Abstract
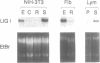
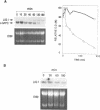
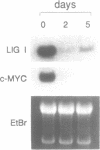
We have studied the regulation of mammalian DNA ligase I gene by using a cDNA probe in Northern blot experiments with RNA extracted from several cell types in different growth conditions. DNA ligase I mRNA is detected in all analysed cell systems, regardless of their proliferation state, including mature rat neurons. A significant increase in DNA ligase I mRNA level is observed when cells are induced to proliferate, in agreement with the raise of DNA joining activity found in the same cell systems. The increase parallels the start of DNA synthesis, but the messenger remains at high level beyond the end of the S phase and is detected also in the presence of aphidicolin. A decrease in DNA ligase I mRNA is observed in HL-60 and NIH-3T3 cells after differentiation. The high stability of DNA ligase I mRNA in both resting and proliferating human fibroblasts suggests a cell proliferation dependent rate of transcription. On the other hand the presence of a basal level of DNA ligase I in nondividing cells, strongly suggests an involvement of this enzyme in DNA repair. This conclusion is supported by a threefold increase in DNA ligase I observed 24 h after UV irradiation of human confluent primary fibroblasts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J. Thermal conversion of nonpriming deoxyribonucleic acid to primer. J Biol Chem. 1959 Oct;234:2733–2734. [PubMed] [Google Scholar]
- Barnes D. E., Johnston L. H., Kodama K., Tomkinson A. E., Lasko D. D., Lindahl T. Human DNA ligase I cDNA: cloning and functional expression in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6679–6683. doi: 10.1073/pnas.87.17.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. E., Tomkinson A. E., Lehmann A. R., Webster A. D., Lindahl T. Mutations in the DNA ligase I gene of an individual with immunodeficiencies and cellular hypersensitivity to DNA-damaging agents. Cell. 1992 May 1;69(3):495–503. doi: 10.1016/0092-8674(92)90450-q. [DOI] [PubMed] [Google Scholar]
- Buvoli M., Biamonti G., Tsoulfas P., Bassi M. T., Ghetti A., Riva S., Morandi C. cDNA cloning of human hnRNP protein A1 reveals the existence of multiple mRNA isoforms. Nucleic Acids Res. 1988 May 11;16(9):3751–3770. doi: 10.1093/nar/16.9.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher M., Rahmsdorf H. J., Litfin M., Karin M., Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal transduction pathways converge to the same enhancer element. Oncogene. 1988 Sep;3(3):301–311. [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Habener J. F. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder R. H., Montecucco A., Ciarrocchi G., Rossignol J. M. Rat liver DNA ligases. Catalytic properties of a novel form of DNA ligase. Eur J Biochem. 1992 Jan 15;203(1-2):53–58. doi: 10.1111/j.1432-1033.1992.tb19826.x. [DOI] [PubMed] [Google Scholar]
- Elder R. H., Rossignol J. M. DNA ligases from rat liver. Purification and partial characterization of two molecular forms. Biochemistry. 1990 Jun 26;29(25):6009–6017. doi: 10.1021/bi00477a019. [DOI] [PubMed] [Google Scholar]
- Englander E. W., Wilson S. H. The cloned promoter of the human DNA beta-polymerase gene contains a cAMP response element functional in HeLa cells. DNA Cell Biol. 1992 Jan-Feb;11(1):61–69. doi: 10.1089/dna.1992.11.61. [DOI] [PubMed] [Google Scholar]
- Fink J. S., Verhave M., Walton K., Mandel G., Goodman R. H. Cyclic AMP- and phorbol ester-induced transcriptional activation are mediated by the same enhancer element in the human vasoactive intestinal peptide gene. J Biol Chem. 1991 Feb 25;266(6):3882–3887. [PubMed] [Google Scholar]
