INTRODUCTION
Colorectal cancer comprises a heterogeneous group of diseases that arise through varying molecular carcinogenic pathways, involving complex interactions between tumour cells and the host microenvironment.[1, 2] It has long been appreciated that developmental and physiological differences exist between anatomic segments of the colorectum, and that colorectal cancers occur with distinctly different frequencies at different subsites.[3] Whilst approaches to surgical and adjuvant therapy have set rectal cancer as a separate entity, colon cancers still tend get lumped together.
In the 1980’s, epidemiological and molecular data suggested that cancers arising at different subsites may be biologically disparate, implying different cancer aetiologies or evolutions. These observations prompted the suggestion that the proximal and distal colon be considered separately in aetiological studies, with the splenic flexure as a demarcation point.[3] This dichotomisation has been propagated by subsequent clinical, translational, and epidemiological studies,[4–10] whilst evolving molecular data have galvanised and lent further support to the two-colon concept.[11–14] It remains uncertain, however, whether the splenic flexure represents a genuine or arbitrary divide in the aetiological spectrum of colon cancers. Notably, our recent study,[15] demonstrating that the frequencies of key tumour molecular features change gradually along the length of the colon (rather than abruptly at the splenic flexure), challenges the current two-colon paradigm. The aim of this article is to review data relevant to the current concept of distinct molecular pathogeneses for proximal vs. distal colon cancers, describe the impact of our recent findings, and discuss the implications for future research.
THE EVOLUTION OF THE TWO-COLON CONCEPT
Historical Overture
There are incontrovertible differences between the ontogeny, morphology, biochemistry and physiology of the proximal and distal colon.[11–14] Furthermore, gross macroscopic differences are well described between proximal and distal colorectal tumours.[9, 11–14] In the early 1980’s, epidemiological studies drew attention to sex and age disparities in site-specific colon cancer incidence, whilst contemporaneous reports highlighted an apparent upward trend in the incidence ratio of proximal to distal cancers.[3, 16, 17] Consequently, it was proposed that proximal and distal cancers may represent distinct disease entities.[3, 16, 18] The credibility of this hypothesis was bolstered by emerging cytogenetic and molecular data on the heterogeneity of colorectal cancer,[18, 19] including evidence that large scale chromosomal aberrations were relatively more frequent in distal cancers.[19]
An authoritative review of the evidence for genetically distinct proximal vs. distal colorectal cancer phenotypes was published by Bufill in 1990.[20] Numerous reports thereafter have supported the concept that proximal and distal cancers arise through distinct molecular pathways, and the two-colon concept has become the prevailing dogma.[11–14]
Genetic and Epigenetic Geography of Colorectal Cancer
In addition to clinical and pathological characteristics, cellular genomic and epigenomic determinants serve as important predictors of tumour evolution and progression.[21] Chromosomal instability (CIN), a common type of genomic instability in colorectal cancer, is characterised by widespread numeric chromosomal aberrations, subchromosomal amplifications, and loss of heterozygosity.[22] CIN is implicated in 60–70% of colorectal cancers, and is more commonly observed in distal compared to proximal cancers.[23, 24]
Microsatellite instability (MSI), characterised by somatic alterations in microsatellite repeat length, represents another distinct form of genomic instability observed in colorectal cancer.[25–27] In contrast to CIN, cancers that display a high degree of MSI (MSI-high) preferentially occur in the proximal colon.[23, 24, 28] MSI-high cancers constitute approximately 15% of all colorectal cancers and most frequently result from aberrant promoter hypermethylation and epigenetic silencing of the mismatch repair (MMR) gene MLH1.[23, 24, 28] MLH1 promoter hypermethylation is most commonly a consequence of the CpG island methylator phenotype (CIMP-high), a form of epigenomic instability.[21, 29–32] MSI-high is also the hallmark of cancers arising in the context of the Lynch syndrome, where genetic predisposition is attributable to germline mutations in MMR genes.[23, 24, 28]
BRAF mutations are also more common in proximal colon cancers.[33–37] BRAF mutations are strongly associated with CIMP-high, which, in turn, correlates strongly with MSI-high.[33–37] Although CIMP-high and MSI-high cancers are both independently associated with proximal colonic location, only CIMP-high cancers are independently associated with BRAF mutation.[33–36]
An Epidemiological Scission
The apparent “left-to-right shift” in colorectal cancer incidence reported by epidemiological studies from the United States in the late 1970s and early 1980s,[16] lead to the adoption of the two-colon model by numerous incidence trend studies internationally.[38, 39] A true site-specific shift in incidence has not been a consistent finding of such studies. Nonetheless, age and sex-specific differences in proximal and distal cancer incidence continue to be described.[40]
Epidemiological studies, including molecular pathological epidemiology research,[1, 2, 41] have also employed the splenic flexure divide when investigating the effect of potential aetiological exposures. For example, prospective data from the Iowa Women’s Health Study demonstrate that the relationship between smoking and colorectal cancer risk is strongest for proximal cancers, and for MSI-high, CIMP-high, and BRAF-mutated phenotypes.[42] The possible association between red or processed meat consumption and colorectal cancer risk is reportedly greater for distal cancers,[4] whilst the protective effect of regular aspirin use appears strongest for proximal cancers.[6]
Two Colons, Two Outcomes?
