Abstract
Spasticity is a condition that can include increased muscle tone, clonus, spasms, and hyperreflexia. In this study, we report the effect of manually stimulating the dorsal lumbosacral skin on spontaneous locomotor-like activity and on a variety of reflex responses in 5 decerebrate chronic spinal cats treated with clonidine. Cats were spinalized 1 month before the terminal experiment. Stretch reflexes were evoked by stretching the left triceps surae muscles. Crossed reflexes were elicited by electrically stimulating the right tibial or superficial peroneal nerves. Windup of reflex responses was evoked by electrically stimulating the left tibial or superficial peroneal nerves. We found that pinching the skin of the back abolished spontaneous locomotor-like activity. We also found that back pinch abolished the rhythmic activity observed during reflex testing without eliminating the reflex responses. Some of the rhythmic episodes of activity observed during reflex testing were consistent with clonus with an oscillation frequency greater than 3 Hz. Pinching the skin of the back effectively abolished rhythmic activity occurring spontaneously or evoked during reflex testing, irrespective of oscillation frequency. The results are consistent with the hypothesis that locomotion and clonus are produced by common central pattern-generators. Stimulating the skin of the back could prove helpful in managing undesired rhythmic activity in spinal cord-injured humans.
Keywords: central pattern generator, locomotion, spinal cord injury, clonus, spinal reflexes, cutaneous, spasticity
Introduction
Spasticity is a term with varying definitions [see (Nielsen et al., 2007) for a discussion]. One of the most cited definitions refers to spasticity as a motor disorder with a velocity-dependent increase in tonic stretch reflexes or muscle tone (Lance, 1980). However, others use the more clinically relevant definition of spasticity, which also includes clonus, spasms, and hyperreflexia (Skold et al., 1999; Dietz, 2000; Nielsen et al., 2007). In the present paper we use the latter definition. Spasticity can be debilitating in patients with spinal cord injury (SCI) but it can be alleviated with various drugs (Adams et al., 2005). However, these drugs can also produce undesired side effects and interfere with functional recovery, such as walking (Adams et al., 2005; Dietz et al., 2007; Kohout et al., 2011). Of obvious interest in treating spasticity after SCI are approaches that eliminate or reduce spasticity without interfering with functional recovery.
As mentioned, clonus, or myoclonus, can be considered as a sign of spasticity. In the present study, clonus is defined as involuntary rhythmic activity in hindlimb muscles crossing the hip, knee, and/or ankle joints (Bussel et al., 1988; Beres-Jones et al., 2003; Nadeau et al., 2010) with an oscillation frequency exceeding 3 Hz (Rack et al., 1984; Hidler et al., 1999; Hidler et al., 2000; Vetrugno et al., 2000). Clonus after SCI can range from manageable to functionally debilitating (Brown et al., 1994). It is thought that the inability of some SCI patients to stand or walk independently is due to severe clonus (Fung et al., 1989; Fung et al., 1990). Therefore, finding effective treatments for clonus that do not interfere with functional recovery (e.g. walking, postural control) is of critical importance.
Recently, it was shown that strongly pinching the skin of the lower back effectively stopped rhythmic spontaneous synchronous discharges of multiple leg muscles (i.e. myoclonus) in a motor complete spinal cord-injured human subject (Nadeau et al., 2010). This is reminiscent of reports in decerebrate curarized rabbits with intact spinal cords whereby applying manual pressure to the dorso-lumbosacral skin abolished locomotor-like activity (Viala et al., 1974; Viala et al., 1978). It was proposed that clonus could be mediated by abnormal regulation of central pattern generators (CPGs) by sensory inputs. Interactions between sensory inputs from the hindlimbs and CPGs have been well described in the spinalized cat [reviewed in (Frigon et al., 2006; Frigon et al., 2008; Rossignol et al., 2011)]. However, other than an inhibitory effect on rhythmic activity, little is known about interactions between cutaneous inputs from the back and CPGs within the injured spinal cord.
The present paper stems from observations obtained in adult cats that were spinal transected (i.e. spinalized) one month before a terminal experiment. The original goal was to evaluate various reflex pathways before and after injecting clonidine (Frigon et al., 2011; Frigon et al., 2012). Clonidine is an alpha-2 noradrenergic agonist known to potentiate locomotor activity in chronic spinal cats (Barbeau et al., 1987). However, after clonidine injection, triceps surae muscle stretch or nerve stimulation often evoked exaggerated reflex responses and/or generated rhythmic activity that could continue unabated for several minutes. Applying manual pressure to the dorso-lumbosacral skin abolished rhythmic activity. In addition, and of potentially greater clinical significance, during reflex testing, manually pinching the dorsal lumbosacral skin removed rhythmic activity without completely abolishing the reflex responses themselves. Therefore, we describe the effects of manually stimulating the dorso-lumbosacral skin of the chronic spinalized cat treated with clonidine on a variety of reflex responses and on rhythmic activity occurring spontaneously or elicited during reflex testing.
The present data provide a template to better understand the mechanisms and circuitry involved in reducing undesired rhythmic activity through sensory stimulation in a well-defined SCI animal model. Identifying and better understanding circuits that might be involved in gating or filtering undesired activity could lead to more effective treatments for spasticity in spinal cord-injured humans.
Methods
Ethical information, animal care, and surgical procedures
All procedures were approved by the Institutional Animal Care and Use Committee of Northwestern University. All animals were obtained from a designated breeding establishment for scientific research. Before the experiments, animals were housed and fed within designated areas, which were monitored daily by veterinarians and trained personnel. The current data set is compiled from 5 adult cats weighing between 2.5 and 5.0 kg. The data presented in the current study are derived from experiments performed for answering other scientific questions (Frigon et al., 2011; Frigon et al., 2012). No additional animals were used to compile the current data set. This is part of our effort to maximize the scientific output from every animal experiment.
Terminal experiment
During the terminal experiment, cats were first placed in a clear plastic cylinder and anesthetized with 1.5–3% isoflurane in a 1:3 mixture of O2 and NO2. After approximately 15 minutes the animal was transferred from the cylinder to a surgical table and anesthesia was continued with a mask. Once the animal was deeply anesthetized, a tracheotomy was performed and cats were intubated to deliver the anesthesia. The right common carotid artery and right jugular vein were cannulated to monitor blood pressure and for fluid administration, respectively. The level of anesthesia was confirmed and adjusted by monitoring blood pressure, applying pressure to the paw to detect limb withdrawal, and by verifying the size and reactivity of the pupils. The animal was then transferred to a stereotaxic frame for further surgery. Following a craniotomy, the cortex and all neural tissue rostral to the colliculi was removed (i.e., a precollicular decerebration). At this point, animals are considered to have complete lack of sentience (Silverman et al., 2005) and anesthesia was discontinued. A lethal injection of potassium chloride (2 mg/kg) was administered at the end of the experiment through the right jugular vein and a bilateral pneumothorax was performed.
Survival surgery
The spinal cord was completely transected at low thoracic levels approximately 1 month before the terminal experiment. The spinalization was performed under aseptic conditions in an operating room with sterilized equipment. Prior to surgery, cats were sedated (Butorphanol, 0.4 mg/kg, i.m.; Acepromazine, 0.05–0.1mg/kg, i.m.; Glycopyrrolate, 0.01mg/kg, subcutaneous). Induction was done with Propofol (2–3 mg/kg, i.v.) or Ketamine/Diazepam (0.11 ml/kg in 1:1 ratio, i.v.). Once anesthetized, the cat was quickly intubated with a flexible endotracheal tube and anesthesia was maintained by adjusting isoflurane concentration as needed (1.5 – 3%). The fur overlying the back was shaved with electric clippers and loose hair was vacuumed. A forelimb was shaved and an i.v. line was placed in a cephalic vein. The level of anesthesia was confirmed and adjusted throughout the surgery by monitoring cardiac rate, respiratory rate, by applying pressure to the paw to detect limb withdrawal, and jaw tone. Body temperature was monitored using a rectal thermometer. Parameters were monitored on a continuous basis (i.e. cardiac and respiratory rates) and recorded every 15 minutes. Cats received fluids i.v. (warmed lactated ringer solution or saline + 2.5% Dextrose) at a rate of 5–10 ml/kg/hr throughout the surgery to maintain hemodynamic stability. Ophthalmic ointment was also applied to the eyes.
A laminectomy was performed at the vertebrae overlying spinal segments T12-T13, the dura was removed, and after local lidocaine application (Xylocaine, 2%), the spinal cord was completely transected with surgical scissors. The spinal cord is transected at low thoracic segments to avoid damaging spinal CPGs that control the hindlimbs, which are thought to be primarily located at L3–L4 in the cat (Marcoux et al., 2000; Delivet-Mongrain et al., 2008). Hemostatic material (Surgicel) was inserted within the gap, and muscles and skin were sewn back to close the opening in anatomic layers. A transdermal fentanyl patch (25 mcg/hr) was taped to the back of the animal 2–3 cm from the base of the tail. During surgery and approximately 7 hours later, an analgesic (Buprenorphine 0.01 mg/kg) was administered subcutaneously. Buprenorphine is administered during and approximately 7 hours after surgery because it is a rapidly acting analgesic, whereas the morphine delivered by the transdermal patch requires a longer time to take effect. Concurrent administration of buprenorphine and morphine maximizes analgesia. An oral antibiotic (Baytril, 5 mg/kg) was given once a day for 5 days after spinalization to prevent infection. The bladder was manually emptied 1–2 times each day up to the terminal experiment. The animals were monitored daily by experienced personnel and veterinarians. The hindlimbs of the cats were frequently cleaned by placing the lower half of the body in a warm soapy bath.
