Abstract
Tight regulation of MHC class I gene expression is critical for CD8 T cell activation and host adaptive immune responses. The promoters of MHC class I genes contain a well-conserved core module, the W/S-X-Y motif, which assembles a nucleoprotein complex termed MHC-enhanceosome. A member of the NLR (nucleotide binding domain, leucin-rich repeat) protein family, NLRC5, is a newly identified transcriptional regulator of MHC class I genes. NLRC5 associates with and transactivates the proximal promoters of MHC class I genes, although the molecular mechanism of transactivation has not been understood. Here, we show that NLRC5-mediated MHC class I gene induction requires the W/S and X1, X2 cis-regulatory elements. The transcription factors RFX5, RFXAP and RFXANK/B, which compose the RFX protein complex and associate with the X1-box, cooperate with NLRC5 for MHC class I expression. Co-immunoprecipitation experiments revealed that NLRC5 specifically interacts with the RFX subunit RFXANK/B via its ankyrin repeats. In addition, we show that NLRC5 can cooperate with ATF1 and the transcriptional co-activators CBP/p300 and GCN5, which display histone acetyltransferase activity. Taken together, our data suggest that NLRC5 participates in an MHC class I specific enhanceosome, which assembles on the conserved W/S-X-Y core module of the MHC class I proximal promoters, including the RFX factor components and CREB/ATF1 family transcription factors to promote MHC class I gene expression.
Keywords: MHC class I, enhanceosome, CITA
Introduction
Major histocompatibility complex (MHC) class I and class II molecules are essential components of the mammalian adaptive immune system. MHC class I molecules present peptide antigens of intracellular origin such as viral or tumor antigens to CD8+ T cells, whereas MHC class II molecules present peptide antigens of extracellular sources such as bacterial antigens to CD4+ T cells (1, 2). MHC class I molecules are composed of MHC-encoded heavy chains and the non-polymorphic subunit β2-microglobulin (β2M) (3). Humans have three classical MHC class Ia molecules (HLA-A, HLA-B and HLA-C), as well as three non-classical MHC class Ib molecules (HLA-E, HLA-F and HLA-G), which have immune regulatory functions (4, 5). MHC class I peptides are mostly produced from the degradation of cytoplasmic proteins by the specialized “immunoproteasome” containing several IFN-γ-inducible subunits, such as LMP2 and LMP7 (6). Peptide loading onto MHC class I requires the peptide loading complex (PLC), which includes the MHC class I heavy chain, β2M, tapasin, ERp57, calreticulin and TAP1/TAP2, a transporter that translocates peptides from the cytoplasm into the ER (6, 7).
MHC class Ia is ubiquitously expressed in almost all nucleated cells, unlike MHC class II, which is found mainly in antigen-presenting cells (3, 8). Both MHC class I and class II, as well as β2M genes, are highly inducible by IFN-γ and share similar cis-regulatory elements in their promoters, termed W/S, X1, X2 and Y-box motifs, which are occupied by similar transcription factor complexes and are critical for transactivation of MHC class I and II genes (9–12). These transcription factors include the X1-box binding trimeric RFX protein complex (composed of RFX5, RFXAP and RFXANK/RFXB) (13–16), members of the X2-box binding CREB/ATF1 family of transcription factors (11, 17), and the Y-box binding NF-Y protein (composed of NF-YA, NF-YB and NF-YC) (18–20). Together, they form a macromolecular nucleoprotein complex called the MHC-enhanceosome (21).
Although the transcription factors directly associated with the W/S-X-Y motif of MHC gene promoters are critical, the formation of an active enhanceosome requires additional transactivators such as the class II transactivator (CIITA). CIITA, a member of the NLR or nucleotide binding domain (NBD), leucine rich repeat (LRR) family of proteins (22, 23), regulates the transcription of MHC class II by associating with the MHC-enhanceosome (21, 24). The expression of CIITA is induced in B cells and dendritic cells as a function of developmental stage and is inducible by IFN-γ or upon activation such as in human T cells (25–29). Therefore, CIITA is important for both constitutive and inducible expression of MHC class II and is referred to as a master regulator of MHC class II genes. In addition to MHC class II genes, CIITA also has a role in the transactivation of MHC class I genes at least in vitro (8–10, 20, 30, 31). However, while mutations in either CIITA, RFX5, RFXAP or RFXANK (or RFX-B) genes cause bare lymphocyte syndrome (BLS), an immunodeficiency characterized by the lack of MHC class II expression, BLS patients with mutations in CIITA but not in RFX genes retain MHC class I expression (32, 33). Similarly, in mice deficient for CIITA, both constitutive and IFN-γ-induced expression of MHC class I molecules are intact, indicating that there should be another mechanism for the activation of MHC class I in vivo (34–36). This niche has been largely filled by the recent finding that another NLR protein, NLRC5, can act as a transactivator of MHC class I genes (37). Similar to CIITA, NLRC5 is IFN-γ-inducible and can translocate into the nucleus due to its nuclear localization signal (NLS). NLRC5 associates with and transactivates MHC class I promoters (37). The expression of NLRC5, or class I transactivator (CITA), as opposed to CIITA, specifically upregulates the expression of MHC class I but not MHC class II genes (37). The NBD is a critical domain for the function of NLRC5, as NBD is required for both nuclear import and transactivation of MHC class I genes (38). In addition to MHC class I, NLRC5 induces the expression of β2M, TAP1 and LMP2 genes, indicating that NLRC5 is a key transcriptional regulator of genes involved in the MHC class I antigen presentation pathway (37, 39).
Although the discovery of NLRC5 as an MHC class I transactivator and its similar function to CIITA are striking, the molecular mechanism by which NLRC5 transactivates MHC class I gene promoters has not been investigated. Here, we demonstrate that NLRC5 cooperates with components of the RFX factor complex, which assembles on the W/S-X-Y motif, to induce MHC class I gene expression. We show that NLRC5-mediated class I gene transactivation requires the X1 cis-regulatory element, the well-known RFX protein complex binding site. We also find that NLRC5 associates with the RFX subunit RFXANK/B through its ankyrin repeats. Furthermore, like CIITA, NLRC5 may also act as a platform for recruitment of histone acetyltransferase activities provided by the general co-activators CBP/p300, GCN5 and PCAF.
Materials and Methods
Cell lines and reagents
The SV40-transformed BLS patient fibroblast cell lines WSI (wild-type), ABI (RFXAP-deficient), OSE (RFX5-deficient), EBA (RFXANK/B-deficient), ATU (CIITA-deficient) as well as the teratocarinoma cell line Tera-2 were described previously (11, 38). Human embryonic kidney 293 (HEK293T) were purchased from ATCC (CRL-11268). All cell lines were cultured in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/ streptomycin (Gibco). Recombinant human IFN-γ was acquired from BioLegend and used at a concentration of 100 Units/ml.
Plasmid construction
Cloning of human GFP-NLRC5 and GFP-CIITA has been described previously (37). The cDNAs of the human RFX factors, RFX5, RFXANK/B, and RFXAP were subcloned into a modified pcDNA3.1 vector (Invitrogen) encoding for an N-terminal HA or RFP tag using standard molecular cloning techniques; The following primers were used: RFXAP, 5’-atatggatccGAGGCGCAGGGTGTAGCGGAG-3’ (forward), 5’-atatgtcgacTCACATTGATGTTCCTGGAAACTG-3’ (reverse); RFX5, 5’-atatatgcggccgcTGGCAGAAGATGAGCCTGATGC-3’ (forward), 5’-atattctagattATGGGGGTGTTGCTTTTGGGTC-3’ (reverse), RFXANK/B, 5’-atatggatccGAGCTTACCCAGCCTGCAGAAG-3’ (forward), 5’-atatctcgagTCACTCAGGGTCAGCGGGCACCAG-3’ (reverse).
The expression vectors pREP4-RFX5, pREP4-RFXANK/B, and pREP4-RFXAP were used as templates (11). For the construction of RFXANK/B deletion mutants the following primers were used: RFXANK/B-ΔC, 5’-atatctcgagTTAGTCGGCTTCGGTGGTGAGGTC-3’ (reverse); RFXANK/B-ΔANK, 5’-atatctcgagTTACTCGTCTGGCTTGTTGACGAG-3’ (reverse); RFXANK/B-ΔPEST, 5’-atatggatccCAGGCAGGCAGCTCCCTGAAG-3’ (forward). All plasmids were verified by sequencing analysis (DFCI molecular biology core facilities).
Transfection and luciferase assay
Cells were transiently transfected using PEI (1 mg/ml, pH 7.2 polyethylenimine, Polysciences, Inc.) at a ratio of DNA:PEI of 1:3–4, or using FuGENE 6 Transfection Reagent (Roche) in serum-free media, following the manufacturer’s instructions. For Western blot and immunoprecipitation experiments 5 × 105 cells were seeded in 2 ml medium into 6 wells, and a total of 3 µg/well of DNA was used per transfection. Medium was changed the following day and cell extracts were prepared 48 hrs post transfection. For co-immunoprecipitation experiments, cells were first transfected with expression vectors for NLRC5 and the following day with plasmids encoding components of the RFX factor complex, to allow for similar expression levels.