Data relating to colon cancer location and mortality are conflicting. Based on data from the German “Colon/Rectal Carcinoma” multicentre observational study, including 17,641 patients, it was reported that proximal colon cancers carry a significantly worse prognosis compared to distal cancers.[5] The size of this effect, however, diminished substantially following adjustment and stratification by disease stage. Furthermore, data from 53,801 colorectal cancer cases in the Surveillance, Epidemiology and End Results (SEER)-Medicare database, demonstrated no overall difference in five-year survival.[8] Interestingly, both of these studies showed a lower mortality for proximal vs. distal cancers for individuals with stage II disease. To date, tumour location has been largely neglected by oncology clinical trialists, and there is therefore a paucity of data on differences in outcome between proximal and distal colon cancers in relation to adjuvant therapy.[7]
A COLORECTAL CONTINUUM
Despite clear epidemiological, molecular, and clinicopathological correlates with colorectal cancer location, many individuals consider it implausible that these associations change abruptly at the splenic flexure. Nonetheless, the two-colon model predominates. It therefore remains uncertain whether the splenic flexure represents a genuine demarcation, or whether it is more a divide of convenience that polarises proximal-distal associations for markers that actually change gradually along the length of the colorectum. LaPointe and colleagues undertook an analysis of normal colonic tissue transcripts by microarray.[43] The gene expression data indicate the existence of two distinct groups of transcripts: a dominant group, whose expression pattern follows the classical dichotomous model, and a second group, where expression levels appear to change gradually along the proximal-distal axis of the bowel.[43] Worthley and colleagues observed a similar gradual transition along the normal colon for certain methylation markers, including ESR1, HIC1, and APBA2.[44]
Recent interest has focused on whether colorectal carcinogenesis might occur, through specific molecular mechanisms as a result of interactions between the gut microbiota, innate immune system, and other host factors, such as diet.[2, 45–48] Luminal contents, including gut microbial communities and their metabolites, might trigger initiating molecular events or, alternatively, influence the tumour microenvironment and promote neoplastic progression. Microbial and biochemical components of the luminal ecosystem, as well as interactions occurring within the mucosal-luminal interface, are likely to vary gradually along the proximal-distal axis of the colorectum.[49, 50] Indeed, this underlying continuum was postulated, by LaPointe and colleagues, to be responsible for the apparent continuity observed in their gene expression data.[43]
Taking into account luminal biogeography, and the potential influence of host-microbial interactions, we recently investigated whether the frequencies of key colorectal cancer-related molecular characteristics also changed gradually through the multiple anatomic subsites.[15] Utilising a database of over 1400 colorectal cancers from two U.S. nationwide prospective cohort studies, we examined the frequencies of several molecular markers (CIMP, MSI, BRAF, KRAS and PIK3CA mutations, and LINE-1 methylation) in cancers arising throughout the colorectum.[15] We assessed the linearity and nonlinearity of molecular relationships by bowel subsite, with subsites defined as caecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, and rectum.[15] Notably, we found that the frequencies of CIMP-high, MSI-high, and BRAF mutation increased in a statistically linear fashion from the rectum to the ascending colon. Interestingly, caecal cancers appeared to represent a unique subtype, characterised by a high frequency of KRAS mutation. Our data demonstrate that the frequencies of molecular pathological changes in colorectal cancer transition gradually through bowel subsites, rather than altering abruptly at the splenic flexure.[15] Figure 1 illustrates the disparity between the prevailing two-colon concept and the continuum model. Importantly, our findings, and the continuum model, are not incompatible with previous observations. Our data do, however, support the necessity for a paradigm shift. We therefore propose a transition from the established dichotomous, or trichotomous (if one distinguishes rectum from distal colon), model towards a more detailed approach to the topography of colorectal carcinogenesis. Translation of the continuum model into a clinically practicable tool must be tempered by an appreciation of the limitations of lesion position reporting by clinicians, and by an awareness of inter-individual variability in colon length and mobility. Nonetheless, the adoption of a multisegmental model should be readily achievable.
