Abstract
PD-1, a member of the CD28 family of immune regulatory molecules, is expressed on activated T cells, interacts with its ligands, PD-L1/B7-H1 and PD-L2/B7-DC on other cells, and delivers inhibitory signals to the T cell. Here we study the role of this pathway in modulating auto-reactive T cell responses in two models of myocarditis. In a CD8+ T cell-mediated adoptive transfer model, we find that compared to Pd1+/+ CD8+ T cells, Pd1−/−CD8+ T cells cause enhanced disease, with increased inflammatory infiltrate, particularly rich in neutrophils. Additionally we show enhanced proliferation in vivo and enhanced cytotoxic activity of PD-1 deficient T lymphocytes against myocardial endothelial cells in vitro. In experimental autoimmune myocarditis, a disease model dependent on CD4+ T cells, we show that mice lacking PD-1 develop enhanced disease compared to wild type mice. PD-1 deficient mice displayed increased inflammation, enhanced serum markers of myocardial damage, and an increased infiltration of inflammatory cells, including CD8+ T cells. Together these studies show that PD-1 plays an important role in limiting T cell responses in the heart.
Introduction
Inflammation in the heart, mediated at least in part by T cells, can result from a variety of infections, including viral, bacterial and fungal, as well as from environmental toxins, drug reactions, and autoimmune disorders. Many cases of myocarditis are idiopathic. Acutely, T cell-mediated myocarditis can cause arrhythmias and heart failure, and chronically may lead to dilated cardiomyopathy. Understanding mechanisms of regulation of T cell responses in the heart is important for developing therapeutic strategies for myocarditis, but these mechanisms are not yet clear.
Various mouse models of T cell-dependent myocarditis exist, and much work has been done in these models to identify important immunologically relevant mediators and regulators of disease. In experimental autoimmune myocarditis (EAM), which is initiated by immunization with cardiac myosin (1), CD4+ T cells are required for disease induction (2), although the relative roles of IL-17 -expressing Th17 cells vs. IFNγ-producing Th1 cells is unresolved (3, 4). CD8+ T cells contribute to the inflammatory infiltrate and severity of EAM, but are not essential for disease induction (5). In coxsackievirus B3-induced myocarditis in mice, CD4+ and CD8+ αβ TCR T cells both appear to play a role in disease, but the interpretation of the effects of experimental deletion of either subset is complicated by the roles of the T cells in viral clearance (6, 7). γδ T cells also contribute to coxsackievirus B3-induced myocarditis (8). Therefore, viral myocarditis may not be the preferred model for studying T cell subset specific mechanisms of regulation of myocarditis. In the cMy-mOva model of myocarditis developed in our laboratory, severe cardiac inflammation and damage are induced exclusively by transferred CD8+ effector T cells specific for a transgenically expressed model antigen in cardiac myocytes (9, 10), providing a unique experimental model in which to asses the contribution of CD8+ T cells to cardiac inflammation, without their confounding role in viral clearance.
PD-1 is a co-inhibitory member of the B7/CD28 superfamily of molecules, and together with its binding partners, PD-L1 and PD-L2, represents an important negative regulator of T cell responses to self and microbial antigens. PD-1 expression is induced by physiologic activation on T cells, B cells and macrophages. PD-1 binds PD-L1, which is broadly expressed on both hematopoietic and non-hematopoietic cells, as well as PD-L2, whose expression is limited to macrophages and dendritic cells (11–13). This pathway has been shown to regulate T cell responses and inflammation in various disease settings, including atherosclerosis (14, 15), allograft vascular disease (16, 17), encephalomyelitis (18), and sepsis (19). PD-1 is described as a mediator of CD8+ T cell exhaustion in the setting of chronic viral infection and cancer (20–22), and blocking anti-PD-1 mAb treatment is in clinical trials for cancer (23).