Experimental design
A schematic illustration of the experimental set-up is shown in a recent publication (Frigon et al., 2012). The Achilles tendon of the left hindlimb was freed from surrounding tissue, leaving only a small piece of the calcaneal bone attached. The tendon was connected through an in-series force transducer attached to a linear motor (Copley Controls) controlled by custom-made software to alter muscle length. Force signals were low-pass filtered at 1000 Hz and sampled at 10000 Hz. Both hindfeet were held with a clamp. The left knee joint was also fixed with a custom-made clamp attached to the femoral epicondyles. Hip, knee, and ankle joint angles were ~120°, 160°, and 90° for both hindlimbs. The hindpaws were not contacting the table surface.
Bipolar wire electrodes were inserted into the soleus (Sol, ankle extensor), lateral gastrocnemius (LG, ankle extensor/knee flexor), semitendinosus (St, knee flexor/hip extensor), anterior sartorius (Srt, hip flexor/knee extensor), and tibialis anterior (TA, ankle flexor) of the left hindlimb and in the right St for electromyography (EMG). EMG signals were amplified (×1000) with a multichannel amplifier (AM Systems Model 3500), bandpass filtered (300–3000 Hz), and sampled at 10000 Hz simultaneously with force data. Bipolar stimulating cuff electrodes were placed around the left and right tibial (Tib) and superficial peroneal (SP) nerves near the ankle joint.
Experimental protocol
Reflex responses were evoked after injecting clonidine through the right jugular vein (i.e. 250–500 µg/kg) with or without pinching the dorso-lumbosacral skin of the back. The actions of clonidine last approximately 5 hours (Chau et al., 1998) and reflex testing was completed within 3 hours of injection. The pinch consisted of holding the skin between the thumb, index, and middle finger at a constant moderate pressure. For the “pinch trials”, the pinch was initiated 20 s before the stretch or nerve stimulation and continued until the end of the trial (i.e. 15 s post-stretch or stimulation). Pinches were also performed during episodes of spontaneous rhythmic activity. The same experimenter performed the pinch in all experiments. Viala et al. 1978 reported that natural stimulation (i.e. exerting manual pressure to the skin) is more effective than electrical stimulation of afferents.
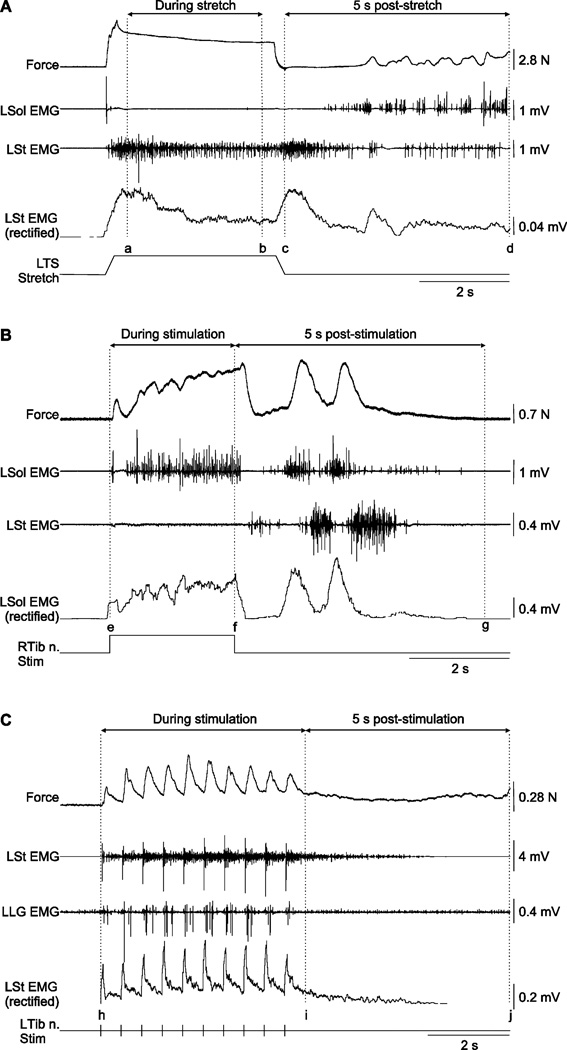
Stretch reflex (Fig. 1A)
Figure 1. Measurements of reflex responses.
A) Left triceps surae (LTS) muscle force and electromyography (EMG) measures during and following stretch of the left triceps surae muscles. The mean force produced by LTS muscles during stretch was calculated as the average force value between cursors a and b, corresponding to a 3 s period centered during the hold. EMG activities were calculated as the area under the curve (modulus function in Spike2) between cursors a and b for all muscles. The same measures were taken starting from the end of the triceps surae muscle stretch to 5 s later (i.e. between cursors c and d). B) Triceps surae muscle force and EMG measures during and following stimulation of the right tibial nerve (RTib n.). Left triceps surae muscle force and EMG activities for all muscles were measured as the area under the curve between cursors e and f. The same measures were taken during a 5 s post-stimulation window starting at stimulation offset (i.e. between cursors f and g). C) Left triceps surae muscle force and EMG measures during and following a series of 10 pulses delivered to the left tibial nerve (LTib n.) nerve. Left triceps surae muscle force and EMG activities for all muscles were measured as the area under the curve between cursors h and i. Cursor i was placed 1 s and 0.5 s following the 10th pulse at stimulation frequencies of 1 Hz and 2 Hz, respectively. The same measures were taken during a 5 s post-stimulation between cursors i and j. L = left, R = right, Sol = soleus, St = semitendinosus, LG = lateral gastrocnemius
Several ramp-hold-return stretches of the left triceps surae muscles were performed. Stretches consisted of a 5 mm lengthening in 0.25 s, a 3.5 s hold, and a return to the initial length in 0.25 s, for a total duration of 4 s. Such stretches are similar in terms of time course and amplitude to postural perturbations used in rabbits (Musienko et al., 2010). The left triceps surae muscles were stretched from an initial muscle length that corresponds to a 90° ankle joint angle. Muscle stretches were performed at intervals of no less than 1 minute.
Crossed reflexes (Fig. 1B)
Crossed responses were evoked by stimulating the right Tib or SP nerves. Motor thresholds (T) were first determined. For the right Tib nerve, the motor threshold was the stimulation intensity required to evoke a small plantarflexion of the right toes. The motor threshold for SP nerve stimulation was the stimulation intensity required to evoke a small flexion response at the knee. Nerves were stimulated at 40 Hz for 2.5 s or 20 s (0.2 ms pulse width) at 2T or 5T at short and long triceps surae muscle lengths. The stimulation intensities required to evoke the motor thresholds were verified throughout the experiment and adjusted accordingly. Nerve stimulations were performed at intervals of no less than 1 minute.
Wind-up (Fig. 1C)
A series of 10 pulses to the left Tib or SP nerves was given to evaluate the presence and modulation of wind-up in chronic spinal cats before and after injecting clonidine. In the present study we only describe the effect of back pinch on the series of 10 pulses. Motor thresholds were determined identically to crossed reflexes but on the left side. The series of 10 pulses (0.2 ms pulse width) was given at 2T or 5T at a stimulation frequency of 1 Hz or 2 Hz.
Measurements
All measurements were done with Spike2 version 6.0. EMG waveforms were rectified and smoothed with a 0.03 time constant for measurements. EMG waveforms with noise or large stimulation artifacts were removed from the analyses. Triceps surae force waveforms were not rectified or smoothed. Windows used for force and EMG measures are shown in Figure 1.
For stretch-evoked responses (Fig. 1A), the mean force produced by the left triceps surae muscles during stretch was calculated as the average force value between cursors a and b. EMG activities were calculated as the area under the curve (modulus function in Spike2) between cursors a and b for all muscles. The same measures were taken starting from the end of the triceps surae muscle stretch to 5 s later (i.e. between cursors c and d).
For crossed reflex response (Fig. 1B) evoked during stimulation of the right Tib and SP nerves, triceps surae muscle force and EMG activities for all muscles were measured as the area under the curve between cursors e and f. The same measures were taken during a 5 s post-stimulation window starting at stimulation offset (i.e. between cursors f and g).
For responses evoked during a series of 10 pulses (Fig. 1C) by stimulating the left Tib or SP nerves, triceps surae muscle force and EMG activities for all muscles were measured as the area under the curve between cursors h and i. Cursor i was placed 1 s and 0.5 s following the 10th pulse at stimulation frequencies of 1 Hz and 2 Hz, respectively. The same measures were taken during a 5 s post-stimulation between cursors i and j.
A trial was considered to have rhythmic activity when at least 5 alternating bursts between extensors (i.e. LG, Sol) and flexors (i.e. St, Srt) were present during or following stretch of left triceps surae muscles or stimulation of the right Tib or SP nerves. Although the TA is an ankle flexor it was not used to determine alternation because it could be co-active with extensors (e.g. Figs. 3A, 5D, or 7A) or flexors (e.g. Fig. 5A). Wind-up stimulations of the left Tib or SP nerves are not included in this analysis because the series of 10 pulses generates bursts and could entrain the rhythm. The time interval from onset of the 1st burst to offset of the 5th burst was determined. Cycle frequency was determined by dividing the number of bursts selected (i.e. 5) by the time interval from burst 1 onset to burst 5 offset and expressed in Hz. Burst onset and offset were determined visually.