Figure 1.
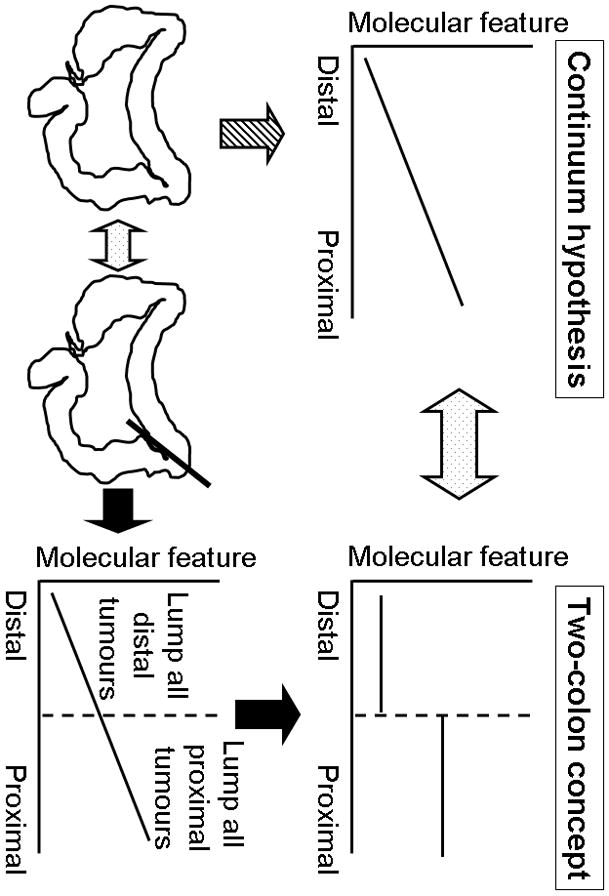
Illustration of the disparity between the continuum model and the two-colon concept. The frequencies of molecular features such as CIMP-high, MSI-high, and BRAF mutation increase linearly from the rectum to the ascending colon according to the continuum model, and as supported by our recent data (left).[15] Previous studies have typically lumped together all distal tumours, and all proximal tumours, and compared the frequencies of various molecular features. Data from numerous previous two-colon studies appear to support the two-colon concept (right, top), even where the likelihood is that a continuum exists for the measured marker along proximal-distal axis of the bowel (right, bottom). The design of two-colon studies does not permit adequate evaluation of the linearity of marker frequency by colon subsite.
FUTURE PERSPECTIVES
The potential limitations of the two-colon model for molecular biomarkers may extend to its application in other arenas. A recent examination of data from 39,568 participants in the German “Colon/Rectal Carcinoma” study suggests that certain clinicopathological features, such as histological subtype, show a seemingly linear correlation with anatomic subsite, whilst others, including disease stage, nodal status and lymphatic invasion, vary by subsite and do not fit neatly into a dichotomous model.[51] Indeed, our findings, suggesting molecular linearity from rectum to ascending colon and a potential unique molecular phenotype for caecal cancers, might have been masked by a two-colon study design.
We are at the dawn of the era of personalised colorectal cancer therapy and prevention.[52] As the oncological armament of chemotherapeutic and chemopreventive agents increases, careful disease phenotyping and subgroup identification is vital in ensuring optimal disease control whilst minimising toxicity.[52] For stage II disease, where site-specific survival differences appear to exist, adjuvant therapy remains controversial.[52] There is therefore clearly a need to define subgroups who might derive benefit from chemotherapy. Few colon cancer outcome studies have, however, considered the influence of tumor location and its potential interactions with molecular characteristics beyond the simple proximal-distal division.[5, 7, 53] In terms of screening, it is generally accepted that colonoscopy impacts less on mortality for proximal cancers compared to cancers arising in the distal colon.[13] If molecular strategies are to be employed in an attempt to increase the sensitivity of colonoscopy for proximal premalignant lesions, then a detailed appreciation of marker variation by subsite is essential.
Thus, a move towards the continuum model and multisegmental research design may prove informative not only for molecular and epidemiological investigators, but also for clinical outcome research. Furthermore, the ability to resolve variation in gut biogeography at subsite level seems imperative for “omics” research, and for a better understanding of effects of gut microbiome and host interactions on disease susceptibility and evolution.