For luciferase assays, cells were split at a density of 2 × 104 / 0.5 ml into 24-wells one day prior to transfection. Unless stated otherwise, cells were co-transfected with 50 ng of either GFP, GFP-NLRC5, GFP-CIITA expression plasmid and 25 ng of the indicated luciferase reporter constructs. 25 ng per well of reporterless Renilla plasmid was included to allow for normalization of transfection efficiency. Cells were harvested 48 hrs post transfection, and cell lysates were analysed using the dual-luciferase reporter assay system (Promega), following the manufacturer’s protocol. Unless stated otherwise, experiments were performed in duplicates, repeated at least twice, and results are given as the mean ± SD. The MHC class I reporter gene constructs as well as the expression plasmids encoding ATF1, CBP, p300, GCN5, and PCAF have been described previously (11, 31, 38, 39).
SDS-PAGE and immunoblotting
Whole cell extracts were prepared using 1x Cell Lysis Buffer (Cell Signaling) supplemented with 1 mM DTT, and 1 mM PMSF, prior to extraction and centrifugation of whole cell lysates. Protein concentration was determined using the Bradford protein assay according to manufacturer’s instructions (Bio-Rad). Cell extracts were subjected to SDS-polyacrylamide gel electrophoresis using 4–12% gradient gels (Invitrogen). Gels were transferred to nitrocellulose membranes (Amersham HyBond ECL) for at least 2 hrs at 80V. Membranes were blocked for 1 hour in 5% BSA in Tris-buffered saline–Tween (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% Tween 20). The following antibodies were used for protein detection: anti-HA (16B12, Covance), anti-β2M (2M2, BioLegend), anti-GFP (JL-8 Clontech), anti-β-actin (I-19, Santa Cruz), and anti-α-Tubulin (TU-02, Santa Cruz). The antibody against MHC class I heavy chain (3B10.7) is a kind gift of Dr. P. Cresswell (Yale University). The following horseradish peroxidase (HRP)-conjugated secondary antibodies were used: anti-mouse IgG and anti-rabbit IgG (GE Healthcare), anti-goat IgG (Santa Cruz) and anti-rat IgG2a (Alpha Diagnostics). Blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), and imaged using the Molecular Imager ChemiDoc XRS+ System (Bio-Rad) or exposed to autoradiography film (Denville). Quantification was performed using ImageQuant (Molecular Dynamics).
Immunoprecipitation
Immunoprecipitation of the HA-tagged RFX subunits and RFXANK/B deletion mutants was performed on HEK293T cell lysates 48 hrs post transfection using an anti-HA antibody (16B12, Covance). After rotating samples at 4°C overnight, Protein A/G UltraLink Resin (Thermo Scientific, Rockford, IL) was added to each tube, and rotated at 4°C for 3 hrs. The beads were washed three times sequentially in cell lysis buffer (Cell Signaling) and washing buffer (20 mM Tris-HCl (pH 7.4), 0.1% Nonidet P-40) and subsequently samples were boiled for 10 min in 20 µl of loading buffer and subjected to SDS/PAGE and immunoblot analysis. Co-immunoprecipitated GFP-NLRC5 was detected by Western blot analysis using an anti-GFP antibody.
Quantitative real-time PCR (qPCR) analysis
RNA was isolated using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. The integrity of isolated RNA was verified by 1% agarose gel electrophoresis. First-strand cDNA was synthesized from 1 µg RNA using the qScript Flex cDNA synthesis kit (Quanta Biosciences), and RNA expression was quantified on the 7300 Real-Time PCR System (Applied Biosystems) using the PerfeCTa SYBR Green SuperMix with ROX (Quanta Biosciences). The following primers were used for amplification: HLA-A, 5’-AAAAGGAGGGAGTTACACTCAGG-3’ (forward), 5’-GCTGTGAGGGACACATCAGAG-3’ (reverse); GAPDH, 5’-GAAGGTGAAGGTCGGAGT-3’ (forward), 5’-GAAGATGGTGATGGGATTTC-3’ (reverse). For analysis of the transiently reconstituted RFXANK/B-deficient cell line EBA, cells were sorted for GFP+, RFP+ or double positive cell populations using a Beckman Coulter MoFlo FACS sorter (DFCI Core facilities).
Statistical Analysis
All experiments were repeated twice or more and data were subjected to Student's t test for analysis of statistical significance using Prism (GraphPad). Results are given as the mean ± SD. A P-value of < 0.05 was considered to be significant.
Results
A functional W/S-X-Y motif is required for NLRC5-mediated MHC class I transactivation
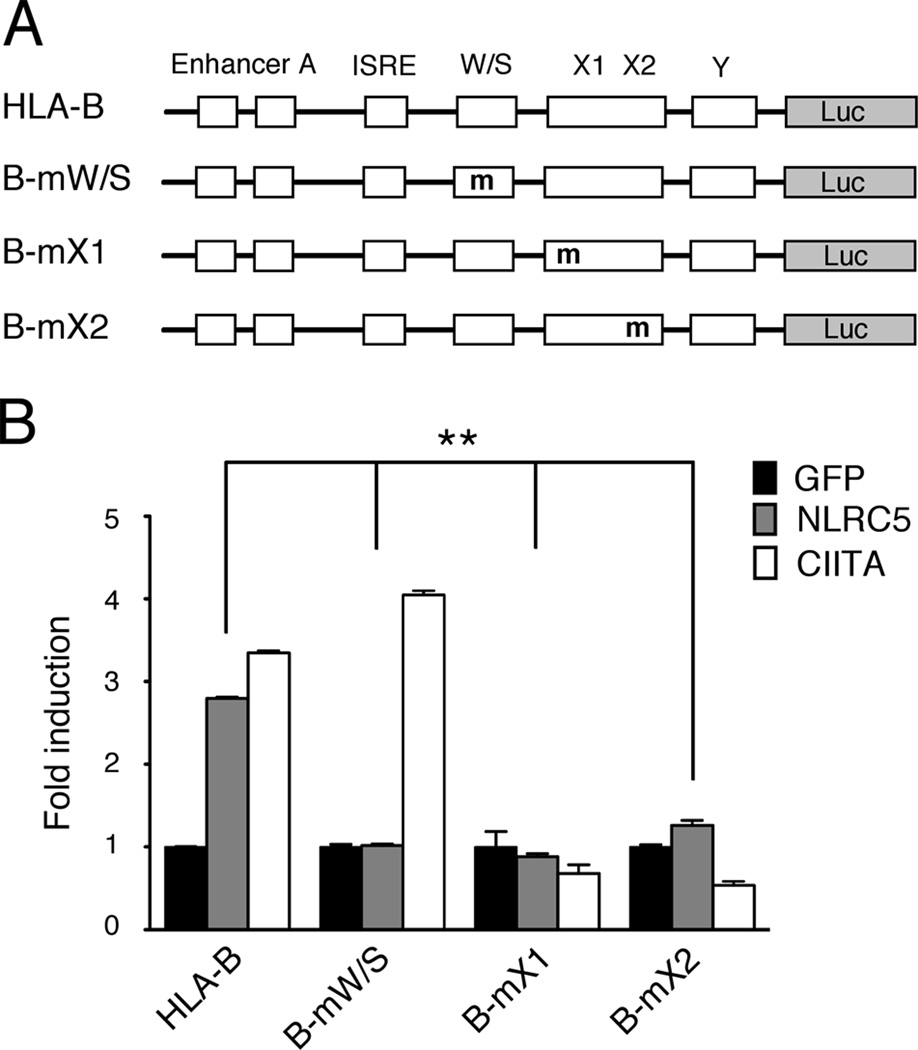
The proximal promoter region of MHC class I genes contains a well-characterized core module consisting of the W/S-X-Y motif. In order to determine which cis-regulatory elements are required for NLRC5-mediated MHC class I gene transactivation, we performed promoter assays using luciferase reporter constructs driven by the HLA-B promoter. An expression vector for NLRC5 was transfected into HEK293T cells together with a reporter gene construct containing the immediate upstream region of the HLA-B gene, and reporter gene activity was compared to that of mutant versions of the HLA-B promoter harboring mutations in the W/S, the X1, or the X2 box (Fig 1A). As control, CIITA and empty GFP expression vectors were transfected and MHC class I promoter activity was compared. As shown in Fig. 1B, NLRC5 induces the transactivation of the wild-type HLA-B promoter to a similar extent to that of CIITA, as previously shown (37). Interestingly, mutation of the W/S box cis-regulatory element (mW/S) specifically abrogated NLRC5-induced HLA-B promoter activity but left CIITA-driven MHC class I transactivation intact. In contrast, mutations of either half of the X box (mX1, mX2), which are known to bind to the trimeric RFX factor complex (for X1 box) and the CREB/ATF1 family transcription factors (for X2 box), abolished both NLRC5 and CIITA-induced HLA-B gene transactivation.