Despite a significant body of work devoted to the pathogenesis of myocarditis in various models, relatively little work has been done on the roles of the B7/CD28 family of molecules that likely regulate the activation of pathogenic T cell responses in these models (24, 25). One group has previously studied the effects of blocking PD-1 and PD-L1 in coxsackievirus-induced myocarditis in mice (24). Additionally PD-L1 or PD-1 deficiency has been implicated in causing spontaneous myocarditis in MRL mice (26, 27). The PD-1 pathway has also been demonstrated to be important in graft arterial disease in cardiac allografts (28). Finally, it has been reported that PD-1 deficiency in mice on the BALB/c background experience spontaneous dilated cardiomyopathy, caused by antibodies to cardiac troponin (29, 30). None of these studies specifically addresses if PD-1 on T cells regulates the response of potentially pathogenic T cells. A previous study from our laboratory, utilizing the cMy-mOva model of CD8+ mediated myocarditis, found a role for PD-L1 on non-hematopoietic cells in dampening T cell responses and secondary neutrophil inflammation (31).
In the study reported here, we assessed the role of PD-1 in regulating cardiac inflammation and damage in two T cell dependent models of myocarditis. Using a modification of our CD8+ T cell-dependent model, we transferred naïve ovalbumin (Ova)-specific CD8+ T cells into cMy-mOva mice, that express a myocyte restricted membrane form of ovalbumin, and then immunize the recipients with ovalumbin, we found increased severity of disease in the absence of PD-1 on CD8+ T cells. Second, we addressed the role of PD-1 (and PD-L1) in EAM, which is mediated largely by CD4+ T cells independent of CD8+ T cells, and again found an augmented disease severity in the absence of PD-1. Together our data indicate an important role for PD-1 in protecting the heart from T cell-mediated damage, which is especially relevant given the interest in PD-1 as a clinical target in therapies for cancer and chronic viral infections.
Materials & Methods
Animal Studies
The OT-I TCR transgenic mouse strain (32) was provided by W. R. Heath and F. Carbone (Walter and Eliza Hall Institute of Medical Research, Melbourne). The OT-I TCR is expressed on CD8+ T cells and is specific for the ovalbumin peptide p.257–264 (SIINFEKL) bound to the class I MHC molecule H2-Kb (32). CMy-mOva transgenic mice on a C57BL/6 background, previously developed in our laboratory (9), express membrane-bound ovalbumin (mOva) exclusively on cardiac myocytes. Pd1−/− mice on a C57BL/6 background, were derived by targeted mutation in C57BL/6 ES cells, which results in deletion of the IgV domain as described (33), and were crossbred with OT-1 TCR transgenic Thy1.1+ mice to generate Pd1−/− OT-1+ Thy1.1+ mice. WT BALB/c mice were purchased from Charles River. The generation of Pdl1−/− and Pd1−/− mice on the BALB/c background has been previously described (34, 35). All mice were given food and water ad libitum and were maintained on a 12/12-hour light/dark cycle under pathogen-free conditions in the Harvard new research building animal facility according to institutional and National Institutes of Health guidelines.
Myocarditis induction in cMy-mOva mice
Male and female C57BL/6 cMy-mOVA transgenic mice between 8 and 20 weeks of age were used as recipients. CD8+ T cells were isolated from spleens of Pd1−/− or Pd1+/+ OT-1+ Thy1.1 mice by magnetic bead separation (Miltenyi), and were adoptively transferred i.v. into recipients at 500,000 cells per mouse. In some experiments naïve CD8 cells were stained with CFSE (Invitrogen). Mice were immunized 24 hours later with a 1:1 emulsion of Complete Freund’s Adjuvant (Sigma) and whole ovalbumin in DPBS, delivering 1mg whole ovalbumin per mouse. Mice were sacrificed 7 days following the immunization for tissue analyses, and 72 hours following immunization for CFSE T cell proliferation analysis.
