-Rad). RNA was isolated from B. japonicum cells using a hot phenol extraction method as previously described (Yang et al., 2006). cDNA was synthesized from 5 μg total RNA using iScript™ cDNA synthesis kit (Bio-Rad). qPCR reactions were carried out as previously described (Hohle & O’Brian, 2009).
Protein overexpression and purification
The pykM gene from B. japonicum and the pykA and pykF genes from E. coli were amplified by PCR and cloned into pET14b vector (Novagen) containing an N-terminal 6xHis tag. The vector with insert was transformed into chemical competent BL21(DE3) (pLysS) E. coli cells. Cells were inoculated from an overnight culture grown in Luria-Bertani media containing 200 μg/ml ampicillin and 25 μg/ml chloramphenicol into 1 liter of fresh 2×YT medium containing 200 μg/ml ampicillin and 25 μg/ml chloramphenicol. Overexpression was induced in cells at the mid-log phase by the addition of 0.5 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG) at 37 °C for 4 hours with shaking. Cells were pelleted by centrifugation, washed in phosphate binding buffer (50 mM NaH2PO4 and 300 mM NaCl, pH 8.0), and subsequently resuspended in 5 ml phosphate binding buffer. Cells were disrupted by passage through a French pressure cell at 1200 p.s.i. and clarified by centrifugation at 13000 x g for 5 minutes. One ml of Ni-NTA slurry (Qiagen) was washed in phosphate binding buffer before being added to the cleared lysate and rocked for 60 minutes at 4 °C.
The Ni-NTA slurry-protein mixture was poured into a column and washed four times with phosphate wash buffer (50 mM NaPO4, 300 mM NaCl, and 20 mM imidazole, pH 8.0) and once with phosphate wash buffer containing 10% glycerol. His-tagged proteins were eluted using phosphate elution buffer (50 mM NaPO4, 300 mM NaCl, and 250 mM imidazole, pH 8.0). The purified proteins were run through an FPLC buffer exchange column so that the final buffer consisted of 50 mM NaPO4, 300 mM NaCl, and 10% glycerol. Nickel contamination from the column was less than 0.02 mol Ni per mol of protein as determined by inductively coupled plasma mass spectrometry (Thermo Scientific X Series 2).
Protein quantity was estimated using the Bradford method. Twenty pmol of each purified protein was run on a 12% denaturing polyacrylamide gel to check purity.
Pyruvate kinase assay
Pyruvate kinase activity was measured using an assay coupled to lactate dehydrogenase. The reaction mixture consisted of 25 mM Bis-Tris Propane, pH 6.9, 20 mM KCl, 5% PEG-8000, 2 mM DTT, 2 mM ADP, 2 units lactate dehydrogenase, 2 mM phosphoenolpyruvate (PEP), 0.15 mM NADH and either 10 μg whole cell extracts or 20 pmol purified protein in a final volume of 1 ml. One mM sodium ascorbate was added to the reactions with Fe2+ to prevent oxidation. Reactions containing B. japonicum pure protein were supplemented with or without 0.1 mM metal. For extracts, the assay was carried out with 0, 0.1 mM or 1 mM MnCl2. Assays of the pure E. coli pyruvate kinase proteins were supplemented with or without 1 mM metal. Where applicable, 1 mM fructose-1,6-bisphosphate or AMP was added to the reaction. The reaction was monitored for 3 minutes.
Circular Dichroism
PykM (4.5 μM) in Na-PO4 buffer (pH 7.0) was measured on a Jasco J-815 CD spectrometer using a 0.1 cm path length at 22°C. Far UV spectra (200 – 260 nm) were collected for the protein alone, or for the protein in the presence of 1 mM MnSO4 or 1 mM MgSO4. Data were processed using the Jasco software and exported to and analyzed in Microsoft Excel.
Bacterial growth studies
The parent strain, mntHΔΩ, pykMΔΩ, sodMΔΩ, pykMΔΩsodMΔ, and katGΔΩ were grown in modified GSY medium under high (50 μM MnCl2) or low (0.4 μM) manganese conditions with glycerol, pyruvate or gluconate as the carbon source as described above. Growth rates were analyzed by measuring the optical density of cells at 540 nm every 8 h until reaching stationary phase.
Superoxide dismutase assays
Fifty μg of cellular extracts were run on a 7.5% polyacrylamide non-denaturing gel at 200V for 2 hours at 4°C. The gel was soaked and rocked at 75 rev/min in a 1 mg/ml nitroblue tetrazolium solution for 15 minutes before being briefly washed twice with distilled water. The gel was then soaked and rocked at 75 revolutions/min in soaking buffer (100 mM potassium phosphate, 28 mM TEMED, and 28 μM riboflavin, pH 7.0) in the dark. The gel was briefly washed 2 times with distilled water. The gel was then exposed to bright light to initiate the photochemical reaction.