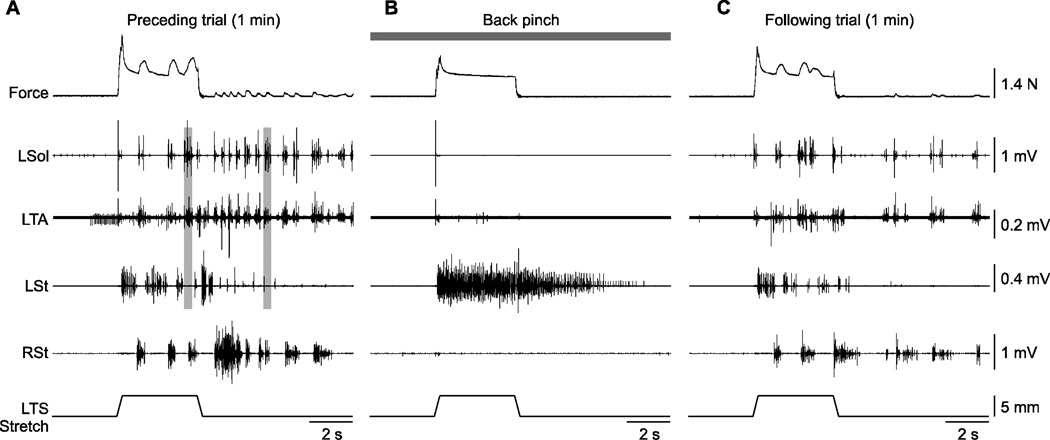
Figure 3. Effect of back pinch on left triceps surae (LTS) muscle force and electromyography (EMG) responses evoked by stretching the LTS muscles in cat 71510.
Shown are 3 trials performed approximately 3 minutes apart without (A, C) and with (B) back pinch. The vertical grey bars in A illustrate co-activation of the left soleus (Sol) and left tibialis (TA) muscles during and following stretch of the LTS muscles. L = left, R = right, St = semitendinosus
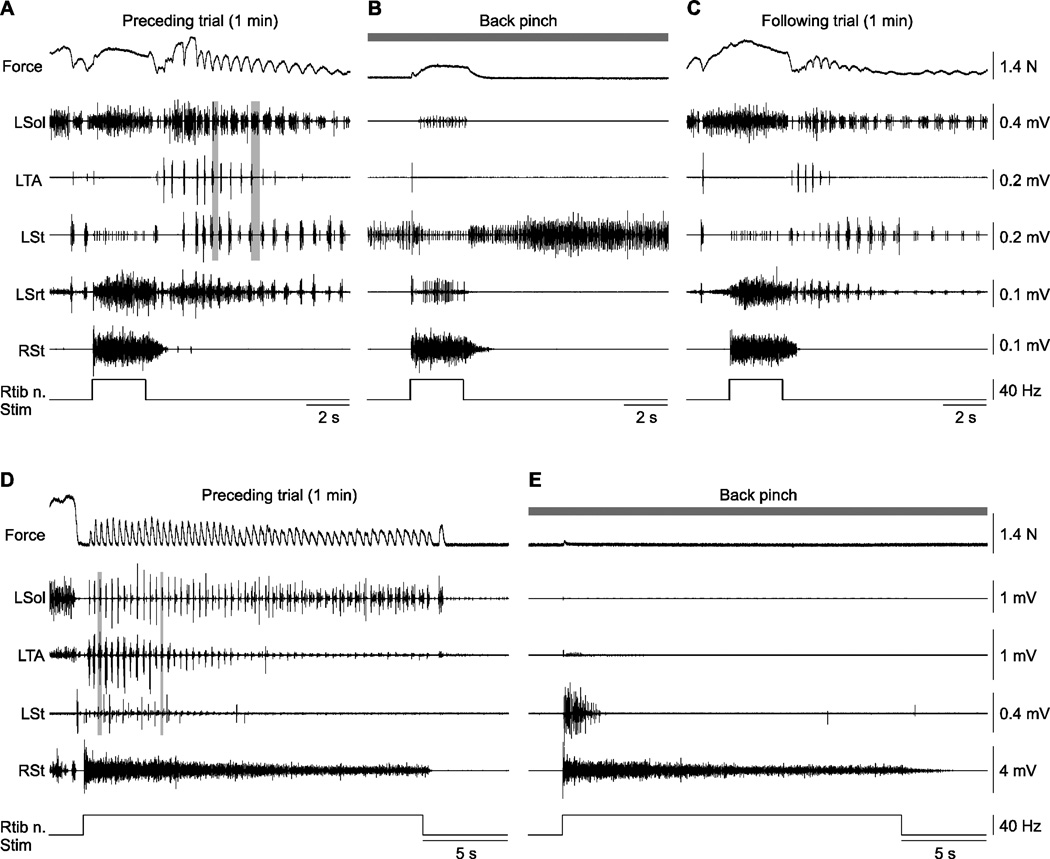
Figure 5. Effect of back pinch on left triceps surae muscle force and electromyography (EMG) responses evoked by stimulating the right tibial nerve (RTib n.) in cats 7155 (A–C) and 71510 (D–E).
A–C) Shown are 3 trials performed approximately 3 minutes apart without (A, C) and with (B) back pinch with stimulation of the RTib n. for 2.5 s at 5T. D–E) Shown are 2 trials with 20 s stimulation of the RTib n. at 2T without (D) and with (E) back pinch. The vertical grey bars in A illustrate alternation of the left soleus (Sol) and left tibialis (TA) muscles following stimulation, whereas the example in D shows co-activation between the left Sol and left TA during stimulation. L = left, R = right, St = semitendinosus, Srt = sartorius
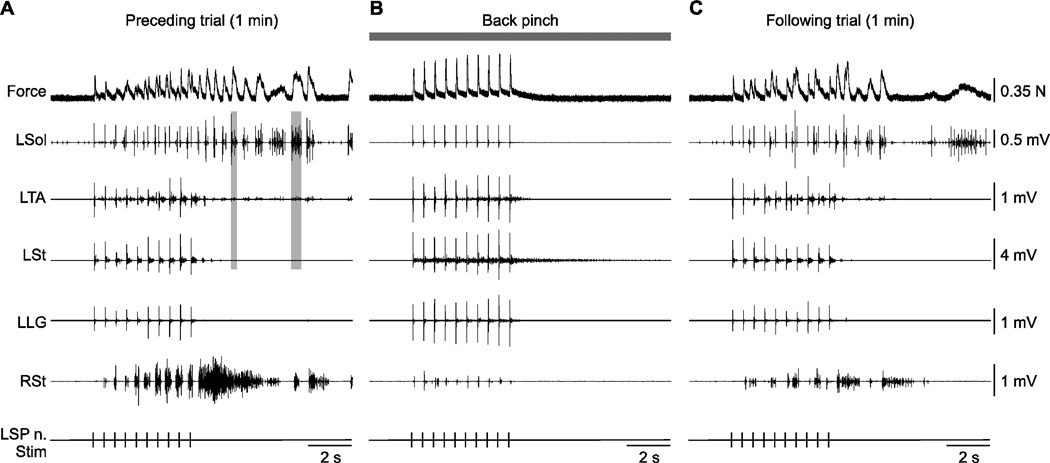
Figure 7. Effect of back pinch on left triceps surae muscle force and electromyography (EMG) responses evoked by stimulating the left superficial peroneal nerve (LSP n.) with a series of 10 pulses in cat 71510.
Shown are 3 trials performed approximately 3 minutes apart without (A, C) and with (B) back pinch. The LSP n. was stimulated at 2 Hz and 5T. The vertical grey bars in A illustrate co-activation between the left soleus (Sol) and left tibialis anterior (TA) following the stimulation. L = left, R = right, St = semitendinosus, LG = lateral gastrocnemius
Statistical analysis
All statistical tests were done with SPSS version 18.0 for measures of force and/or EMG. Statistical analyses were performed using the Wilcoxon signed-rank test for paired data to determine statistical differences between pinch trials and preceding trials. For crossed reflex responses, data obtained from right Tib or SP nerve stimulation at 2T and 5T were pooled for statistical analyses. For wind-up responses, data from left Tib and left SP nerves at 2T, 5T, 1 Hz, and 2 Hz were pooled for statistical analyses. Statistical significance was set at p ≤ 0.05. All values in the text and figures are the mean ± standard deviation (SD).
Results
Episodes of rhythmic activity were termed “locomotor-like” when they occurred spontaneously and with an oscillation frequency of 1.5 Hz or less (Frigon, 2011). Seven episodes of spontaneous locomotor-like activity were recorded in 4 cats with an average cycle frequency of 0.54 ± 0.29 Hz (range 0.26 – 1.11 Hz). Pinching the back of the skin effectively abolished locomotor-like activity in 100% of trials. Episodes of rhythmic activity were classified as “clonus-like” when they were triggered by left triceps surae muscle stretch or by stimulating the right Tib or SP nerves and with an oscillation frequency greater than 3 Hz (Rack et al., 1984; Hidler et al., 1999; Hidler et al., 2000; Vetrugno et al., 2000). Overall, 42 of 59 reflex trials without back pinch possessed episodes of rhythmic activity with an average cycle frequency of 2.45 ± 1.08 Hz. Of those 42 episodes of rhythmic activity, 12 could be classified as clonus-like (i.e. cycle frequency ≥ 3 Hz). No stretch or crossed reflex trials (0/37) with back pinch had rhythmic activity. Therefore, manually stimulating cutaneous inputs from the back consistently abolishes locomotor-like and clonus-like activity in the chronic spinalized cat, irrespective of oscillation frequency.