Experimental autoimmune myocarditis
BALB/C WT mice, and Pd1−/− and Pdl1−/− mice on the BALB/C background between 8 and 20 weeks of age were immunized with a peptide derived from murine a-myosin heavy chain, Myhc-α614–634 – Ac-SLKLMATLFSTYASAD-OH, (Anaspec), as described (36). The peptide was diluted in DPBS, 1mg/mL, and emulsified 1:1 with Complete Freund’s Adjuvant. 100μg of the peptide was injected in 200μL total volume of the emulsion, subcutaneously in the flank, on day 0 and day 7. Mice were sacrificed at day 21.
CTL Killing Assay
Mouse heart endothelial cells (MHEC) were prepared from juvenile mouse hearts by Collagenase I digestion (Worthington), followed by sequential magnetic bead sorting (Dynal), using beads coated with antibodies to CD31 and CD102 (BD Pharmingen). WT OT-1+ and Pd1−/− OT-1+ T cell cultures were prepared by isolating CD8+ cells by magnetic beads (Miltenyi), and culturing with mitomycin-c (Sigma) treated splenic APCs for 5 days in the presence of 666ng/mL SIINFEKL peptide, 50U/mL IL-2 (R&D), 10ng/mL IL-12 (R&D), and 2μg/mL anti-CD28 (BioExpress). T cells were rested in fresh media for 24 hours before being co-cultured with MHEC. MHEC were plated on fibronectin-coated 12 well plates and grown to confluence. Monolayers were pretreated with IFNγ, (Peprotech), and 300ng SIINFEKL peptide for 2 hours, washed twice with DPBS, and then incubated with activated, rested CD8+ effector cells from either WT or Pd1−/− OT-1+ for one hour. Plates were then washed twice in DPBS, and detached from the plate using Trypsin-Versene (Lonza). Cells were surface stained using CD90.1-APC (Biolegend), in order to identify and exclude T cells from the analysis. Cells were washed twice more in DPBS and then stained with AnnexinV-PE and 7-AAD in Annexin binding buffer, and analyzed by flow cytometry.
Cytokine measurements
ELISAs to detect IFNγ and granzyme B in culture supernatants were performed using kits from Biolegend and eBioscience. Sera and culture supernatants were analyzed for cytokine concentrations using Luminex bead-based multiplex assays.
Immunohistochemistry
Frozen heart sections were stained with antibodies specific for CD4, CD8, F4/80 for macrophages, and GR1 for neutrophils, as described. (37)
Flow Cytometry
Whole hearts were digested in a bicarbonate-based buffer with 0.895 mg/mL collagenase I (Sigma), and 0.5 mg/mL Elastase XIV (Sigma) as described (3). Whole heart digestions, spleens and cardiac draining lymph nodes were made into single cell suspensions, and filtered through 0.22 micron cell strainers (BD). Cells were fixed in 1% paraformaldehyde prior to staining. Antibodies for CD4, CD8, IFNγ, IL-17A, Ly-6G, CD11b were purchased from Biolegend. Antibodies were diluted 1:100 for staining. In some staining cells were permeabilized using wash/perm buffer (BD).