- Focher F., Mazzarello P., Verri A., Hübscher U., Spadari S. Activity profiles of enzymes that control the uracil incorporation into DNA during neuronal development. Mutat Res. 1990 Mar;237(2):65–73. doi: 10.1016/0921-8734(90)90012-g. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Variation of DNA polymerases-alpha, -beta. and -gamma during perinatal tissue growth and differentiation. Nucleic Acids Res. 1977 Aug;4(8):2917–2929. doi: 10.1093/nar/4.8.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N., Ono T., Kato T. Ligation and synthesis of chromatin deoxyribonucleic acid in vitro in neuronal, glial and liver nuclei isolated from adult guinea pig. Biochem J. 1979 Jun 15;180(3):471–480. doi: 10.1042/bj1800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. L., Barker D. G., Johnston L. H. Induction of yeast DNA ligase genes in exponential and stationary phase cultures in response to DNA damaging agents. Curr Genet. 1986;11(2):107–112. doi: 10.1007/BF00378201. [DOI] [PubMed] [Google Scholar]
- Kantor G. J., Setlow R. B. Rate and extent of DNA repair in nondividing human diploid fibroblasts. Cancer Res. 1981 Mar;41(3):819–825. [PubMed] [Google Scholar]
- Kedar P. S., Widen S. G., Englander E. W., Fornace A. J., Jr, Wilson S. H. The ATF/CREB transcription factor-binding site in the polymerase beta promoter mediates the positive effect of N-methyl-N'-nitro-N-nitrosoguanidine on transcription. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3729–3733. doi: 10.1073/pnas.88.9.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko D. D., Tomkinson A. E., Lindahl T. Eukaryotic DNA ligases. Mutat Res. 1990 Sep-Nov;236(2-3):277–287. doi: 10.1016/0921-8777(90)90011-s. [DOI] [PubMed] [Google Scholar]
- Lasko D. D., Tomkinson A. E., Lindahl T. Mammalian DNA ligases. Biosynthesis and intracellular localization of DNA ligase I. J Biol Chem. 1990 Jul 25;265(21):12618–12622. [PubMed] [Google Scholar]
- Lehman I. R. DNA ligase: structure, mechanism, and function. Science. 1974 Nov 29;186(4166):790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]
- Mazzarello P., Focher F., Verri A., Spadari S. Misincorporation of uracil into DNA as possible contributor to neuronal aging and abiotrophy. Int J Neurosci. 1990 Feb;50(3-4):169–174. doi: 10.3109/00207459008987169. [DOI] [PubMed] [Google Scholar]
- Montecucco A., Biamonti G., Ciarrocchi G. A new sequence variant of the human DNA ligase type I gene (LIG I). Nucleic Acids Res. 1991 Nov 25;19(22):6347–6347. doi: 10.1093/nar/19.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco A., Ciarrocchi G. AMP-dependent DNA relaxation catalyzed by DNA ligase occurs by a nicking-closing mechanism. Nucleic Acids Res. 1988 Aug 11;16(15):7369–7381. doi: 10.1093/nar/16.15.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco A., Lestingi M., Pedrali-Noy G., Spadari S., Ciarrocchi G. Use of ATP, dATP and their alpha-thio derivatives to study DNA ligase adenylation. Biochem J. 1990 Oct 1;271(1):265–268. doi: 10.1042/bj2710265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco A., Pedrali-Noy G., Spadari S., Ciarrocchi G. Multiple roles of DNA ligase at the replication fork. Biochim Biophys Acta. 1988 Dec 20;951(2-3):330–334. doi: 10.1016/0167-4781(88)90103-0. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C., Slor H., Thomas G., Cleaver J. E. Defective thymine dimer excision by cell-free extracts of xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2757–2761. doi: 10.1073/pnas.73.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya N., Sawasaki Y., Teraoka H., Nakajima H., Tsukada K. Changes of DNA ligase in developing rat brain. J Biochem. 1977 May;81(5):1575–1577. [PubMed] [Google Scholar]