Total SOD activity in cell extracts was measured by the xanthine oxidase-cytochrome c method (McCord & Fridovich, 1969).
Catalase assay
Total catalase activity in cell extracts was measured as previously described (Panek & O’Brian, 2004).
ACKNOWLEDGMENTS
We thank Sarah Popadowski for construction of pET14b-pykM. This work was supported by NIH grant R01 GM099667. We acknowledge the NSF MRI Program (CHE 0959565), which supported the purchase of the ICP-MS.
REFERENCES
- Ainsworth S, Macfarlane N. Activation and inhibition of rabbit muscle pyruvate kinase by transition-metal ions. Biochem J. 1975;145:63–71. doi: 10.1042/bj1450063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ES, Paulley JT, Gaines JM, Valderas MW, Martin DW, Menscher E, Brown TD, Burns CS, Roop RM., 2nd The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect Immun. 2009;77:3466–3474. doi: 10.1128/IAI.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao TC, Becker A, Buhrmester J, Puhler A, Weidner S. The Sinorhizobium meliloti fur gene regulates, with dependence on Mn(II), transcription of the sitABCD operon, encoding a metal-type transporter. J Bacteriol. 2004;186:3609–3620. doi: 10.1128/JB.186.11.3609-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BW, Walker GC. Disruption of sitA compromises Sinorhizobium meliloti for manganese uptake required for protection against oxidative stress. J Bacteriol. 2007;189:2101–2109. doi: 10.1128/JB.01377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Mireles E, Wexler M, Todd JD, Bellini D, Johnston AW, Sawers RG. The manganese-responsive repressor Mur of Rhizobium leguminosarum is a member of the Fur-superfamily that recognizes an unusual operator sequence. Microbiology. 2005;151:4071–4078. doi: 10.1099/mic.0.28342-0. [DOI] [PubMed] [Google Scholar]
- Domenech P, Pym AS, Cellier M, Barry CE, 3rd, Cole ST. Inactivation of the Mycobacterium tuberculosis Nramp orthologue (mntH) does not affect virulence in a mouse model of tuberculosis. FEMS Microbiol Lett. 2002;207:81–86. doi: 10.1111/j.1574-6968.2002.tb11032.x. [DOI] [PubMed] [Google Scholar]
- Frustaci JM, Sangwan I, O’Brian MR. Aerobic growth and respiration of a δ-aminolevulinic acid synthase (hemA) mutant of Bradyrhizobium japonicum. J. Bacteriol. 1991;173:1145–1150. doi: 10.1128/jb.173.3.1145-1150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett DJ, Schweizer HP, Ohman DE. Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J. Bacteriol. 1995;177:6330–6337. doi: 10.1128/jb.177.22.6330-6337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohle TH, Franck WL, Stacey G, O’Brian MR. Bacterial outer membrane channel for divalent metal ion acquisition. Proc Natl Acad Sci U S A. 2011;108:15390–15395. doi: 10.1073/pnas.1110137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohle TH, O’Brian MR. The mntH gene encodes the major Mn2+ transporter in Bradyrhizobium japonicum and is regulated by manganese via the Fur protein. Mol Microbiol. 2009;72:399–409. doi: 10.1111/j.1365-2958.2009.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohle TH, O’Brian MR. Transcriptional control of the Bradyrhizobium japonicum irr gene requires repression by Fur and antirepression by Irr. J Biol Chem. 2010;285:26074–26080. doi: 10.1074/jbc.M110.145979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubovics NS, Jenkinson HF. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology. 2001;147:1709–1718. doi: 10.1099/00221287-147-7-1709. [DOI] [PubMed] [Google Scholar]
- Jedrzejas MJ, Chander M, Setlow P, Krishnasamy G. Structure and mechanism of action of a novel phosphoglycerate mutase from Bacillus stearothermophilus. EMBO J. 2000;19:1419–1431. doi: 10.1093/emboj/19.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Nakamura Y, Sato S, Minamisawa K, Uchiumi T, Sasamoto S, Watanabe A, Idesawa K, Iriguchi M, Kawashima K, Kohara M, Matsumoto M, Shimpo S, Tsuruoka H, Wada T, Yamada M, Tabata S. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 2002;9:189–197. doi: 10.1093/dnares/9.6.189. [DOI] [PubMed] [Google Scholar]
- Larsen TM, Benning MM, Rayment I, Reed GH. Structure of the Bis(Mg2+)–ATP–Oxalate Complex of the Rabbit Muscle Pyruvate Kinase at 2.1 Å Resolution: ATP Binding over a Barrel†,‡. Biochemistry. 1998;37:6247–6255. doi: 10.1021/bi980243s. [DOI] [PubMed] [Google Scholar]
- Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MF. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- Malcovati M, Valentini G. AMP- and fructose 1,6-bisphosphate-activated pyruvate kinases from Escherichia coli. Methods Enzymol. 1982;90(Pt E):170–179. doi: 10.1016/s0076-6879(82)90123-9. [DOI] [PubMed] [Google Scholar]