qRT-PCR of heart tissue
RNA isolation and qRT-PCR analyses was performed as described (9,31). Briefly, the apex of the heart was removed following perfusion and snap frozen. RNA was prepared from whole heart tissue using the RNeasy Kit and DNASE I (Qiagen). cDNA was prepared using ThermoScript RT-PCR cDNA Synthesis Kit (Invitrogen). RT-PCR was performed using Applied Biosystems. The primer sequences we uses are: Actb F: 5′-TCC TTC GTT GCC GGT CCA CCA-3′, Actb R: 5′-ACC AGC GCA GCG ATA TCG TC TC-3′; Ifng F: 5′-AAC GCT ACA CAC TGC ATC TTG G G-3′, Ifng R: 5′-GCC GTG GCA GTA ACA GCC GCC-3′; Tnfa F: 5′-GCA CAG AAA GCA TGA CCC G G-3′, Tnfa R: 5′-GCC CCC CAT CTT TTG GG GG-3′; Ccl3 F: 5′-CCA AGT CTT CTC AGC GCC AT-3′, Ccl3 R: 5′-TCC GGC TGT AGG AGA AGC AG-3′; Ccl5 F: 5′-CAA GTG CTC CAA TCT TGC AGT C-3′, Ccl5 R: 5′-TTC TCT GGG TTG GCA CAC AC-3; Cxcl10 F: 5′-GCC GTC ATT TTC TGC CTC A-3′, Cxcl10 R: 5′-CGT CCT TGC GAG AGG GAT C-3′; Rorc F: 5′-CCGCTGAGAGGGCTTCAC-3′, Rorc R: 5′-TGCAGGAGTAGGCCACATTACA-3′; Il17a F: 5′-GCT CCA GAA GGC CCT CAG A-3′, Il17a R: 5′-AGC TTT CCC TCC GCA TTG A-3′; Nos2 F: 5′-GGA GTG ACG GCA AAC ATG ACT-3′, Nos2 R: 5′-TAG CCA GCG TAC CGG ATG A-3′.
Serum troponis determinations
Blood was collected from mice at time of sacrifice; serum levels of cardiac troponin-I (cTnI) were measured by a clinical quantitative immunoassay technique(TnI-Ultra, Siemens).
Statistics
All statistical analyses were performed using Prism software. Differences between 2 groups of mice were analyzed by Student t test and expressed as mean±SEM or by the Mann-Whitney test (for nonparametric data). For experiments with 3 or more groups, ANOVA with the Bonferonni multiple comparison post test was used. A value of P<0.05 was considered significant.
Results
PD-1 deficiency increases CD8+ T cell mediated cardiac inflammation and damage
To investigate the role of PD-1 signaling in myocarditis we first used the cMy-mOVA mouse model of myocarditis, where transgenic ovalbumin is constitutively expressed as membrane bound protein by cardiac myocytes, under the control of the α-myosin heavy chain promoter (9). Naïve CD8+ T cells were isolated from Pd1−/− or Pd1+/+ OT-I mice, and transferred to cMy-mOVA mice intravenously. Recipient mice were immunized 24 hours later with adjuvant and whole ovalbumin, and were sacrificed 7 days later. Histological analysis revealed significantly more myocardial inflammation in recipients receiving PD-1 deficient T cells (Fig. 1A), as well as increased levels of circulating cardiac troponin I (Fig 1B), a clinical marker of myocyte damage. We have previously established and reconfirmed in controls in this study that cMy-mOVa mice are tolerant to ovalbumin, and no disease develops spontaneously without OT-1 transfer (9).
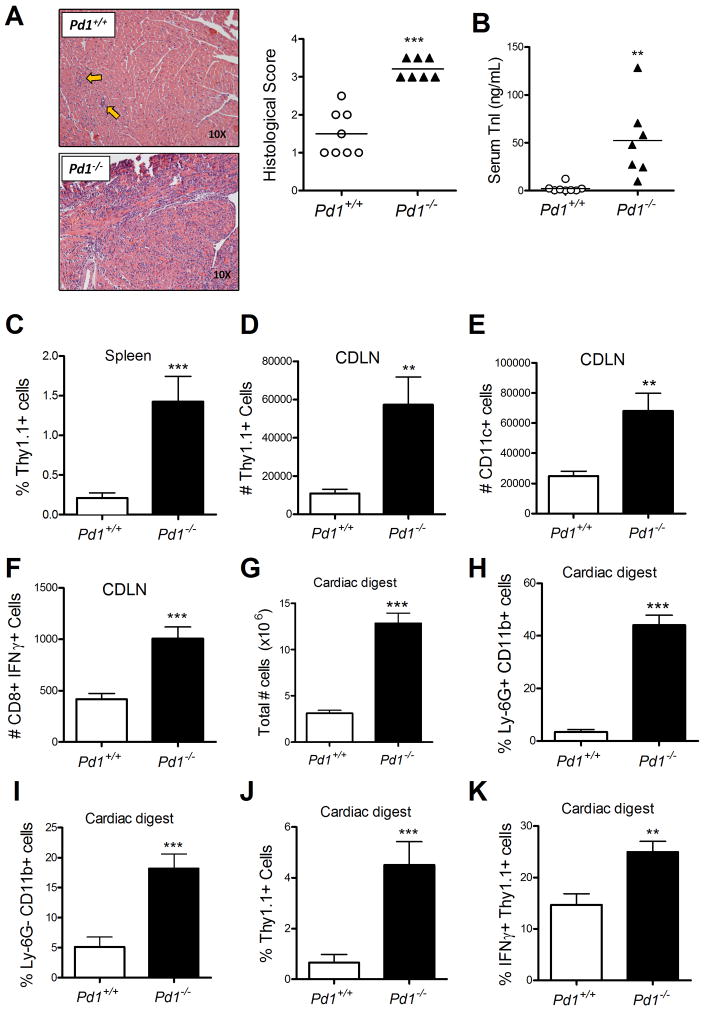
Figure 1. PD-1 controls pathologic CD8+ T cell responses in the heart.