Effect of back pinch on locomotor-like activity
Figure 2 shows a representative example of the effect of manually pinching the dorsal lumbosacral skin during an episode of spontaneous locomotor-like activity in one cat. The pattern consisted of alternation between the extensors (Sol, LG) and the ipsilateral St and alternation between the left and right St, consistent with locomotor activity. However, the TA was co-activated with Sol and LG, which can be observed during clonus in spinal cord-injured humans (Cook, Jr., 1967; Gottlieb et al., 1977; Latash et al., 1989; Hidler et al., 2000; Beres-Jones et al., 2003).
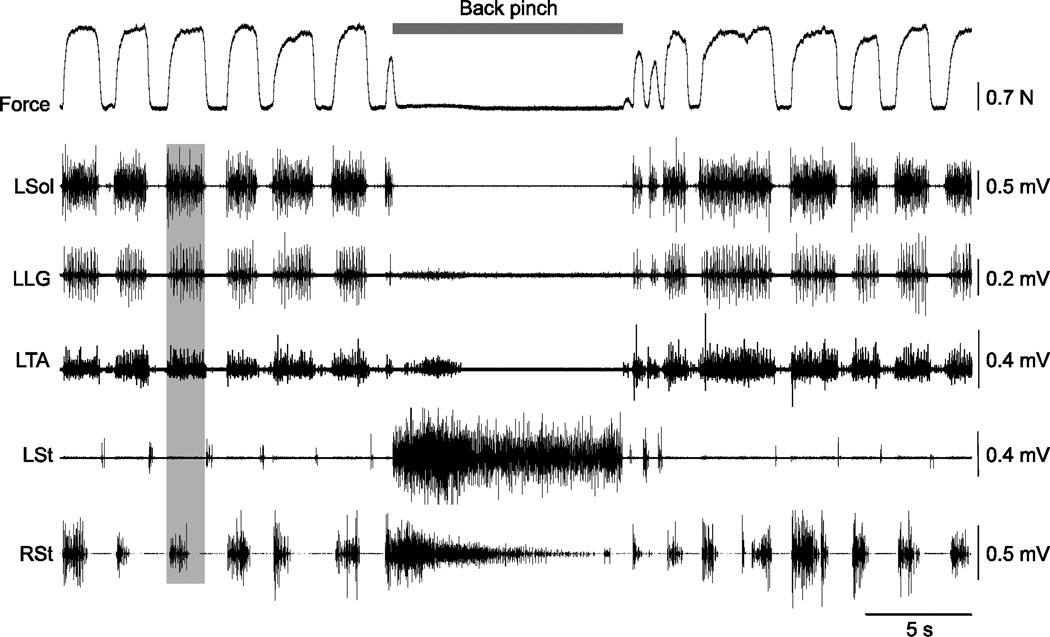
Figure 2. Effect of back pinch during locomotor-like activity in cat 71510.
The vertical grey bar illustrates synchronous activation of the left soleus (Sol), lateral gastrocnemius (LG), and tibialis anterior (TA) muscles along with the right semitendinosus (St). L = left, R = right
As can be seen, back pinch stopped the bursting pattern in left Sol, LG, and TA while producing sustained activity in St bilaterally. The locomotor-like pattern resumed immediately after terminating the back pinch. The reset to flexion suggests that sensory inputs generated by pinching the skin of the back have direct access to the rhythm-generating circuitry [recently reviewed in (Frigon et al., 2010a; Frigon et al., 2010b; Gossard et al., 2011; Frigon, 2012)]. Spontaneous locomotor-like activity was observed in all cats following clonidine administration and pinching the back effectively stopped locomotor-like activity in 100% of trials.
Effect of back pinch on stretch-evoked responses
We recently showed that hindlimb muscle responses evoked by stretching the left triceps surae muscles were considerably altered in acute and chronic spinal cats (Frigon et al., 2011). We also showed that clonidine produced no consistent changes in stretch-evoked responses (Frigon et al., 2012). After clonidine injection, some cats showed an increase in stretch-evoked responses while other cats showed a decrease or no change. In the present study we compared the effect of back pinch on stretch-evoked responses in chronic spinal cats treated with clonidine.
Figure 3 shows the effect of back pinch during and after stretch of the left triceps surae muscles in one cat. In the trial that preceded back pinch, stretching the triceps surae muscles evoked bilateral rhythmic bursts during and after stretch (Fig. 3A). While the dorsal lumbosacral skin was manually pinched the same stretch primarily produced sustained activity in the left St and no rhythmic bursts were present (Fig. 3B). One minute following the back pinch trial the left triceps surae muscles were again stretched and rhythmic bursts reappeared bilaterally (Fig. 3C).
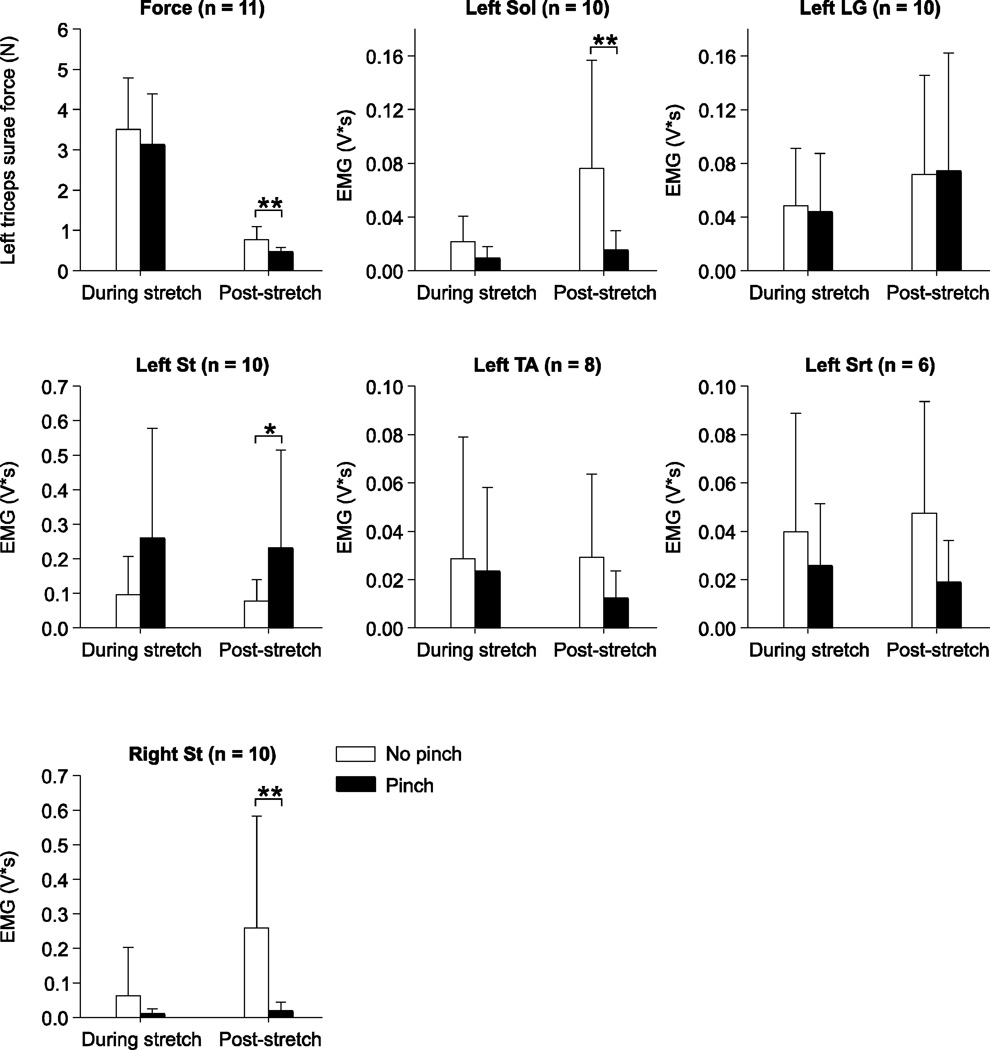
Pooled data show that back pinch had no significant effect on left triceps surae muscle force or EMG measures during left triceps surae muscle stretch (Fig. 4). However, left triceps surae muscle force and EMG activities for the left Sol and right St produced during the 5 sec period following stretch were significantly diminished while the EMG activity for the left St was increased. Therefore, stimulating cutaneous inputs from the lumbosacral back reduces some reflex responses without influencing the main stretch reflex component, which is the homonymous force and EMG responses from the left triceps surae muscles.
Figure 4. Pooled data showing the effect of back pinch on left triceps surae muscle force and electromyography (EMG) responses evoked during and following stretch of the left triceps surae muscles.