FIGURE 1. NLRC5-mediated transactivation of the HLA-B promoter requires the W/S and X box cis-regulatory elements.
A, Schematic representation of the HLA-B luciferase reporter construct and the indicated mutant versions used in this study. Mutated cis-regulatory elements are marked (m). B, Reporter gene analysis of the HLA-B promoter. HEK293T cells were transiently transfected with either expression vectors for GFP (black bar), GFP-NLRC5 (grey bar), or GFP-CIITA (white bar), along with the indicated HLA-B luciferase reporter constructs. Cell lysates were analyzed 48 hrs post transfection by dual-luciferase assay. Data are a representative of several independent experiments performed in duplicates and are plotted as fold induction with respect to the GFP control vector. Error bars represent ± SD. *p < 0.05; **p < 0.01.
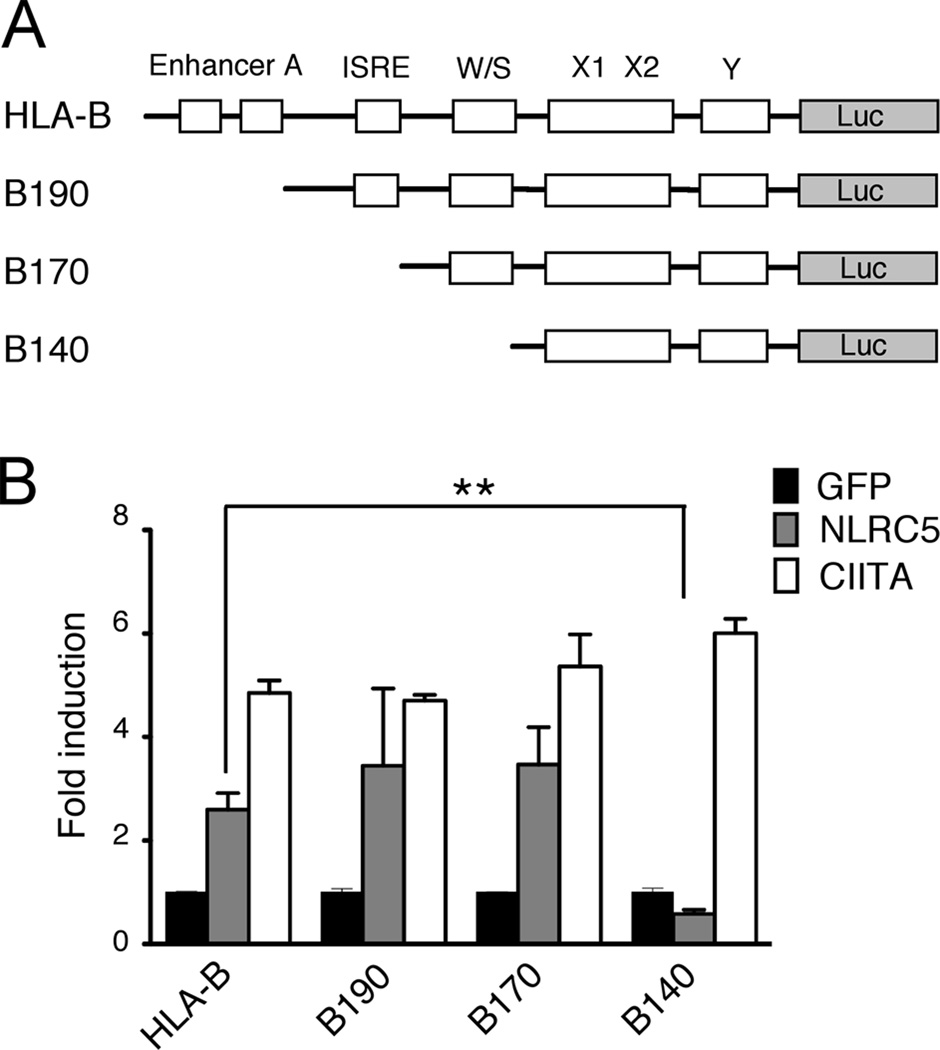
In a different set of experiments, we used deletion mutants of the HLA-B promoter and observed that NLRC5 can induce MHC class I expression even in the absence of the Enhancer A and ISRE cis-regulatory elements, which are conserved in the proximal promoters of the classical MHC class I genes (Fig. 2, B190: ΔEnhancer A; B170: ΔEnhancer A, ΔISRE). Again, deletion of the W/S box selectively abolished NLRC5-induced MHC class I induction, whereas CIITA-mediated activity remained intact (B140: ΔEnhancer A, ΔISRE and ΔW/S). Taken together, NLRC5-mediated MHC class I gene activation requires the conserved X1 and X2 box, as well as the W/S motif, which is dispensable for CIITA-mediated MHC class I gene activation.
FIGURE 2. HLA-B transactivation by NLRC5 occurs in the absence of the Enhancer A and ISRE.
A, Schematic representation of the HLA-B promoter deletion mutants used in this study. B190 (ΔEnhancer A), B170 (ΔEnhancer A, ΔISRE), B140 (ΔEnhancer A, ΔISRE, ΔW/S). B, NLRC5-mediated transactivation of the HLA-B promoter and the indicated promoter mutants (grey bar) is compared to empty vector control (GFP, black bar), and CIITA (white bar). Transiently transfected HEK293T cells were analyzed 48 hrs post transfection by dual-luciferase assay. The data was plotted as fold induction relative to the empty GFP expression vector control as the mean of duplicates from one representative experiment. The experiment was repeated twice. Error bars are given as ± SD. *p < 0.05; **p < 0.01.
NLRC5 cooperates with ATF1 and the transcriptional co-activators CBP, p300, and GCN5
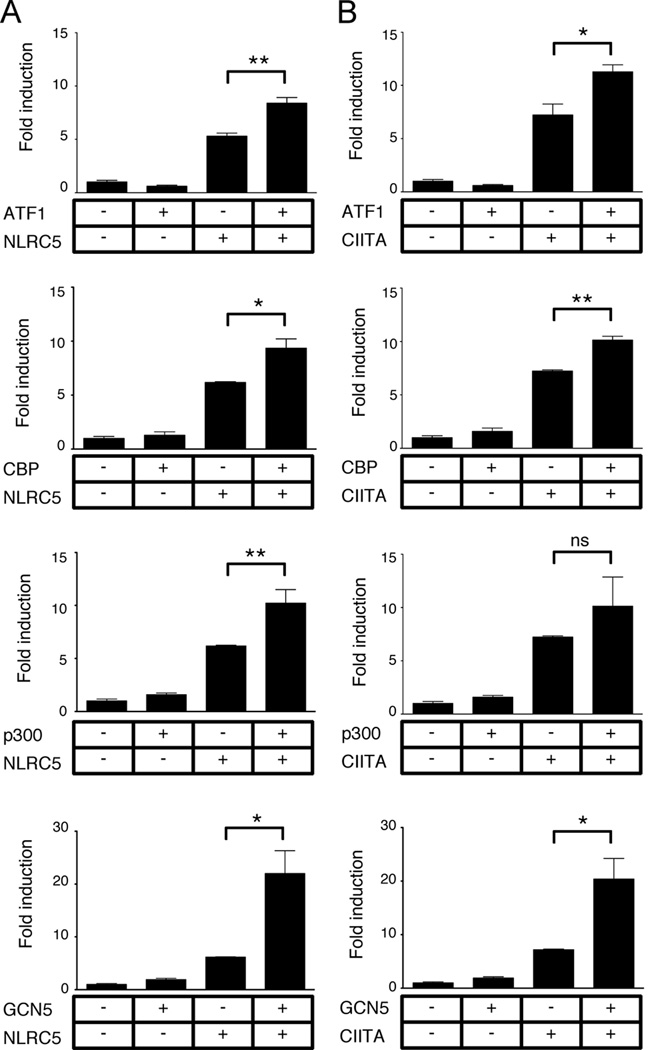
Next, we examined whether NLRC5 cooperates with other transcriptional regulators involved in MHC class I transactivation. It has been well established that CIITA interacts with other transcriptional co-regulators and is able to recruit chromatin modifying enzymes, such as histone acetyltransferases, to the MHC promoters. In order to compare the impact of NLRC5-mediated MHC class I expression to previously reported data for CIITA (11), we used the well characterized teratocarcinoma cell line Tera-2. Both NLRC5 and CIITA activated the HLA-B promoter in this cell line to a similar extent (Fig. 3). In agreement to what has been reported for CIITA(11), we observed a synergistic induction of the HLA-B promoter with NLRC5 and the X2 box binding protein ATF1, a member of the CREB family of transcriptional activators. Similarly, co-expression of NLRC5 and the transcriptional co-activators CBP (CREB-binding protein), p300, GCN5 (Fig. 3) and PCAF (data not shown) resulted in enhanced reporter activity, comparable to that seen for CIITA (Fig. 3). Overexpression of the individual co-factors had no significant impact on the HLA-B promoter, probably because they are constitutively expressed in most cell types. In summary, similar to CIITA, NLRC5 cooperates with the X2-box binding factor ATF1 and the transcriptional co-activators CBP/p300, GCN5 and PCAF, which all display histone acetyltransferase activity, to transactivate MHC class I genes.