Naive WT or PD-1 deficient CD8+ OT-1+ T cells were adoptively transferred into cMy-mOVA mice, immunized 24 hours later with CFA/Ovalbumin, and sacrificed 7 days later. Whole heart H&E sections were prepared and scored for myocarditis (A), and circulating troponin was measured in the serum (B). Flow cytometric analysis was performed for identification of transferred cells in the spleen (C), and cardiac draining lymph node (D), as well as for identification of dendritic cells (E), and IFNγ-producing cells (F), in draining lymph node. Enzymatic digestion of whole hearts was performed and total cell number (G), infiltrating neutrophils (H), infiltrating monocytes (I), infiltrating transferred OT-1 cells (J), and IFNγ-producing transferred cells (K), were enumerated by flow cytometry. In A and B data are pooled form three experiments, each one with its own Pd1+/+ and Pd1−/− OT-1 T cell preparations. Each symbol represent the mean of replicate measurements of individual mice, and the horizontal bars are the S.E.M. of all the mice. Data in C-K represent mean ± S.E.M, N=3 mice, of samples from one of the three experiments,. * P<0.05, ** P<0.01 *** P< 0.001.
Flow cytometric analysis of spleen cells from the cMy-mOVA mice revealed more Thy1.1+ cells in spleens from mice injected with Pd1−/− OT-1 cells compared to recipients of Pd1+/+ OT-1 cells (Fig 1C), indicating that T cells lacking PD-1 survived longer and/or proliferated more in the immunized hosts. Cardiac draining lymph nodes (9) were harvested and analyzed, revealing increases in the total cellularity of the node, with a concomitant increase in transferred (Thy1.1+) cells when the OT-1 cells lacked PD-1 (Fig 1D). We also observed an increase in the total number of CD11c+ dendritic cells, possibly reflecting an increase in tissue-resident dendritic cells migrating into the draining lymph node (Fig 1E). Additionally, the draining lymph node of mice receiving PD-1 null T cells had more CD8+ IFNγ+ T cells compared to the WT group (Fig 1F). Analysis of enzymatic digests of whole hearts revealed an increase in the total number of infiltrating leukocytes in the mice that received Pd1−/− T cells (Fig 1G). An increase in both percentage and total number of Ly-6G+CD11b+ as well as Ly-6G−CD11b+ cells was observed, indicating an enhanced recruitment of neutrophils and macrophages, respectively, at the site of inflammation (Fig 1H,I). The total number of OT-I cells in the heart, and the percentage of these cells that expressed IFNγ were greater in mice that received PD-1 deficient OT-I cells compared to PD-1 expressing OT-I cells (Fig 1J, 1K). Together this data demonstrate an enhanced T cell response, as well as an enhanced infiltration of innate immune cells to the site of inflammation, suggesting that PD-1 is responsible for controlling these responses in vivo.