- Noguiez P., Barnes D. E., Mohrenweiser H. W., Lindahl T. Structure of the human DNA ligase I gene. Nucleic Acids Res. 1992 Aug 11;20(15):3845–3850. doi: 10.1093/nar/20.15.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy G. C., Dalpra L., Pedrini A. M., Ciarrocchi G., Giulotto E., Nuzzo F., Falaschi A. Evidence for two waves of induction of DNA enzymes in stimulated human lymphocytes. Nucleic Acids Res. 1974 Sep;1(9):1183–1199. doi: 10.1093/nar/1.9.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson B. E., Nasheuer H. P., Wang T. S. Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol Cell Biol. 1991 Apr;11(4):2081–2095. doi: 10.1128/mcb.11.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S., Miller-Faurès A., Miller A. O., Kruppa J., Koch G. Synchronization of HeLa cell cultures by inhibition of DNA polymerase alpha with aphidicolin. Nucleic Acids Res. 1980 Jan 25;8(2):377–387. doi: 10.1093/nar/8.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini A. M., Nuzzo F., Ciarrocchi G., Dalprà L., Falaschi A. Induction of polynucleotide ligase in human lymphocytes stimulated by phytohemoagglutinin. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1221–1227. doi: 10.1016/0006-291x(72)90965-5. [DOI] [PubMed] [Google Scholar]
- Petrini J. H., Huwiler K. G., Weaver D. T. A wild-type DNA ligase I gene is expressed in Bloom's syndrome cells. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7615–7619. doi: 10.1073/pnas.88.17.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent C., Lasko D. D., Kodama K., Woodgett J. R., Lindahl T. Activation of mammalian DNA ligase I through phosphorylation by casein kinase II. EMBO J. 1992 Aug;11(8):2925–2933. doi: 10.1002/j.1460-2075.1992.tb05362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Bressler P., Kelly K. Two distinct mechanisms of transcriptional control operate on c-myc during differentiation of HL60 cells. Mol Cell Biol. 1988 Feb;8(2):867–874. doi: 10.1128/mcb.8.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S., Focher F., Hübscher U. Developmental activity profile of DNA polymerases delta and alpha in rat neurons suggests a coordinated in vivo function. In Vivo. 1988 Sep-Oct;2(5):317–320. [PubMed] [Google Scholar]
- Spadari S., Montecucco A., Pedrali-Noy G., Ciarrocchi G., Focher F., Hübscher U. A double-loop model for the replication of eukaryotic DNA. Mutat Res. 1989 May;219(3):147–156. doi: 10.1016/0921-8734(89)90009-x. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. DNA ligases of eukaryotes. FEBS Lett. 1976 Aug 1;67(1):1–8. doi: 10.1016/0014-5793(76)80858-7. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Tsukada K. Eukaryotic DNA ligase. Purification and properties of the enzyme from bovine thymus, and immunochemical studies of the enzyme from animal tissues. J Biol Chem. 1982 May 10;257(9):4758–4763. [PubMed] [Google Scholar]
- Tomkinson A. E., Roberts E., Daly G., Totty N. F., Lindahl T. Three distinct DNA ligases in mammalian cells. J Biol Chem. 1991 Nov 15;266(32):21728–21735. [PubMed] [Google Scholar]
- Wahl A. F., Geis A. M., Spain B. H., Wong S. W., Korn D., Wang T. S. Gene expression of human DNA polymerase alpha during cell proliferation and the cell cycle. Mol Cell Biol. 1988 Nov;8(11):5016–5025. doi: 10.1128/mcb.8.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudzka B. Z., Fornace A., Collins J., Wilson S. H. Characterization of DNA polymerase beta mRNA: cell-cycle and growth response in cultured human cells. Nucleic Acids Res. 1988 Oct 25;16(20):9587–9596. doi: 10.1093/nar/16.20.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]