FIGURE 3. NLRC5 cooperates with ATF1 and the transcriptional co-activators CBP/p300, and GCN5.
Transactivation of the HLA-B promoter by NLRC5 (A) or CIITA (B) with co-expression of ATF1 and the transcriptional co-activators CBP, p300 and GCN5 was examined. Tera-2 cells were transiently transfected with either empty control vector or the indicated expression plasmids and cells were harvested 48 hrs post transfection. Cell lysates were analyzed using the dual-luciferase assay and normalized against Renilla firefly activity. Data are a representative of several independent experiments performed in duplicates and are plotted as fold induction with respect to the control vector. Error bars represent ± SD. ns, not significant; *p < 0.05; **p < 0.01.
NLRC5 cooperates with the RFX factor complex to promote MHC class I expression
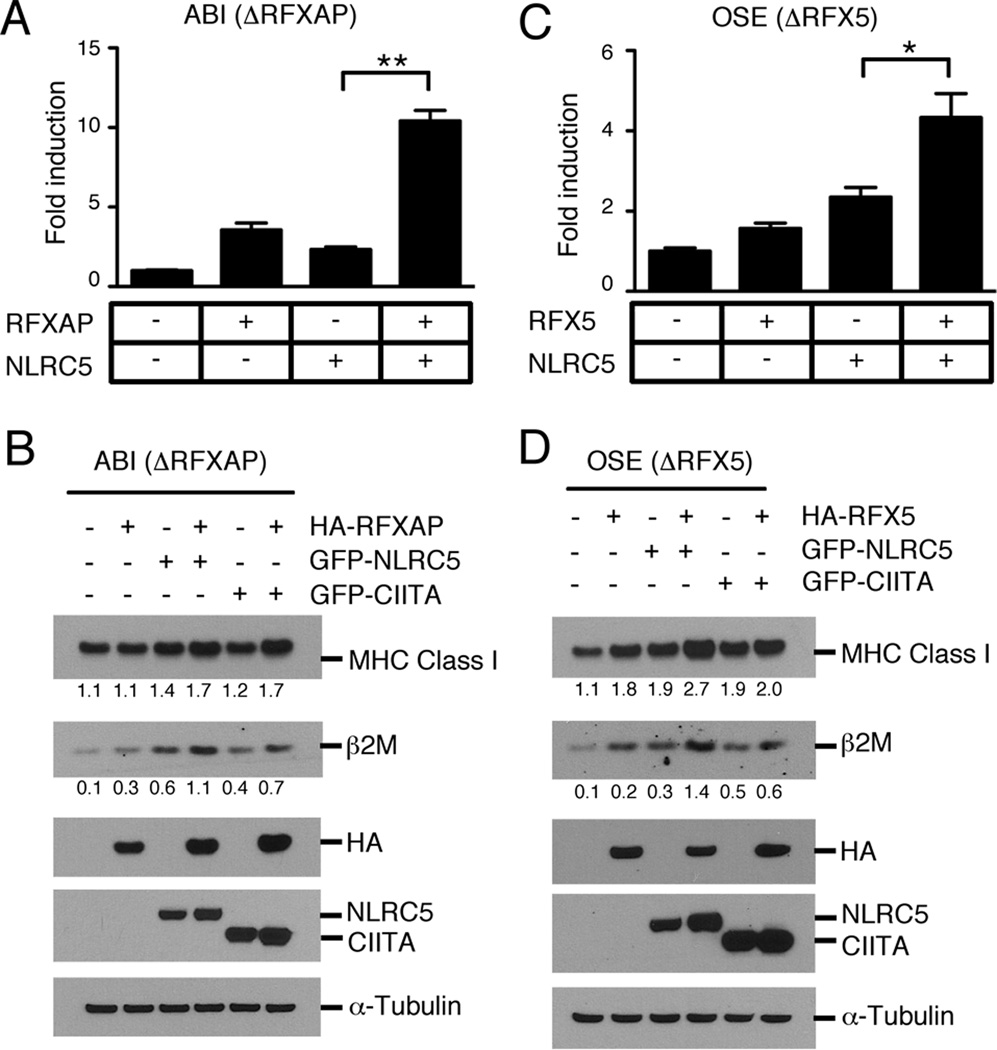
We have previously demonstrated by chromatin immunoprecipitation experiments that NLRC5 specifically binds to the proximal promoter of MHC class I genes (37). The observation that NLRC5-mediated induction of the HLA-B promoter required the X1 box, the binding site for the RFX factor complex, prompted us to investigate the role of the RFX factor components in this process. In order to dissect the role of each RFX transcription factor for NLRC5-mediated MHC class I transactivation, we used wild-type (WSI), RFX5-deficient (OSE), RFXAP-deficient (ABI) and RFXANK/B-deficient (EBA) fibroblasts derived from BLS patients. These cell lines have previously been characterized for their levels of constitutive and inducible MHC class I and β2M expression (40–42). We confirmed reduced MHC class I expression by qPCR analysis using primers specific for HLA-A and on the protein level by Western blot analysis using a pan-HLA(Hc) antibody (Supplemental Fig. 1A and B). Our results confirm previous data that MHC class I expression is reduced in all three cell lines investigated, OSE, ABI, EBA, when compared to wild-type cells (WSI). Next, we assessed whether NLRC5-mediated MHC class I induction requires individual components of the RFX complex using these cell lines. In line with previous observations (11), reconstitution of the RFXAP-deficient cell line (ABI) with an expression plasmid encoding RFXAP resulted in increased reporter gene activity of the HLA-B promoter (Fig. 4A). Similarly, transfection of NLRC5 into the same cell line induced the HLA-B promoter, although only to a moderate extent. RFXAP/NLRC5 co-transfection into the RFXAP-deficient cell line, however, resulted in a synergistic activation of the reporter gene construct. This synergy was also detected on the protein level by Western blot for MHC class I and β2M (Fig. 4B). While RFXAP transfection into the ABI cell line alone did not change MHC class I expression levels to a noticeable extent, co-transfection of RFXAP together with NLRC5 significantly increased MHC class I and β2M protein levels. A similar synergy was also observed when CIITA was co-transfected with RFXAP into the ABI cell line (Fig. 4B).
FIGURE 4. NLRC5 cooperates with the RFX components RFXAP and RFX5 for the induction of MHC class I.
(A, C) Analysis of HLA-B reporter activity in human fibroblast cell lines derived from BLS patients. ABI (RFXAP deficient) or OSE (RFX5 deficient) fibroblasts were used in (A) and (C), respectively. The indicated expression plasmids were co-transfected with the HLA-B promoter reporter gene construct, and luciferase activity was measured 48 hrs post transfection using the dual-luciferase assay. The data was plotted as fold induction relative to the empty vector control as the mean of triplicates from one representative experiment out of several independent experiments. Error bars are given as ±SD. *p < 0.05; **p < 0.01. (B, D) Cell extracts of the indicated BLS patient cell line, transiently transfected with the specified expression plasmids, were examined for MHC class I and β2M protein expression by immunoblotting 48 hrs post transfection. Signal intensities were quantified using ImageQuant. Expression levels of α-Tubulin levels are presented as a loading control.
Next, we examined whether the RFX component RFX5 is essential for NLRC5-induced MHC class I expression by reconstituting the RFX5-deficient cell line OSE with RFX5 in the presence or absence of NLRC5. Again, we observed a synergistic activation of the HLA-B reporter when both components were transfected into the OSE cell line together with the HLA-B reporter gene construct (Fig. 4C) and on the protein level using anti-MHC class I (Hc) and anti-β2M antibodies (Fig. 4D). Yet still, both NLRC5 as well as CIITA can induce MHC class I expression even in the absence of RFX5 (Fig. 4C, D), suggesting a possible redundancy among the RFX factor components.