The EMG activities of the left soleus (Sol), lateral gastrocnemius (LG), semitendinosus (St), tibialis anterior (TA), left sartorius (Srt), and of the right St were measured as described in figure 1A and in the methods. The number of paired trials is indicated in brackets at the top for each graph. Asterisks indicate significant differences between trials without and with back pinch using the Wilcoxon signed-rank test for paired data at p ≤ 0.05. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
Effect of back pinch on crossed responses
Stimulating the right Tib or SP nerves at high frequency for a few seconds evokes crossed extensor reflex responses in cats with an intact spinal cord. Crossed extensor reflexes are abolished following acute spinalization but show signs of recovery in 1 month chronic spinalized cats and clonidine potentiates these responses (Frigon et al., 2012). In the present study we compared the effect of back pinch on crossed reflex responses in chronic spinalized cats treated with clonidine.
Figure 5 shows the effect of back pinch during and after stimulating the right Tib nerve at 40 Hz for 2.5 s in one cat (Fig. 5A–C) and at 40 Hz for 20 s in another cat (Fig. 5D–E). In the trial that preceded back pinch, Tib nerve stimulation evoked a large response in the right St and crossed activation of the left Sol and Srt muscles with weak activation of the left St (Fig. 5A). A spontaneous locomotor-like rhythm was observed before stimulation. Following stimulation the rhythm was faster. Pinching the back abolished spontaneous locomotor-like activity and diminished crossed activation of the left Sol and Srt muscles during stimulation without visibly altering the response in the right St (Fig. 5B). The left St was tonically active before, during, and after stimulation. Stimulating the Tib nerve one minute following the back pinch trial produced similar crossed activation and rhythmic activity as observed in the trial preceding the back pinch trial (compare Fig. 5C with 5A).
Stimulating the Tib nerve for 20 s before and during back pinch was performed in 3 cats (6 back pinch trials with preceding controls) and yielded qualitatively similar results. In the example shown in Figure 5D the stimulation generated a rhythm characterized by co-activation of Sol and TA muscles on the left and sustained activation of the right St. With back pinch, rhythmic activity was abolished, a brief burst was observed in the left St, and sustained activation of the right St was unaffected (Fig. 5E). Thus, back pinch potently abolishes rhythmic activity that occurs spontaneously or that is triggered by nerve stimulation.
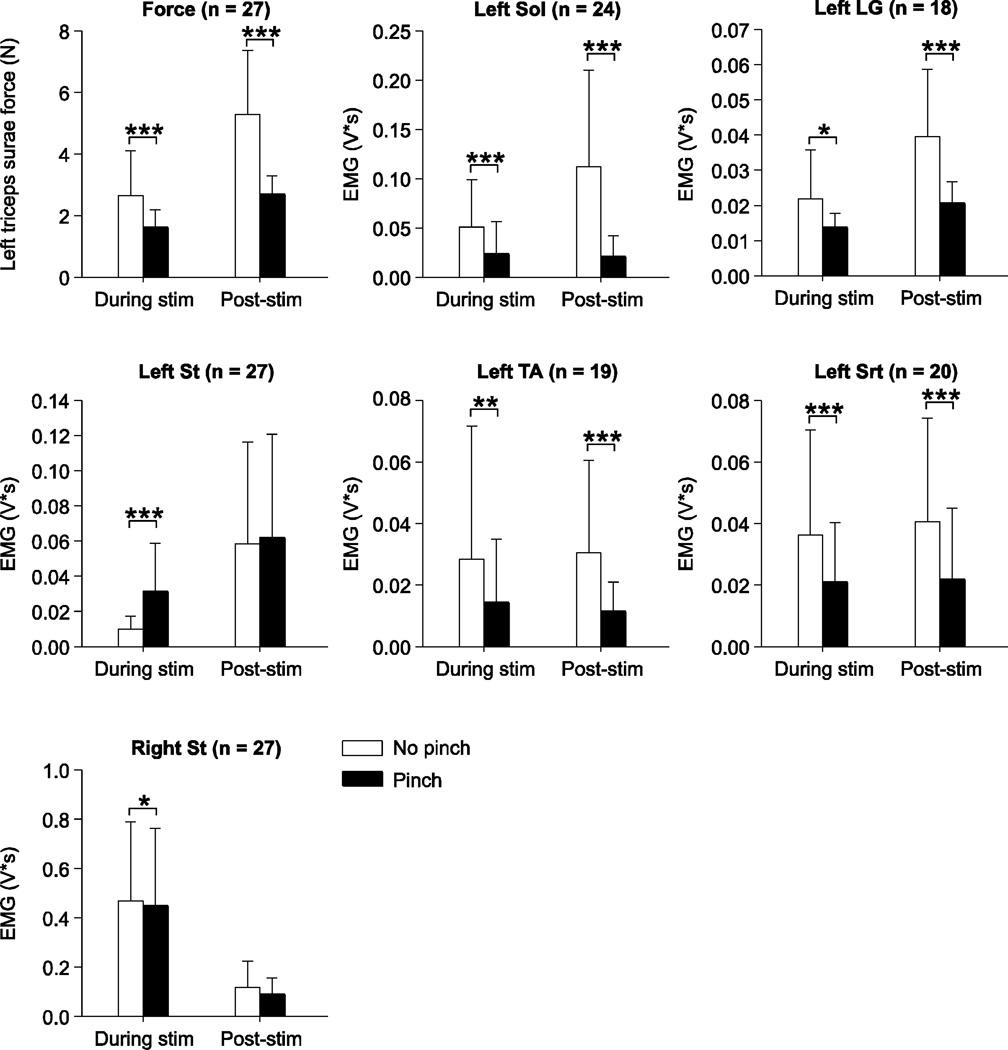
Pooled data show that back pinch reduced left triceps surae muscle force and the EMG activity of left Sol, left LG, left TA, left Srt, and right St evoked during stimulation of the right Tib and SP nerves (Fig. 6). On the other hand, activity in the left St was increased by back pinch during stimulation. In the 5 s window following nerve stimulation, back pinch reduced left triceps surae muscle force and the EMG activity in left Sol, left LG, left TA, and left Srt. Therefore, stimulating cutaneous inputs from the lumbosacral back reduces crossed reflex responses to most muscles during and after nerve stimulation but produces an increase in the St. This indicates independent modulation of crossed inputs to various motor pools.
Figure 6. Pooled data showing the effect of back pinch on left triceps surae muscle force and electromyography (EMG) responses evoked during and following stimulation of the right tibial (n = 15) or superficial peroneal (n = 12) nerves.
The EMG activities of the left soleus (Sol), lateral gastrocnemius (LG), semitendinosus (St), tibialis anterior (TA), left sartorius (Srt), and of the right St were measured as described in figure 1B and in the methods. The number of paired trials is indicated in brackets at the top for each graph. Asterisks indicate significant differences between trials without and with back pinch using the Wilcoxon signed-rank test for paired data at p ≤ 0.05. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
Effect of back pinch on wind-up
Wind-up of reflex responses is used to indirectly evaluate persistent inward currents that mediate sustained activity of sensory or motor neurons within the spinal cord [reviewed in (Perrier et al., 2002)]. In the present study, wind-up was evoked by stimulating the left Tib or SP nerves with 10 electrical pulses (1 or 2 Hz at 2T or 5T). Figure 7 shows 10 stimulations of the left SP nerve at 2 Hz and 5T in one chronic spinal cat before, during, and after back pinch. In the trial shown in Figure 7A, SP nerve stimulation triggered rhythmic activity that persisted following the stimulation. With back pinch, the force produced was smooth and peak force increased with each successive pulse (i.e. wind-up) reaching a plateau around the 7th stimulation (Fig. 7B). Although no rhythmic activity was present during or after stimulation with back pinch it returned in the following trial (Fig. 7C). From these data it is clear that back pinch does not abolish reflex responses evoked by cutaneous nerve stimulation or the wind-up phenomenon.