In the first instance, multisegmental research design demands the collection of detailed colorectal subsite data for each neoplastic lesion in clinical, epidemiologic and pathological studies. One inevitable consequence of this study design will be the requirement for an increase in total sample size. This will be necessary in order to provide adequate statistical power necessary to permit meaningful subsite analyses. The larger sample size demanded by this model is therefore likely to promote pooling projects and multi-centre or multi-cohort collaborations in the near future.
CONCLUSION
The two-colon model has served us for over three decades.[11–14] Nonetheless, the emergence of data suggesting linearity in the frequencies of certain tumour molecular characteristics, and subsite-specific clinicopathological differences beyond the simple proximal-distal divide, support the need for a paradigm shift. In the era of personalised cancer therapy and prevention, future studies should consider adopting a multisegmental approach to bowel subsite in order both to advance our understanding of the complex aetiology of colorectal carcinogenesis, and to improve tailored preventative and therapeutic strategies.
Acknowledgments
Funding: This work was supported by U.S. National Institute of Health grants [P01 CA87969 (to S.E. Hankinson), P01 CA55075 (to W.C. Willett), P50 CA127003 (to C.S.F.), R01 CA118553 (to C.S.F.), R01 CA151993 (to S.O.), and R01 CA137178 (to A.T.C.)] and a U.S. National Science Foundation grant [DBI-1053486 (to C.H.)]. P.L. is a Scottish Government Clinical Academic Fellow and is supported by a Harvard University Knox Memorial Fellowship. T.M. was supported by a fellowship grant from the Japan Society for Promotion of Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of US NIH. Funding agencies did not have any role in the design of the study; the collection, analysis, or interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
Abbreviations
- CIN
chromosomal instability
- CIMP
CpG island methylator phenotype
- MMR
mismatch repair
- MSI
microsatellite instability
- SEER
Surveillance, Epidemiology and End Results
Footnotes
Competing Interests: None directly relevant to this work.
Licence Statement: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Group and co-owners or contracting owning societies (where published by the BMJ Group on their behalf), and its Licensees to permit this article (if accepted) to be published in Gut and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence.
References
- 1.Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogino S, Galon J, Fuchs CS, et al. Cancer immunology-analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–19. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen OM. Different age and sex relationship for cancer of subsites of the large bowel. Br J Cancer. 1984;50:825–9. doi: 10.1038/bjc.1984.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–64. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 5.Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–50. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 7.Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–70. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 8.Weiss JM, Pfau PR, O'Connor ES, et al. Mortality by Stage for Right- Versus Left-Sided Colon Cancer: Analysis of Surveillance, Epidemiology, and End Results-Medicare Data. J Clin Oncol. 2011;29:4401–9. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghazi S, Lindforss U, Lindberg G, et al. Analysis of colorectal cancer morphology in relation to sex, age, location, and family history. J Gastroenterol. 2012 doi: 10.1007/s00535-011-0520-9. in press. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Ward EM, Jemal A. Trends in Colorectal Cancer Incidence Rates in the U.S. by Tumor Location and Stage, 1992–2008. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/055-9965.EPI-11-1020. in press. [DOI] [PubMed] [Google Scholar]
- 11.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 12.Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004;88:261–6. doi: 10.1002/jso.20156. [DOI] [PubMed] [Google Scholar]
- 13.Carethers JM. One colon lumen but two organs. Gastroenterology. 2011;141:411–2. doi: 10.1053/j.gastro.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albuquerque C, Bakker ER, van Veelen W, et al. Colorectal cancers choosing sides. Biochim Biophys Acta. 2011;1816:219–31. doi: 10.1016/j.bbcan.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal vs. distal colorectum. Gut. 2012 doi: 10.1136/gutjnl-2011-300865. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beart RW, Melton LJ, 3rd, Maruta M, et al. Trends in right and left-sided colon cancer. Dis Colon Rectum. 1983;26:393–8. doi: 10.1007/BF02553382. [DOI] [PubMed] [Google Scholar]
- 17.Butcher D, Hassanein K, Dudgeon M, et al. Female gender is a major determinant of changing subsite distribution of colorectal cancer with age. Cancer. 1985;56:714–6. doi: 10.1002/1097-0142(19850801)56:3<714::aid-cncr2820560345>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Rothberg PG, Spandorfer JM, Erisman MD, et al. Evidence that c-myc expression defines two genetically distinct forms of colorectal adenocarcinoma. Br J Cancer. 1985;52:629–32. doi: 10.1038/bjc.1985.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delattre O, Olschwang S, Law DJ, et al. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet. 1989;2:353–6. doi: 10.1016/s0140-6736(89)90537-0. [DOI] [PubMed] [Google Scholar]