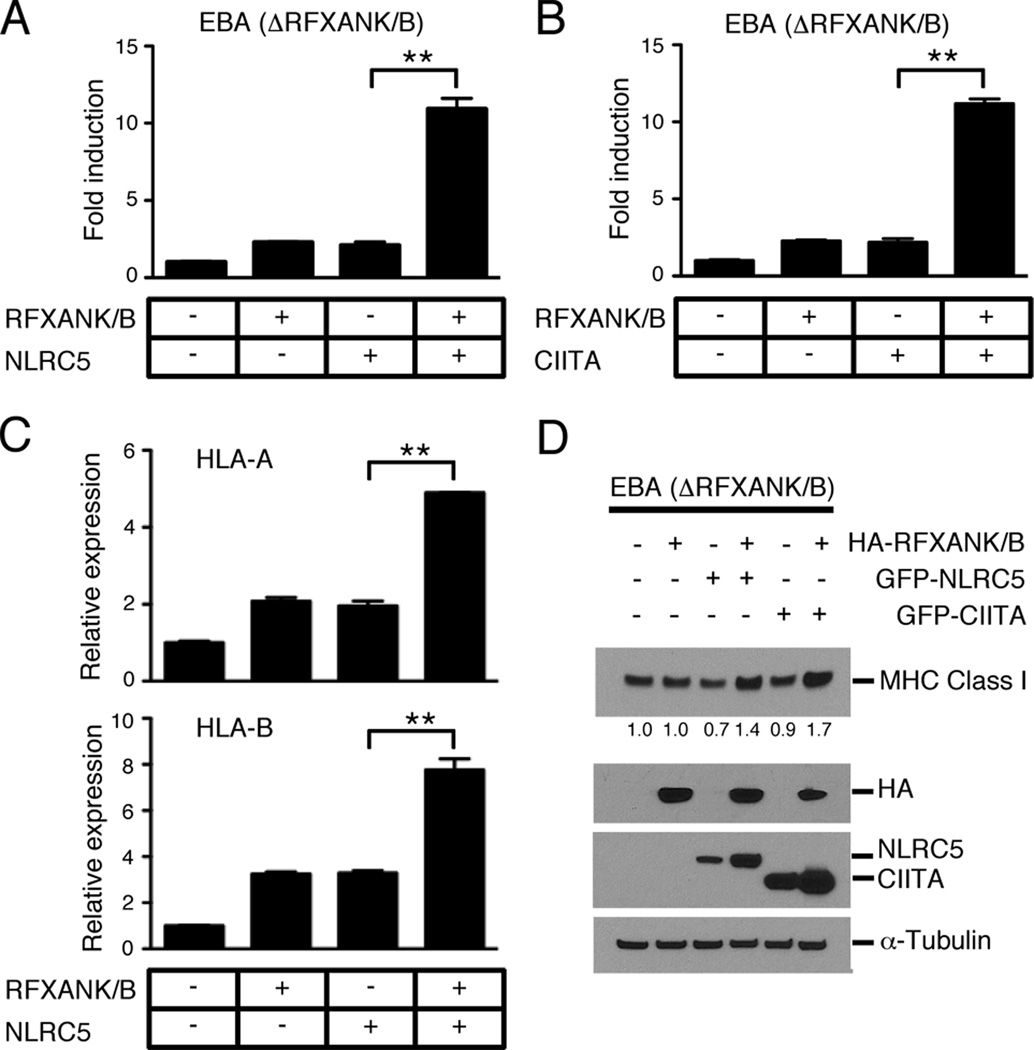
A similar set of experiments using the RFXANK/B-deficient cell line (EBA) revealed that efficient induction of NLRC5-mediated MHC class I expression requires the presence of the RFX factor component RFXANK/B (Fig. 5). We observed a strong synergy between NLRC5 and RFXANK/B in the HLA-B reporter gene assay while expression of RFXANK/B or NLRC5 alone activated the MHC class I promoter poorly (Fig. 5A). Only upon co-transfection of RFXANK/B together with NLRC5 or CIITA did we observe an efficient MHC class I promoter activation (Fig. 5A,B). Cooperative induction of MHC class I genes by RFXANK/B and NLRC5 was confirmed both at the RNA level by qPCR for HLA-A and HLA-B, and at the protein level by Western blot analysis using the anti-MHC class I (Hc) antibody (Fig 5C, D). Together, these results support the view that the RFX component RFXANK/B is important for efficient NLRC5-induced transactivation of MHC class I genes.
FIGURE 5. RFXANK/B is required for efficient MHC class I induction by NLRC5.
HLA-B reporter gene activity induced by either NLRC5 (A) or CIITA (B) in the RFXANK/B-deficient cell line EBA. The indicated expression plasmids were co-transfected together with the HLA-B promoter reporter gene construct, and luciferase activity was determined 48 hrs post transfection using the dual-luciferase assay. Data are a representative of several independent experiments performed in triplicates, and are plotted as fold induction with respect to the empty vector control. Error bars represent ± SD. *p < 0.05; **p < 0.01. (C) Analysis of MHC class I gene expression in the RFXANK/B-deficient cell line EBA using gene specific primers for HLA-A and HLA-B. Cells were reconstituted with either RFP-RFXANK/B, GFP-NLRC5, or both expression plasmids and sorted for GFP+, RFP+ or double positive cell population 48 hrs after transfection prior to RNA isolation. Error bars represent ± SD of duplicates. *p < 0.05; **p < 0.01. (D) MHC class I protein expression in RFXANK/B-deficient fibroblasts, transiently transfected with the indicated expression plasmids. Cell lysates were prepared 48 hrs post transfection and analysed by Western blotting. α-Tubulin levels are shown as a loading control.
NLRC5 specifically binds to the RFX component RFXANK/B via its ankyrin repeats
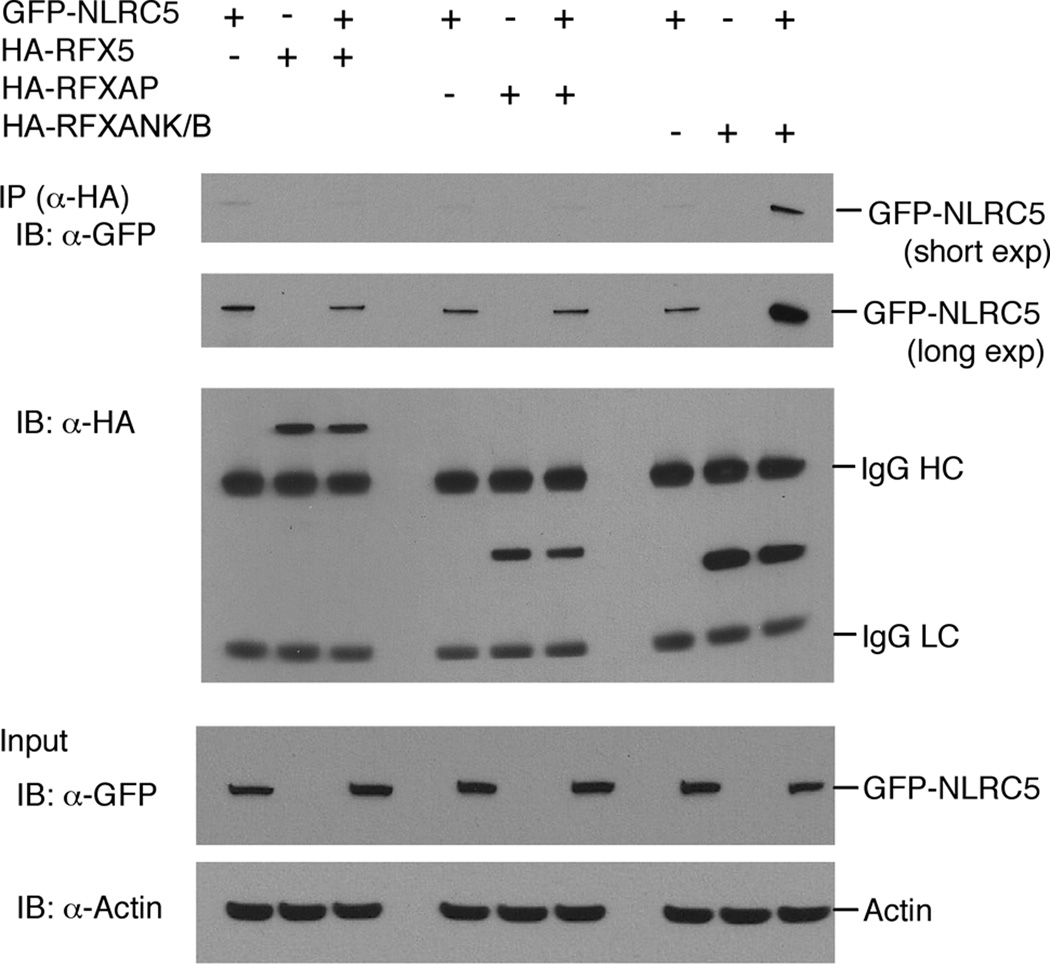
The requirement for the X1 box in NLRC5-induced MHC class I induction and synergy between NLRC5 and the RFX proteins prompted us to investigate whether NLRC5 can directly associate with RFX factor components. An expression vector for GFP-NLRC5 was co-transfected with expression vectors for HA-tagged RFX5, RFXAP or RFXANK/B into HEK293T cells. RFX proteins were immunoprecipitated with an anti-HA-antibody and associated GFP-NLRC5 was detected by Western blot. Interestingly, we only observed an enrichment of GFP-NLRC5 above background levels in the immunoprecipitates of HA-RFXANK/B, not in the immunoprecipitates of RFX5 and RFXAP (Fig. 6).
FIGURE 6. Specific binding of NLRC5 to the RFX component RFXANK/B.