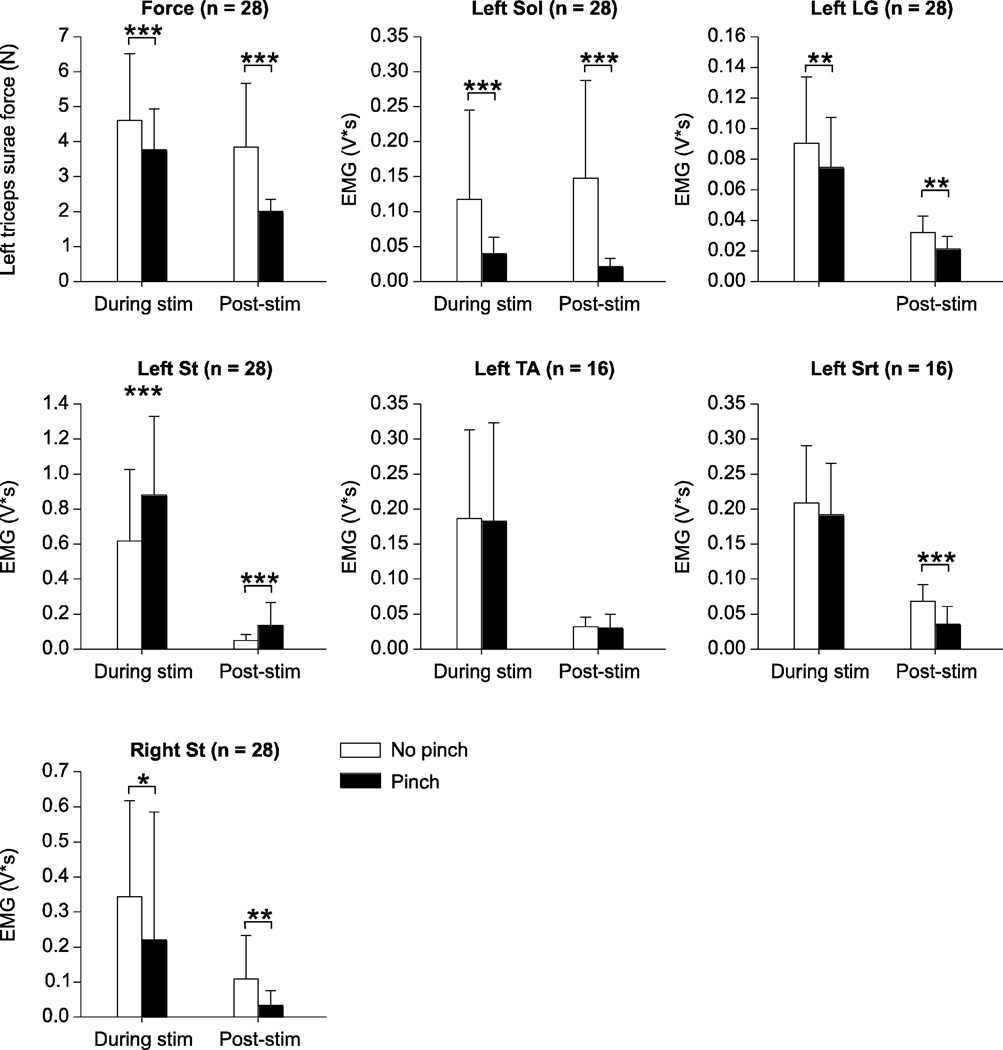
Pooled data show that back pinch reduced triceps surae muscle force and the EMG activity of left Sol, left LG, and right St during 10 stimuli delivered to the left Tib and SP nerves (Fig. 8). On the other hand, activity in the left St was increased by back pinch during stimulation. In the 5 s window following nerve stimulation, back pinch reduced triceps surae muscle force and the EMG activity in left Sol, left LG, left Srt, and right St. Back pinch also increased EMG activity of the left St in the 5 s post-stimulation.
Figure 8. Pooled data showing the effect of back pinch on left triceps surae muscle force and electromyography (EMG) responses evoked during and following a series of 10 pulses delivered to the left tibial (n = 20) or superficial peroneal (n = 8) nerves.
The EMG activities of the left soleus (Sol), lateral gastrocnemius (LG), semitendinosus (St), tibialis anterior (TA), left sartorius (Srt), and of the right St were measured as described in figure 1C and in the methods. The number of paired trials is indicated in brackets at the top for each graph. Asterisks indicate significant differences between trials without and with back pinch using the Wilcoxon signed-rank test for paired data at p ≤ 0.05. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
In summary, back pinch abolished locomotor-like activity and rhythmic bursts that occurred during and following muscle stretch or nerve stimulation. The force generated by the left triceps surae muscles and the EMG activity of most muscles were reduced with back pinch, with the notable exception of the activity of the left St, which was generally increased during and after triceps surae muscle stretch or various nerve stimulations.
Discussion
The present paper extends previous findings that showed that stimulating the lumbosacral skin of the back can abolish locomotor-like activity (Viala et al., 1974; Viala et al., 1978; Nadeau et al., 2010). However, we further show that manually stimulating the dorsal lumbosacral skin did not simply inhibit locomotor-like activity. Instead it filtered the rhythmic activity during reflex testing that occurred during and following muscle stretch or nerve stimulation.
Central pattern generation and clonus
The hypothesis that abnormal function within central pattern-generating circuitry and its regulation by peripheral sensory inputs is responsible for clonus, or other signs of spasticity, has been discussed (Dimitrijevic et al., 1980; Walsh et al., 1987; Rossi et al., 1990; Beres-Jones et al., 2003; Dietz, 2003; Dietz et al., 2007). For instance, Beres-Jones et al. (2003) found that 1) clonus was frequently present following muscle stretch, 2) imposed stretch of ankle plantarflexors could generate clonus throughout the legs, and 3) phasic sensory inputs were not required to trigger clonus. In other studies, Walsh and colleagues (Walsh, 1976; Walsh et al., 1987) found that ankle clonus was not entrained by imposed oscillations of the ankle joint. Clonus can also be induced by cutaneous stimulation (Dimitrijevic et al., 1968; Dimitrijevic et al., 1980; Latash et al., 1989), by attempting volitional movement (Latash et al., 1989), and by nociceptive flexor withdrawal stimuli (Guthrie et al., 1954). These data are not consistent with the hypothesis that clonus results from recurrent activation of stretch reflexes [see (Hidler et al., 1999; Hidler et al., 2000)] and instead point to a central origin for clonus.
In the present study, rhythmic activity consistent with clonus could be evoked during reflex testing. Back pinch was equally effective in abolishing rhythmic activity irrespective of its oscillation frequency and how it was initiated. Therefore, the rhythmic activity that occurred spontaneously or that was evoked during reflex testing is most likely produced by common CPGs. However, because the present work was done in an animal model, we cannot conclusively state that clonus-like activity is the same as the clonus observed clinically in spinal cord-injured humans.
Regulation of “flexor” activities
During normal locomotion, swing onset is marked by a brief burst in St followed shortly thereafter by activation of TA (Rossignol, 1996). During spontaneous fictive locomotion, electroneurograms reveal that St and TA are activated during the flexion phase and alternate with extensors [see (Frigon et al., 2010b)], suggesting that they normally receive a “flexor-related” locomotor command from central pattern-generating circuitry. In the present study, TA could be co-active with extensors during rhythmic activity, whereas St always remained in alternation with extensors (e.g. Fig. 2) in chronic spinal cats treated with clonidine.
The spinal transection itself could account for changes in TA activation with respect to extensors. For instance, SCI alters reciprocal connections between TA and ankle extensors. Indeed, reciprocal inhibition between TA and ankle extensors is reduced after SCI and reciprocal facilitation can appear (Boorman et al., 1996; Crone et al., 2003; Xia et al., 2005). The mechanisms involved in this functional reorganization are unclear but appear to involve changes in Cl- homeostasis by the potassium-chloride co-transporter KCC2, which is down-regulated following SCI (Boulenguez et al., 2010; Viemari et al., 2011).
Neuromodulation of central pattern-generating circuitry can also influence activation patterns across hindlimb muscles [see (Harris-Warrick, 2011; Frigon, 2012) for recent reviews]. The synchronous activation of extensors, such as LG and Sol, with the ankle flexor TA could be mediated by clonidine. Indeed, synchronized bursts of flexors and extensors were shown to appear with high doses of clonidine (4–8 mg/kg) in curarized cats acutely spinalized at C1 (Zangger, 1981). However, in that study, St, TA and LG discharged synchronously whereas in the present study extensors remained in alternation with St. It is unclear if higher doses of clonidine would have produced a similar effect or if the level of spinalization is a factor in a more widespread synchronization.
Inhibitory process
In previous studies in decerebrate curarized rabbits with intact spinal cords, pinching or clipping the dorsal lumbar skin was the most effective for inhibition while some sites, such as the face and plantar surface of the paws had an excitatory effect on locomotor-like discharges (Viala et al., 1974). What is the basis for this inhibitory circuit from cutaneous receptors of the lumbosacral back to CPGs controlling the hindlimbs? A variety of vertebrate species have what are termed “hypnotic responses” to external stimuli (Gallup, Jr., 1974). Animal hypnosis is defined as a behavioural state where the animal is immobilised and desensitised to external stimuli (Fleischmann et al., 1988; Pozza et al., 2008). Although this can be achieved in several ways, in mammals, immobilisation can be induced by pinching or clipping the skin at certain sites, such as the neck (Pozza et al., 2008). In mammals, this behaviour facilitates the carrying of a newborn by its mother. Moreover, when a rabbit is turned on its back, the animal becomes immobile after the lumbar skin touches a rigid surface, remaining motionless for several minutes (Viala et al., 1978). In certain mammals, an inhibitory pathway from the skin of the back to CPGs could be a means to facilitate mating behaviour [see (Van der Horst et al., 1998)]. This circuit appears to have been conserved in humans (Nadeau et al., 2010), although its purpose other than an evolutionary remnant is unclear.
The inhibitory circuit is evidently contained within the spinal cord. The fact that pinching the back abolished spontaneous locomotor-like activity and filtered rhythmic activity during reflex testing in spinal transected cats indicates a more or less direct action of cutaneous inputs on spinal CPGs. Mapping cutaneous projections from the pinched sites from the dorso-lumbosacral skin to spinal neurons involved in rhythm-generation, presumably at L3-L4 in the cat (Marcoux et al., 2000; Delivet-Mongrain et al., 2008), is needed to identify the connectivity of this circuitry. It is also possible that the effect of back pinch is magnified following spinal lesions due to an expansion of receptive fields (Schouenborg et al., 1992; Andersen et al., 2004). Pinching the skin reset the locomotor-like rhythm (Fig. 2), suggesting that neurons receiving direct inputs from cutaneous afferents of the back could be part of the spinal locomotor CPGs (Conway et al., 1987; Guertin et al., 1995; Hultborn et al., 1998; Pearson et al., 1998; Schomburg et al., 1998; Rybak et al., 2006; Frigon et al., 2010a; Frigon et al., 2010b).