- Martin JE, Imlay JA. The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol Microbiol. 2011;80:319–334. doi: 10.1111/j.1365-2958.2011.07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Mulongoy K, Elkan GH. Glucose catabolism in two derivatives of a Rhizobium japonicum strain differing in nitrogen-fixing efficiency. J Bacteriol. 1977;131:179–187. doi: 10.1128/jb.131.1.179-187.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E, Ponce E. Pyruvate kinase: current status of regulatory and functional properties. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2003;135:197–218. doi: 10.1016/s1096-4959(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Nath KA, Ngo EO, Hebbel RP, Croatt AJ, Zhou B, Nutter LM. Alpha-ketoacids scavenge H2O2 in vitro and in vivo and reduce menadione-induced DNA injury and cytotoxicity. The American journal of physiology. 1995;268:C227–236. doi: 10.1152/ajpcell.1995.268.1.C227. [DOI] [PubMed] [Google Scholar]
- O’Brian MR, Fabiano E. Mechanisms and regulation of iron homeostasis in the Rhizobia. In: Cornelis P, Andrews SC, editors. Iron Uptake and Homeostasis in Microorganisms. Caister Academic Press; Norfolk: 2010. pp. 37–63. [Google Scholar]
- Panek HR, O’Brian MR. KatG is the primary detoxifier of hydrogen peroxide produced by aerobic metabolism in Bradyrhizobium japonicum. J Bacteriol. 2004;186:7874–7880. doi: 10.1128/JB.186.23.7874-7880.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertierra AG, Cooper RA. Pyruvate formation during the catabolism of simple hexose sugars by Escherichia coli: studies with pyruvate kinase-negative mutants. J Bacteriol. 1977;129:1208–1214. doi: 10.1128/jb.129.3.1208-1214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platero R, de Lorenzo V, Garat B, Fabiano E. Sinorhizobium meliloti fur-like (Mur) protein binds a fur box-like sequence present in the mntA promoter in a manganese-responsive manner. Appl Environ Microbiol. 2007;73:4832–4838. doi: 10.1128/AEM.00686-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platero RA, Jaureguy M, Battistoni FJ, Fabiano ER. Mutations in sitB and sitD genes affect manganese-growth requirements in Sinorhizobium meliloti. FEMS Microbiol Lett. 2003;218:65–70. doi: 10.1111/j.1574-6968.2003.tb11499.x. [DOI] [PubMed] [Google Scholar]
- Ponce E, Flores N, Martinez A, Valle F, Bolivar F. Cloning of the two pyruvate kinase isoenzyme structural genes from Escherichia coli: the relative roles of these enzymes in pyruvate biosynthesis. J Bacteriol. 1995;177:5719–5722. doi: 10.1128/jb.177.19.5719-5722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- Prentki P, Krisch HM. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;28:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Puri S, Hohle TH, O’Brian MR. Control of bacterial iron homeostasis by manganese. Proc Natl Acad Sci USA. 2010;107:10691–10695. doi: 10.1073/pnas.1002342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, O’Brian MR. Interaction between the bacterial iron response regulator and ferrochelatase mediates genetic control of heme biosynthesis. Mol. Cell. 2002;9:155–162. doi: 10.1016/s1097-2765(01)00431-2. [DOI] [PubMed] [Google Scholar]
- Que Q, Helmann JD. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol. 2000;35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- Santos R, Herouart D, Sigaud S, Touati D, Puppo A. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol Plant Microbe Interact. 2001;14:86–89. doi: 10.1094/MPMI.2001.14.1.86. [DOI] [PubMed] [Google Scholar]
- Sobota JM, Imlay JA. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci U S A. 2011;108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini G, Chiarelli L, Fortin R, Speranza ML, Galizzi A, Mattevi A. The Allosteric Regulation of Pyruvate Kinase. J. Biol. Chem. 2000;275:18145–18152. doi: 10.1074/jbc.M001870200. [DOI] [PubMed] [Google Scholar]
- Yang J, Sangwan I, Lindemann A, Hauser F, Hennecke H, Fischer HM, O’Brian MR. Bradyrhizobium japonicum senses iron through the status of haem to regulate iron homeostasis and metabolism. Mol Microbiol. 2006;60:427–437. doi: 10.1111/j.1365-2958.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoraghi R, See RH, Gong H, Lian T, Swayze R, Finlay BB, Brunham RC, McMaster WR, Reiner NE. Functional analysis, overexpression, and kinetic characterization of pyruvate kinase from methicillin-resistant Staphylococcus aureus. Biochemistry. 2010;49:7733–7747. doi: 10.1021/bi100780t. [DOI] [PubMed] [Google Scholar]