Association of NLRC5 with components of the RFX factor complex was analysed by co-immunoprecipitation. A GFP-NLRC5 expression plasmid was co-transfected with expression vectors for HA-tagged RFX5, RFXAP or RFXANK/B into HEK293T cells. Cell extracts were prepared 48 hrs post transfection. RFX proteins were immunoprecipitated using anti-HA antibody and associated GFP-NLRC5 was detected using an anti-GFP antibody. The blots shown are a representative of three independent experiments. Short exp; short exposure, long exp; long exposure.
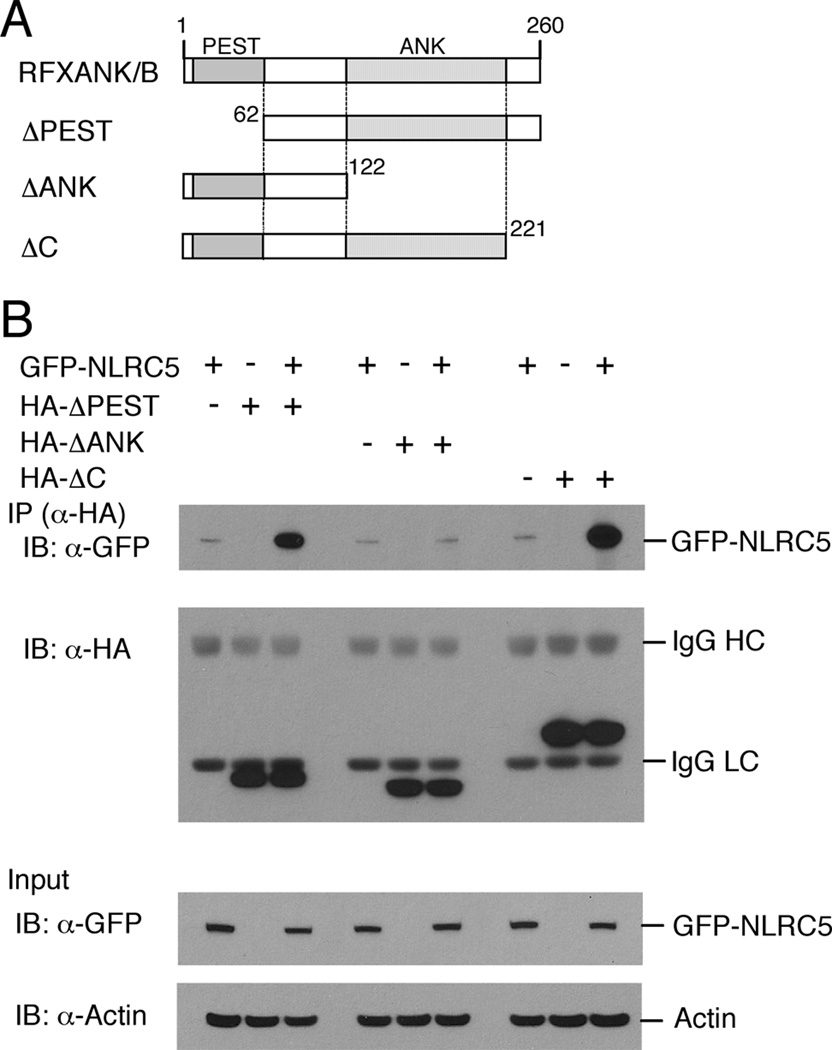
To characterize which domain of RFXANK/B is required for binding to NLRC5, we performed co-immunoprecipitation experiments with RFXANK/B deletion mutants lacking the following domains: RFXANK/B-ΔPEST (aa 62–260, lacking the N-terminal PEST domain), RFXANK/B-ΔANK (aa 1–122, C-terminal truncation of the ankyrin repeats) and RFXANK/B-ΔC (aa 1–221, lacking the very C-terminus) (Fig. 7A). The HA-tagged RFXANK/B deletion mutants were co-expressed with GFP-tagged NLRC5 in HEK293T cells. Binding of NLRC5 was retained even in the absence of the very C-terminal portion of RFXANK/B and did not require the presence of the N-terminal PEST (Proline, Glutamate, Serine, Threonine-rich) domain. In contrast, deletion of the ankyrin repeats (ΔANK), which comprise well-known protein-protein interaction modules, resulted in loss of NLRC5 binding (Fig. 7B). These results support a role of the ankyrin-repeats of RFXANK/B in recruiting NLRC5 to the RFX factor complex that assembles on the X1 cis-regulatory element in the MHC-class I gene promoters.
FIGURE 7. NLRC5 associates with RFXANK/B via its ankyrin repeats.
A, Schematic representation of the domain structure of RFXANK/B and the indicated deletion mutants: RFXANK-ΔPEST (aa 62–260, lacking the N-terminal PEST domain), RFXANK-ΔANK (aa 1–122, C-terminal truncation of the ankyrin repeats) and RFXANK-ΔC (aa 1–221, lacking the very C-terminus).
B, Characterization of the NLRC5 binding domain using RFXANK/B deletion mutants. HEK293T cells were transiently transfected with the indicated expression plasmids, and cell extracts were prepared 48 hrs post transfection. RFXANK/B mutants were immunoprecipitated using an anti-HA antibody and associated GFP-NLRC5 was detected by immunoblotting with an anti-GFP antibody. The blots shown are a representative of two independent experiments.
Discussion
Although CIITA can transactivate both MHC class I and class II promoters in in vitro experiments, the contribution of CIITA to the regulation of MHC class I gene expression in vivo remained unclear. It has been observed previously that loss of CIITA in BLS patients and CIITA-deficient mice does not seem to significantly affect the expression levels of MHC class I genes (32–36). The recent identification of NLRC5 as an MHC class I transactivator (CITA) significantly improved our understanding of the regulation of MHC class I genes (37). The molecular mechanism how NLRC5 transactivates the promoters of MHC class I and functionally related genes, however, had not been investigated in greater detail. Similar to CIITA, NLRC5 is lacking a known DNA-binding domain (37). NLRC5 will thus rely on other transcription factors, which directly associate with MHC class I promoters to perform its function as an MHC class I transactivator. Regulation of both MHC class I and class II genes depends on the W/S-X-Y motif, which contains conserved cis–regulatory elements found in their proximal promoters (9, 10). These cis-regulatory elements are constitutively occupied by the RFX transcription factor complex, members of the CREB/ATF1 family and the NF-Y transcription factor complex. Together, they form a stable DNA-protein complex, which remains inactive without the expression of an additional transactivator such as CIITA (43). In this study, we demonstrate that NLRC5 also requires a functional W/S-X-Y motif for an efficient transactivation of MHC class I genes. Reporter gene assays showed that the W/S, X1 and X2 box are essential for NLRC5-mediated MHC class I transactivation. In line with this observation, NLRC5 can cooperate with the X1 box binding transcription factor RFX and the X2 box binding transcription factor ATF1 in MHC class I promoter activation. In addition, we demonstrate that NLRC5 specifically associates with the RFX subunit RFXANK/B via its ankyrin repeats. Taken together, our findings suggest that the requirement of NLRC5 for the X-box found in the MHC class I promoters is similar to that of CIITA. Both molecules cooperate with RFX proteins and ATF1/CREB family members bound to the X1 and X2 box to transactivate MHC class I genes.
Interestingly, we observed that NLRC5 interacts with the RFX component RFXANK/B but not with other RFX proteins, suggesting that RFXANK/B might assist in the recruitment of NLRC5 to the MHC class I promoter. Similarly, binding of CIITA to RFXANK/B through the ankyrin repeats has been reported previously (44). However, CIITA has been found to interact with all three components of the RFX complex, although CIITA can associate with the RFX complex more strongly when all three subunits exist (44–47). While it is still possible that NLRC5 may associate with all individual RFX proteins under less stringent condition, cooperative binding to the RFX factors is an alternative possibility; NLRC5 might be recruited and associate more tightly with the RFX complex in the presence of all three components, RFXANK/B, RFX5, and RFXAP together, as seen in the association between CIITA and the RFX complex. Curiously, we were unable to determine which domain of NLRC5 is interacting with RFXANK/B, as deletion of any domain of NLRC5 abolished binding to RFXANK/B (data not shown). These findings suggest that the overall conformational integrity of NLRC5 might be required for a robust interaction with the RFXANK/B subunit of the RFX complex.
Another interesting difference between NLRC5 and CIITA-induced MHC class I induction is that NLRC5 requires the W/S box for efficient transactivation (Fig. 1 and 2) (11). The role of the W-box, which inside contains the highly conserved S-box, is still not well understood compared to well-established roles of the X1-, X2- and Y-box. According to one study, the W/S-box might serve as a binding site for a second RFX complex (48). Alternatively the W/S-box has been suggested to represent a binding site for an as yet unidentified factor(s) (48, 49). It has previously been shown that recruitment of CIITA to the MHC-enhanceosome on MHC class II promoters requires both the conserved W/S box and a stringent spacing between the W/S and X boxes (49). By analogy, the W/S-box in MHC class I promoters may be important for the recruitment of NLRC5 to the MHC-enhanceosome on MHC class I promoters. Again, the fact that NLRC5 does not contain a discernable DNA binding domain makes it unlikely that NLRC5 directly binds to the W/S box, although this hypothesis has not been tested experimentally. Instead, it is more likely that NLRC5 will associate with the as yet unknown DNA-binding protein bound to the W/S-box and this interaction might prove pivotal for the recruitment and interaction of NLRC5 with the MHC-enhanceosome on the MHC class I promoter.