Afferents activated by back pinch
Although our experimental paradigm did not allow distinguishing the afferents mediating the back pinch effect, Viala and colleagues (1978) showed that stimulating Aα, Aβ, or C fibres from the dorsal lumbosacral skin had an excitatory effect on fictive locomotor-like discharges, whereas activation of Aδ fibres produced inhibition. It is likely that the inhibitory influence on rhythmic activity observed in the present study is mediated by Aδ afferents from the lumbosacral skin. A more systematic investigation of the afferents from the lumbosacral skin involved in modulating rhythmic and reflex activity in chronic spinal cats is however required.
Functional and clinical considerations
Nadeau et al. 2010 found that pinching the skin of the back stopped rhythmic synchronized discharges in a motor complete spinal cord-injured human, suggesting that the circuitry presently reported in decerebrate chronic spinal cats and in decerebrate curarized rabbits (Viala et al., 1974; Viala et al., 1978) is conserved in humans. However, further studies in human subjects are required to replicate the results of Nadeau and colleagues.
Manually pinching the skin to reduce signs of spasticity is similar in principle to electrically stimulating the skin with surface electrodes, also called transcutaneous electrical nerve stimulation (TENS). It is thought that TENS activates sensory afferents that modulate spinal inhibitory circuits (Crone et al., 1994; Perez et al., 2003; Ping Ho et al., 2010) but precise underlying mechanisms remain largely unclear. TENS applied to the legs can effectively manage signs of lower limb spasticity in spinal cord-injured patients with few side effects (Goulet et al., 1996; Aydin et al., 2005; Ping Ho et al., 2010). Whether TENS applied over several days or weeks can induce long-term neuroplastic changes within the spinal cord remains to be determined.
At present the lack of research on TENS, particularly its underlying mechanisms and the circuitry involved at the spinal level, limits its use and effectiveness to treat spasticity in spinal cord-injured subjects. The results presented herein are a first step in understanding the spinal circuitry that inhibits or filters rhythmic motor activity in a well-defined SCI animal model and could lead to more effective TENS to manage spasticity in human patients. TENS applied over the lumbosacral regions could have more widespread effect on spasticity than focalized stimulation applied over the legs.
Conclusions
In summary, manually stimulating the dorsal lumbosacral skin abolished rhythmic bursts that occurred spontaneously or were evoked during reflex testing in chronic spinalized cats treated with clonidine. This suggests that cutaneous inputs from the back have access to spinal CPGs. Importantly, cutaneous inputs from the back did not abolish reflex responses. Such stimulation could be useful in treating clonus in spinal cord-injured humans. The 1-month chronic spinalized cat treated with clonidine appears to be an ideal model to study the genesis, regulation, and underlying mechanisms of rhythmic activity that resembles clonus.
Highlights.
Pinching the skin of chronic spinal cats abolished locomotor-like activity
Pinching the skin abolished rhythmic activity during reflex testing
Locomotion and clonus could be produced by shared pattern generators
Acknowledgments
The authors would like to thank Marin Manuel and Jack Miller for technical assistance. The present research was funded by an individual grant from the Wings for Life Foundation and by a postdoctoral fellowship from the Canadian Institutes of Health Research to A. Frigon, as well as by an NIH grant (NS034382) to C.J. Heckman.
Abbreviations
- CPGs
central pattern generators
- EMG
electromyography
- LG
lateral gastrocnemius
- SCI
spinal cord injury
- SD
standard deviation
- Sol
soleus
- SP
superficial peroneal
- St
semitendinosus
- T
motor threshold
- TA
tibialis anterior
- Tib
tibial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alain Frigon, Email: alain.frigon@usherbrooke.ca.
Yann Thibaudier, Email: yann.thibaudier@usherbrooke.ca.
Michael D. Johnson, Email: m-johnson16@northwestern.edu.
C.J. Heckman, Email: c-heckman@northwestern.edu.
Marie-France Hurteau, Email: marie-france.hurteau@usherbrooke.ca.
References
- Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–586. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- Andersen OK, Finnerup NB, Spaich EG, Jensen TS, Arendt-Nielsen L. Expansion of nociceptive withdrawal reflex receptive fields in spinal cord injured humans. Clin. Neurophysiol. 2004;115:2798–2810. doi: 10.1016/j.clinph.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Aydin G, Tomruk S, Keles I, Demir SO, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am. J. Phys. Med. Rehabil. 2005;84:584–592. doi: 10.1097/01.phm.0000171173.86312.69. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Julien C, Rossignol S. The effects of clonidine and yohimbine on locomotion and cutaneous reflexes in the adult chronic spinal cat. Brain Res. 1987;437:83–96. doi: 10.1016/0006-8993(87)91529-0. [DOI] [PubMed] [Google Scholar]
- Beres-Jones JA, Johnson TD, Harkema SJ. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp. Brain Res. 2003;149:222–236. doi: 10.1007/s00221-002-1349-5. [DOI] [PubMed] [Google Scholar]
- Boorman GI, Lee RG, Becker WJ, Windhorst UR. Impaired "natural reciprocal inhibition" in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr. Clin. Neurophysiol. 1996;101:84–92. doi: 10.1016/0924-980x(95)00262-j. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Marsden CD. Propriospinal myoclonus: evidence for spinal "pattern" generators in humans. Mov Disord. 1994;9:571–576. doi: 10.1002/mds.870090511. [DOI] [PubMed] [Google Scholar]
- Bussel B, Roby-Brami A, Azouvi P, Biraben A, Yakovleff A, Held JP. Myoclonus in a patient with spinal cord transection. Possible involvement of the spinal stepping generator. Brain. 1988;111(Pt 5):1235–1245. doi: 10.1093/brain/111.5.1235. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Effects of intrathecal alpha1- and alpha2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J. Neurophysiol. 1998;79:2941–2963. doi: 10.1152/jn.1998.79.6.2941. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp. Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Cook WA., Jr Antagonistic muscles in the production of clonus in man. Neurology. 1967;17:779–781. 796. doi: 10.1212/wnl.17.8.779. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J, Petersen N, Ballegaard M, Hultborn H. Disynaptic reciprocal inhibition of ankle extensors in spastic patients. Brain. 1994;117(Pt 5):1161–1168. doi: 10.1093/brain/117.5.1161. [DOI] [PubMed] [Google Scholar]
- Delivet-Mongrain H, Leblond H, Rossignol S. Effects of localized intraspinal injections of a noradrenergic blocker on locomotion of high decerebrate cats. J. Neurophysiol. 2008;100:907–921. doi: 10.1152/jn.90454.2008. [DOI] [PubMed] [Google Scholar]
- Dietz V. Spastic movement disorder. Spinal Cord. 2000;38:389–393. doi: 10.1038/sj.sc.3101030. [DOI] [PubMed] [Google Scholar]
- Dietz V. Spinal cord pattern generators for locomotion. Clin. Neurophysiol. 2003;114:1379–1389. doi: 10.1016/s1388-2457(03)00120-2. [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6:725–733. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Nathan PW. Studies of spasticity in man. 3. Analysis of revlex activity evoked by noxious cutaneous stimulation. Brain. 1968;91:349–368. doi: 10.1093/brain/91.2.349. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Nathan PW, Sherwood AM. Clonus: the role of central mechanisms. J. Neurol. Neurosurg. Psychiatry. 1980;43:321–332. doi: 10.1136/jnnp.43.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A, Urca G. Clip-induced analgesia and immobility in the mouse: pharmacological characterization. Neuropharmacology. 1988;27:641–648. doi: 10.1016/0028-3908(88)90187-6. [DOI] [PubMed] [Google Scholar]
- Frigon A. Chapter 7--interindividual variability and its implications for locomotor adaptation following peripheral nerve and/or spinal cord injury. Prog. Brain Res. 2011;188:101–118. doi: 10.1016/B978-0-444-53825-3.00012-7. [DOI] [PubMed] [Google Scholar]
- Frigon A. Central Pattern Generators of the Mammalian Spinal Cord. Neuroscientist. 2012;18:56–69. doi: 10.1177/1073858410396101. [DOI] [PubMed] [Google Scholar]
- Frigon A, Gossard JP. Evidence for specialized rhythm-generating mechanisms in the adult mammalian spinal cord. J. Neurosci. 2010a;30:7061–7071. doi: 10.1523/JNEUROSCI.0450-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Johnson MD, Heckman CJ. Altered activation patterns by triceps surae stretch reflex pathways in acute and chronic spinal cord injury. J. Neurophysiol. 2011;106:1669–1678. doi: 10.1152/jn.00504.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Johnson MD, Heckman CJ. Differential modulation of crossed and uncrossed reflex pathways by clonidine in adult cats following complete spinal cord injury. J. Physiol. 2012 doi: 10.1113/jphysiol.2011.222208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog. Brain Res. 2006;157:231–260. doi: 10.1016/s0079-6123(06)57016-5. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Adaptive changes of the locomotor pattern and cutaneous reflexes during locomotion studied in the same cats before and after spinalization. J. Physiol. 2008;586:2927–2945. doi: 10.1113/jphysiol.2008.152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Sirois J, Gossard JP. Effects of ankle and hip muscle afferent inputs on rhythm generation during fictive locomotion. J. Neurophysiol. 2010b;103:1591–1605. doi: 10.1152/jn.01028.2009. [DOI] [PubMed] [Google Scholar]