- 20.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–88. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 21.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–7. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. 2011;60:116–29. doi: 10.1136/gut.2009.206250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaltonen LA, Peltomaki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–6. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 26.Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–61. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 27.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–9. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 28.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–87. e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtin K, Slattery ML, Samowitz WS. CpG island methylation in colorectal cancer: past, present and future. Pathol Res Int. 2011:902674. doi: 10.4061/2011/902674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong JJ, Hawkins NJ, Ward RL, et al. Methylation of the 3p22 region encompassing MLH1 is representative of the CpG island methylator phenotype in colorectal cancer. Mod Pathol. 2011;24:396–411. doi: 10.1038/modpathol.2010.212. [DOI] [PubMed] [Google Scholar]
- 32.Hughes LA, Khalid-de Bakker CA, Smits KM, et al. The CpG island methylator phenotype in colorectal cancer: Progress and problems. Biochim Biophys Acta. 2012;1825:77–85. doi: 10.1016/j.bbcan.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–45. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 35.Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3:e3698. doi: 10.1371/journal.pone.0003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka N, Huttenhower C, Nosho K, et al. Novel application of structural equation modeling to correlation structure analysis of CpG island methylation in colorectal cancer. Am J Pathol. 2010;177:2731–40. doi: 10.2353/ajpath.2010.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zlobec I, Bihl M, Foerster A, et al. Comprehensive analysis of CpG island methylator phenotype (CIMP)-high, -low, and -negative colorectal cancers based on protein marker expression and molecular features. J Pathol. 2011;225:336–43. doi: 10.1002/path.2879. [DOI] [PubMed] [Google Scholar]
- 38.Thorn M, Bergstrom R, Kressner U, et al. Trends in colorectal cancer incidence in Sweden 1959–93 by gender, localization, time period, and birth cohort. Cancer Causes Control. 1998;9:145–52. doi: 10.1023/a:1008826109697. [DOI] [PubMed] [Google Scholar]
- 39.Chauvenet M, Cottet V, Lepage C, et al. Trends in colorectal cancer incidence: a period and birth-cohort analysis in a well-defined French population. BMC Cancer. 2011;11:282. doi: 10.1186/1471-2407-11-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy G, Devesa SS, Cross AJ, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668–75. doi: 10.1002/ijc.25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365–7. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012–22. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaPointe LC, Dunne R, Brown GS, et al. Map of differential transcript expression in the normal human large intestine. Physiol Genomics. 2008;33:50–64. doi: 10.1152/physiolgenomics.00185.2006. [DOI] [PubMed] [Google Scholar]
- 44.Worthley DL, Whitehall VL, Buttenshaw RL, et al. DNA methylation within the normal colorectal mucosa is associated with pathway-specific predisposition to cancer. Oncogene. 2010;29:1653–62. doi: 10.1038/onc.2009.449. [DOI] [PubMed] [Google Scholar]
- 45.Schernhammer ES, Giovannucci E, Kawasaki T, et al. Dietary folate, alcohol, and B vitamins in relation to LINE-1 hypomethylation in colon cancer. Gut. 2010;59:794–99. doi: 10.1136/gut.2009.183707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes LA, Simons CC, van den Brandt PA, et al. Body size, physical activity and risk of colorectal cancer with or without the CpG island methylator phenotype (CIMP) PLoS One. 2011;6:e18571. doi: 10.1371/journal.pone.0018571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012 doi: 10.1101/gr.126573.111. in press. (published online in 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012 doi: 10.1101/gr.126516.111. in press (published online in 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Leblanc J, Truong A, et al. A metaproteomic approach to study human-microbial ecosystems at the mucosal luminal interface. PLoS One. 2011;6:e26542. doi: 10.1371/journal.pone.0026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stearns JC, Lynch MDJ, Senadheera DB, et al. Bacterial biogeography of the human digestive tract. Scientific Reports. 2011;1:Article number: 170. doi: 10.1038/srep00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benedix F, Schmidt U, Mroczkowski P, et al. Colon carcinoma - Classification into right and left sided cancer or according to colonic subsite? - Analysis of 29 568 patients. Eur J Surg Oncol. 2011;37:134–39. doi: 10.1016/j.ejso.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Catenacci DV, Kozloff M, Kindler HL, et al. Personalized colon cancer care in 2010. Semin Oncol. 2011;38:284–308. doi: 10.1053/j.seminoncol.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wray CM, Ziogas A, Hinojosa MW, et al. Tumor subsite location within the colon is prognostic for survival after colon cancer diagnosis. Dis Colon Rectum. 2009;52:1359–66. doi: 10.1007/DCR.0b013e3181a7b7de. [DOI] [PubMed] [Google Scholar]