As previously shown and confirmed in this report, CIITA can transactivate MHC class I promoters at least in vitro (11, 30, 31, 37). It is an interesting question, therefore, whether NLRC5 requires the presence of CIITA to transactivate MHC class I promoters, possibly by forming a NLRC5-CIITA complex. However, our observation that NLRC5 alone can induce MHC class I expression in cell lines that do not express CIITA, such as HEK293T cells, suggests that this scenario is less likely (Fig. 1 and 2) (37). Moreover, expression of NLRC5 in a CIITA-deficient fibroblast cell line (ATU cells) can still activate MHC class I promoters and induce MHC class I gene expression (data not shown). These findings support the notion that NLRC5 acts as a CITA independently of CIITA to induce MHC class I gene expression.
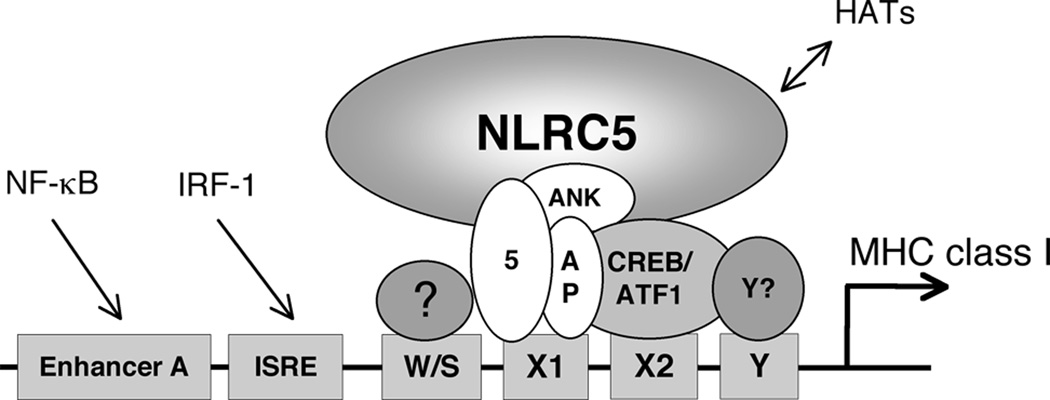
We propose the following model describing the molecular composition of the NLRC5/CITA enhanceosome, which is required for the transactivation of MHC class I and related genes (Fig. 8). Similar to CIITA, NLRC5 relies on the presence of the X1-box-bound RFX factor complex, which is composed of the RFX5, RFXAP and RFXANK/B subunits, for efficient MHC class I gene induction. NLRC5 also synergizes with additional transcription factors of the CREB/ATF1 family bound to the X2-box. Moreover, NLRC5 strictly requires the W/S-box, although the factor binding to the W/S box remains unknown. Additional cis-regulatory elements (Enhancer A, ISRE) in the MHC class I promoter also modulate MHC class I promoter activity. Once the CITA nhanceosome is established on the MHC class I proximal promoter, NLRC5 may recruit other transcriptional co-activators such as CBP/p300, GCN5 and PCAF to promote the transcription of MHC class I genes.
FIGURE 8. Model of the NLRC5 enhanceosome.
Schematic representation of the NLRC5 (‘CITA’) enhanceosome. NLRC5 activates MHC class I genes by generating a nucleoprotein complex with other transcription factors bound to the conserved W/S-X-Y module found in the proximal promoter region of MHC class I genes. The trimeric RFX factor complex and the transcription factor ATF1/CREB are components of the CITA enhanceosome and bind to the X1 and X2 box cis-regulatory elements, respectively. The CITA enhanceosome requires the W/S motif, yet the factor binding to the W/S motif remains unknown. Additional cis-regulatory elements (Enhancer A, ISRE) in the MHC class I promoter also modulate the activity of MHC class I promoters. 5, RFX5; ANK, RFXANK/B; AP, RFXAP; Y, NF-Y; HAT, histone acetyltransferase.
In summary, we have demonstrated that NLRC5 is a crucial component of an MHC class I enhanceosome, which assembles on the conserved W/S-X-Y motif. This CITA enhanceosome is specific to MHC class I gene promoters. Given the important roles of MHC class I genes in immunity, further characterization of the CITA enhanceosome may lead to the development of therapeutics beneficial in anti-viral treatment, tumor immunity and transplantation.
Supplementary Material
Acknowledgments
The authors thank Dr. Peter Cresswell for providing reagents; Jonathan Kagan for helpful discussions; Andrea Dearth for technical assistance.
Abbreviations used in this paper
- NLR proteins
nucleotide binding domain (NBD) leucine rich repeat (LRR) containing proteins
- ER
endoplasmic reticulum
- β2M
β2-microglobulin
- LMP
large multifunctional protease
- Ii
invariant chain
- NLS
nuclear localization signal
- RFX
regulatory factor X
- ATF1
activating transcription factor 1
- NF-Y
nuclear factor-Y
- PCAF
P300/CBP-associated factor
- GCN5
general control nonderepressible 5
- HAT
histone acetyltransferase
Footnotes
This work was supported by grants from the NIH and the Crohn’s and Colitis Foundation of America. K.S.K. is a recipient of the Investigator Award from the Cancer Research Institute and the Claudia Adams Barr Award. T.B.M. has been a recipient of the EMBO Long Term fellowship and A.B. is a recipient of Crohn’s Colitis Foundation of America fellowship.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu. Rev. Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 2.Oldstone MB. Virus-lymphoid cell interactions. Proc. Natl. Acad. Sci. USA. 1996;93:12756–12758. doi: 10.1073/pnas.93.23.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pamer E, Cresswell P. Mechanisms of MHC class I--restricted antigen processing. Annu. Rev. Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 4.Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr. Opin. Immunol. 1999;11:100–108. doi: 10.1016/s0952-7915(99)80018-1. [DOI] [PubMed] [Google Scholar]
- 5.Le Bouteiller P, Solier C. Is antigen presentation the primary function of HLA-G? Microbes Infect. 2001;3:323–332. doi: 10.1016/s1286-4579(01)01386-7. [DOI] [PubMed] [Google Scholar]
- 6.Shastri N, Cardinaud S, Schwab SR, Serwold T, Kunisawa J. All the peptides that fit: the beginning, the middle, and the end of the MHC class I antigen-processing pathway. Immunol. Rev. 2005;207:31–41. doi: 10.1111/j.0105-2896.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 7.Peaper DR, Cresswell P. Regulation of MHC class I assembly and peptide binding. Annu. Rev. Cell Dev. Biol. 2008;24:343–368. doi: 10.1146/annurev.cellbio.24.110707.175347. [DOI] [PubMed] [Google Scholar]
- 8.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- 9.van den Elsen PJ, Gobin SJ, van Eggermond MC, Peijnenburg A. Regulation of MHC class I and II gene transcription: differences and similarities. Immunogenetics. 1998;48:208–221. doi: 10.1007/s002510050425. [DOI] [PubMed] [Google Scholar]
- 10.van den Elsen PJ, Peijnenburg A, van Eggermond MC, Gobin SJ. Shared regulatory elements in the promoters of MHC class I and class II genes. Immunol. Today. 1998;19:308–312. doi: 10.1016/s0167-5699(98)01287-0. [DOI] [PubMed] [Google Scholar]
- 11.Gobin SJ, van Zutphen M, Westerheide SD, Boss JM, van den Elsen PJ. The MHC-specific enhanceosome and its role in MHC class I and beta(2)-microglobulin gene transactivation. J. Immunol. 2001;167:5175–5184. doi: 10.4049/jimmunol.167.9.5175. [DOI] [PubMed] [Google Scholar]
- 12.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109(Suppl.):S21–S33. doi: 10.1016/s0092-8674(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 13.Steimle V, Durand B, Barras E, Zufferey M, Hadam MR, Mach B, Reith W. A novel DNA-binding regulatory factor is mutated in primary MHC class II deficiency (bare lymphocyte syndrome) Genes Dev. 1995;9:1021–1032. doi: 10.1101/gad.9.9.1021. [DOI] [PubMed] [Google Scholar]
- 14.Durand B, Sperisen P, Emery P, Barras E, Zufferey M, Mach B, Reith W. RFXAP, a novel subunit of the RFX DNA binding complex is mutated in MHC class II deficiency. EMBO J. 1997;16:1045–1055. doi: 10.1093/emboj/16.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masternak K, Barras E, Zufferey M, Conrad B, Corthals G, Aebersold R, Sanchez JC, Hochstrasser DF, Mach B, Reith W. A gene encoding a novel RFX-associated transactivator is mutated in the majority of MHC class II deficiency patients. Nat. Gen. 1998;20:273–277. doi: 10.1038/3081. [DOI] [PubMed] [Google Scholar]
- 16.Nagarajan UM, Louis-Plence P, DeSandro A, Nilsen R, Bushey A, Boss JM. RFX-B is the gene responsible for the most common cause of the bare lymphocyte syndrome, an MHC class II immunodeficiency. Immunity. 1999;10:153–162. doi: 10.1016/s1074-7613(00)80016-3. [DOI] [PubMed] [Google Scholar]
- 17.Moreno CS, Beresford GW, Louis-Plence P, Morris AC, Boss JM. CREB regulates MHC class II expression in a CIITA-dependent manner. Immunity. 1999;10:143–151. doi: 10.1016/s1074-7613(00)80015-1. [DOI] [PubMed] [Google Scholar]