- Fung J, Barbeau H. A dynamic EMG profile index to quantify muscular activation disorder in spastic paretic gait. Electroencephalogr. Clin. Neurophysiol. 1989;73:233–244. doi: 10.1016/0013-4694(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Fung J, Stewart JE, Barbeau H. The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal cord injured subjects. J. Neurol. Sci. 1990;100:85–93. doi: 10.1016/0022-510x(90)90017-h. [DOI] [PubMed] [Google Scholar]
- Gallup GG., Jr Animal hypnosis: factual status of a fictional concept. Psychol. Bull. 1974;81:836–853. doi: 10.1037/h0037227. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Sirois J, Noue P, Cote MP, Menard A, Leblond H, Frigon A. Chapter 2--the spinal generation of phases and cycle duration. Prog. Brain Res. 2011;188:15–29. doi: 10.1016/B978-0-444-53825-3.00007-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Agarwal GC. Physiological clonus in man. Exp. Neurol. 1977;54:616–621. doi: 10.1016/0014-4886(77)90260-6. [DOI] [PubMed] [Google Scholar]
- Goulet C, Arsenault AB, Bourbonnais D, Laramee MT, Lepage Y. Effects of transcutaneous electrical nerve stimulation on H-reflex and spinal spasticity. Scand. J. Rehabil. Med. 1996;28:169–176. [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J. Physiol. 1995;487(Pt 1):197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie TC, Krout RM, Shires EB. A study of hyper-reflexia, clonus, and involuntary reflex movement using a multichannel electromyograph. Trans. Am. Neurol. Assoc. 1954;13:194–196. [PubMed] [Google Scholar]
- Harris-Warrick RM. Neuromodulation and flexibility in Central Pattern Generator networks. Curr. Opin. Neurobiol. 2011;21:685–692. doi: 10.1016/j.conb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidler JM, Rymer WZ. A simulation study of reflex instability in spasticity: origins of clonus. IEEE Trans. Rehabil. Eng. 1999;7:327–340. doi: 10.1109/86.788469. [DOI] [PubMed] [Google Scholar]
- Hidler JM, Rymer WZ. Limit cycle behavior in spasticity: analysis and evaluation. IEEE Trans. Biomed. Eng. 2000;47:1565–1575. doi: 10.1109/10.887937. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard JP, Brownstone R, Fedirchuk B, Schomburg ED, Enriquez-Denton M, Perreault MC. How do we approach the locomotor network in the mammalian spinal cord? Ann. N. Y. Acad. Sci. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- Kohout RK, Saunders LL, Krause JS. The relationship between prescription medication use and ability to ambulate distances after spinal cord injury. Arch. Phys. Med. Rehabil. 2011;92:1246–1249. doi: 10.1016/j.apmr.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WW, editors. Spasticity: Disordered Motor Control. Symposia Specialists. Miami. Chicago: Year Book Medical Publishers; 1980. pp. 485–500. [Google Scholar]
- Latash ML, Penn RD, Corcos DM, Gottlieb GL. Short-term effects of intrathecal baclofen in spasticity. Exp. Neurol. 1989;103:165–172. doi: 10.1016/0014-4886(89)90078-2. [DOI] [PubMed] [Google Scholar]
- Marcoux J, Rossignol S. Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. J. Neurosci. 2000;20:8577–8585. doi: 10.1523/JNEUROSCI.20-22-08577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Orlovsky GN, Deliagina TG. Facilitation of postural limb reflexes with epidural stimulation in spinal rabbits. J. Neurophysiol. 2010;103:1080–1092. doi: 10.1152/jn.00575.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Jacquemin G, Fournier C, Lamarre Y, Rossignol S. Spontaneous motor rhythms of the back and legs in a patient with a complete spinal cord transection. Neurorehabil. Neural Repair. 2010;24:377–383. doi: 10.1177/1545968309349945. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity--from a basic science point of view. Acta Physiol (Oxf) 2007;189:171–180. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Ann. N. Y. Acad. Sci. 1998;860:203–215. doi: 10.1111/j.1749-6632.1998.tb09050.x. [DOI] [PubMed] [Google Scholar]
- Perez MA, Field-Fote EC, Floeter MK. Patterned sensory stimulation induces plasticity in reciprocal ia inhibition in humans. J. Neurosci. 2003;23:2014–2018. doi: 10.1523/JNEUROSCI.23-06-02014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res. Brain Res. Rev. 2002;40:223–229. doi: 10.1016/s0165-0173(02)00204-7. [DOI] [PubMed] [Google Scholar]
- Ping Ho CB, Kam Kwan CB. Immediate effect of transcutaneous electrical nerve stimulation on spasticity in patients with spinal cord injury. Clin. Rehabil. 2010;24:202–210. doi: 10.1177/0269215509343235. [DOI] [PubMed] [Google Scholar]
- Pozza ME, Stella JL, Chappuis-Gagnon AC, Wagner SO, Buffington CA. Pinch-induced behavioral inhibition ('clipnosis') in domestic cats. J. Feline. Med. Surg. 2008;10:82–87. doi: 10.1016/j.jfms.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack PM, Ross HF, Thilmann AF. The ankle stretch reflexes in normal and spastic subjects. The response to sinusoidal movement. Brain. 1984;107(Pt 2):637–654. doi: 10.1093/brain/107.2.637. [DOI] [PubMed] [Google Scholar]
- Rossi A, Mazzocchio R, Scarpini C. Clonus in man: a rhythmic oscillation maintained by a reflex mechanism. Electroencephalogr. Clin. Neurophysiol. 1990;75:56–63. doi: 10.1016/0013-4694(90)90152-a. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology, Section 12. Exercise: Regulation and Integration of Multiple Systems. Oxford: American Physiological Society; 1996. pp. 173–216. [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu. Rev. Neurosci. 2011;34:413–440. doi: 10.1146/annurev-neuro-061010-113746. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Stecina K, Shevtsova NA, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. J. Physiol. 2006;577:641–658. doi: 10.1113/jphysiol.2006.118711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp. Brain Res. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Schouenborg J, Holmberg H, Weng HR. Functional organization of the nociceptive withdrawal reflexes. II. Changes of excitability and receptive fields after spinalization in the rat. Exp. Brain Res. 1992;90:469–478. doi: 10.1007/BF00230929. [DOI] [PubMed] [Google Scholar]
- Silverman J, Garnett NL, Giszter SF, Heckman CJ, Kulpa-Eddy JA, Lemay MA, Perry CK, Pinter M. Decerebrate mammalian preparations: unalleviated or fully alleviated pain? A review and opinion. Contemp. Top. Lab Anim Sci. 2005;44:34–36. [PubMed] [Google Scholar]
- Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch. Phys. Med. Rehabil. 1999;80:1548–1557. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- Van der Horst VG, Holstege G. Sensory and motor components of reproductive behavior: pathways and plasticity. Behav. Brain Res. 1998;92:157–167. doi: 10.1016/s0166-4328(97)00188-5. [DOI] [PubMed] [Google Scholar]
- Vetrugno R, Provini F, Plazzi G, Valentino ML, Liguori R, Lugaresi E, Montagna P. Focal myoclonus and propriospinal propagation. Clin. Neurophysiol. 2000;111:2175–2179. doi: 10.1016/s1388-2457(00)00471-5. [DOI] [PubMed] [Google Scholar]
- Viala G, Buser P. [Inhibition of spinal locomotor activity by a special method of somatic stimulation in rabbits] Exp. Brain Res. 1974;21:275–284. doi: 10.1007/BF00235747. [DOI] [PubMed] [Google Scholar]
- Viala G, Orsal D, Buser P. Cutaneous fiber groups involved in the inhibition of fictive locomotion in the rabbit. Exp. Brain Res. 1978;33:257–267. doi: 10.1007/BF00238064. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Bos R, Boulenguez P, Brocard C, Brocard F, Bras H, Coulon P, Liabeuf S, Pearlstein E, Sadlaoud K, Stil A, Tazerart S, Vinay L. Chapter 1--importance of chloride homeostasis in the operation of rhythmic motor networks. Prog. Brain Res. 2011;188:3–14. doi: 10.1016/B978-0-444-53825-3.00006-1. [DOI] [PubMed] [Google Scholar]
- Walsh EG. Clonus: beats provoked by the application of a rhythmic force. J. Neurol. Neurosurg. Psychiatry. 1976;39:266–274. doi: 10.1136/jnnp.39.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EG, Wright GW. Patellar clonus: an autonomous central generator. J. Neurol. Neurosurg. Psychiatry. 1987;50:1225–1227. doi: 10.1136/jnnp.50.9.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R, Rymer WZ. Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord. 2005;43:14–21. doi: 10.1038/sj.sc.3101656. [DOI] [PubMed] [Google Scholar]
- Zangger P. The effect of 4-aminopyridine on the spinal locomotor rhythm induced by L-DOPA. Brain Res. 1981;215:211–223. doi: 10.1016/0006-8993(81)90503-5. [DOI] [PubMed] [Google Scholar]