- 18.Louis-Plence P, Moreno CS, Boss JM. Formation of a regulatory factor X/X2 box-binding protein/nuclear factor-Y multiprotein complex on the conserved regulatory regions of HLA class II genes. J. Immunol. 1997;159:3899–3909. [PubMed] [Google Scholar]
- 19.Jabrane-Ferrat N, Nekrep N, Tosi G, Esserman LJ, Peterlin BM. Major histocompatibility complex class II transcriptional platform: assembly of nuclear factor Y and regulatory factor X (RFX) on DNA requires RFX5 dimers. Mol. Cell. Biol. 2002;22:5616–5625. doi: 10.1128/MCB.22.15.5616-5625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boss JM, Jensen PE. Transcriptional regulation of the MHC class II antigen presentation pathway. Curr. Opin. Immunol. 2003;15:105–111. doi: 10.1016/s0952-7915(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 21.Masternak K, Muhlethaler-Mottet A, Villard J, Zufferey M, Steimle V, Reith W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. 2000;14:1156–1166. [PMC free article] [PubMed] [Google Scholar]
- 22.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J. Leukocyte Biol. 2008;83:13–30. doi: 10.1189/jlb.0607402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beresford GW, Boss JM. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat. Immunol. 2001;2:652–657. doi: 10.1038/89810. [DOI] [PubMed] [Google Scholar]
- 25.DeSandro A, Nagarajan UM, Boss JM. The bare lymphocyte syndrome: molecular clues to the transcriptional regulation of major histocompatibility complex class II genes. Am. J. Hum. Genet. 1999;65:279–286. doi: 10.1086/302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 27.LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 28.Wong AW, Ghosh N, McKinnon KP, Reed W, Piskurich JF, Wright KL, Ting JP. Regulation and specificity of MHC2TA promoter usage in human primary T lymphocytes and cell line. J. Immunol. 2002;169:3112–3119. doi: 10.4049/jimmunol.169.6.3112. [DOI] [PubMed] [Google Scholar]
- 29.Holling TM, van der Stoep N, Quinten E, van den Elsen PJ. Activated human T cells accomplish MHC class II expression through T cellspecific occupation of class II transactivator promoter III. J. Immunol. 2002;168:763–770. doi: 10.4049/jimmunol.168.2.763. [DOI] [PubMed] [Google Scholar]
- 30.Martin BK, Chin KC, Olsen JC, Skinner CA, Dey A, Ozato K, Ting JP. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/s1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- 31.Gobin SJ, Peijnenburg A, Keijsers V, van den Elsen PJ. Site alpha is crucial for two routes of IFN gamma-induced MHC class I transactivation: the ISRE-mediated route and a novel pathway involving CIITA. Immunity. 1997;6:601–611. doi: 10.1016/s1074-7613(00)80348-9. [DOI] [PubMed] [Google Scholar]
- 32.Benichou B, Strominger JL. Class II-antigen-negative patient and mutant B-cell lines represent at least three, and probably four, distinct genetic defects defined by complementation analysis. Proc. Natl. Acad. Sci. USA. 1991;88:4285–4288. doi: 10.1073/pnas.88.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 34.Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 35.Itoh-Lindstrom Y, Piskurich JF, Felix NJ, Wang Y, Brickey WJ, Platt JL, Koller BH, Ting JP. Reduced IL-4-, lipopolysaccharide-, and IFN-gamma-induced MHC class II expression in mice lacking class II transactivator due to targeted deletion of the GTP-binding domain. J. Immunol. 1999;163:2425–2431. [PubMed] [Google Scholar]
- 36.Williams GS, Malin M, Vremec D, Chang CH, Boyd R, Benoist C, Mathis D. Mice lacking the transcription factor CIITA--a second look. Int. Immunol. 1998;10:1957–1967. doi: 10.1093/intimm/10.12.1957. [DOI] [PubMed] [Google Scholar]
- 37.Meissner TB, Li A, Biswas A, Lee KH, Liu YJ, Bayir E, Iliopoulos D, van den Elsen PJ, Kobayashi KS. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl. Acad. Sci. USA. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meissner TB, Li A, Liu YJ, Gagnon E, Kobayashi KS. The nucleotide-binding domain of NLRC5 is critical for nuclear import and transactivation activity. Biochem. BiophysRes, Commun. 2012 doi: 10.1016/j.bbrc.2012.01.104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meissner TB, Li A, Kobayashi KS. NLRC5: a newly discovered MHC class I transactivator (CITA) Microbes Infect. 2012 doi: 10.1016/j.micinf.2011.12.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gobin SJ, Peijnenburg A, van Eggermond M, van Zutphen M, van den Berg R, van den Elsen PJ. The RFX complex is crucial for the constitutive and CIITA-mediated transactivation of MHC class I and beta2-microglobulin genes. Immunity. 1998;9:531–541. doi: 10.1016/s1074-7613(00)80636-6. [DOI] [PubMed] [Google Scholar]
- 41.Gobin SJ, van Zutphen M, Woltman AM, van den Elsen PJ. Transactivation of classical and nonclassical HLA class I genes through the IFN-stimulated response element. J. Immunol. 1999;163:1428–1434. [PubMed] [Google Scholar]
- 42.Villard J, Lisowska-Grospierre B, van den Elsen P, Fischer A, Reith W, Mach B. Mutation of RFXAP, a regulator of MHC class II genes, in primary MHC class II deficiency. N. Engl. J.Med. 1997;337:748–753. doi: 10.1056/NEJM199709113371104. [DOI] [PubMed] [Google Scholar]
- 43.Peijnenburg A, Van Eggermond MC, Van den Berg R, Sanal O, Vossen JM, Van den Elsen PJ. Molecular analysis of an MHC class II deficiency patient reveals a novel mutation in the RFX5 gene. Immunogenetics. 1999;49:338–345. doi: 10.1007/s002510050501. [DOI] [PubMed] [Google Scholar]
- 44.Nagarajan UM, Peijnenburg A, Gobin SJ, Boss JM, van den elsen PJ. Novel mutations within the RFX-B gene and partial rescue of MHC and related genes through exogenous class II transactivator in RFX-B-deficient cells. J. Immunol. 2000;164:3666–3674. doi: 10.4049/jimmunol.164.7.3666. [DOI] [PubMed] [Google Scholar]
- 45.Choi NM, Majumder P, Boss JM. Regulation of major histocompatibility complex class II genes. Curr. Opin. Immunol. 2011;23:81–87. doi: 10.1016/j.coi.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nekrep N, Geyer M, Jabrane-Ferrat N, Peterlin BM. Analysis of ankyrin repeats reveals how a single point mutation in RFXANK results in bare lymphocyte syndrome. Mol. Cell. Biol. 2001;21:5566–5576. doi: 10.1128/MCB.21.16.5566-5576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholl T, Mahanta SK, Strominger JL. Specific complex formation between the type II bare lymphocyte syndrome-associated transactivators CIITA and RFX5. Proc. Natl. Acad. Sci. USA. 1997;94:6330–6334. doi: 10.1073/pnas.94.12.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu XS, Linhoff Mw, Li G, Chin KC, Maity SN, Ting JP. Transcriptional scaffold: CIITA interacts with NF-Y, RFX, CREB to cause stereospecific regulation of the class II major histocompatibility complex promoter. Mol. Cell. Biol. 2000;20:6051–6061. doi: 10.1128/mcb.20.16.6051-6061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeSandro AM, Nagarajan UM, Boss JM. Associations and interactions between bare lymphocyte syndrome factors. Mol. Cell. Biol. 2000;20:6587–6599. doi: 10.1128/mcb.20.17.6587-6599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garvie CW, Stagno JR, Reid S, Singh A, Harrington E, Boss JM. Characterization of the RFX complex and the RFX5(L66A) mutant: implications for the regulation of MHC class II gene expression. Biochemistry. 2007;46:1597–1611. doi: 10.1021/bi6023868. [DOI] [PubMed] [Google Scholar]
- 51.Muhlethaler-Mottet A, Krawczyk M, Masternak K, Spilianakis C, Kretsovali A, Papamatheakis J, Reith W. The S box of major histocompatibility complex class II promoters is a key determinant for recruitment of the transcriptional co-activator CIITA. J. Biol. Chem. 2004;279:40529–40535. doi: 10.1074/jbc.M406585200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.