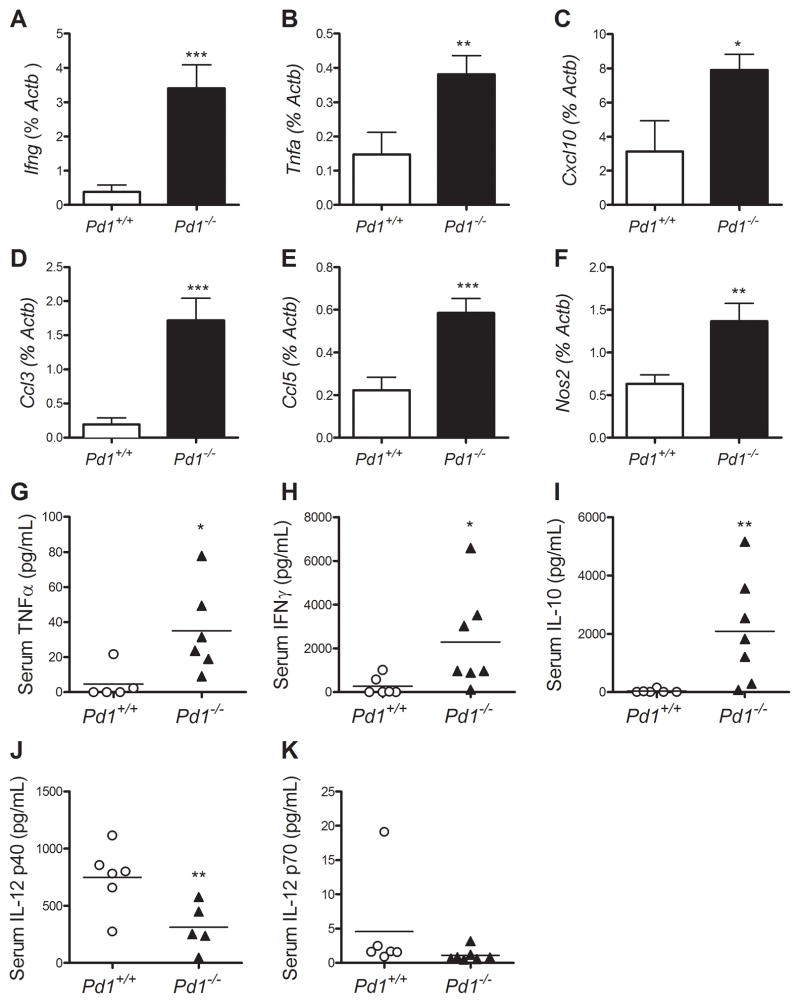
We performed qRT-PCR analysis for selected genes in cMy-mOva heart tissue at day 7 after immunization, as well as serum cytokine analyses by a cytokine bead assay, in order to further compare both inflammatory cell and endogenous tissue responses to Pd1−/− and control T cell mediated myocardial injury (Fig 2). There were significantly increased levels of IFNγ (Fig 2A) and TNFα (Fig 2B) mRNA in hearts of mice receiving Pd1−/− OT-1 cells, two signature cytokines of the CD8+ T cell response, as well as an increase in the interferon-inducible chemokine IP-10/CXCL10 (Fig 2C). Additionally we found an increase in mRNA expression of MIP1α/CCL3 and RANTES/CCL5 in hearts of mice receiving Pd1−/− OT-1 cells (Fig 2D,E); these chemokines are important in neutrophil and monocyte/macrophage recruitment to inflammatory sites. The PD-1 deficient group also had elevated expression levels of iNOS compared to the control group (Fig. 2F). Analyses of serum cytokines revealed increased levels of TNFα, IFNγ, and IL-10 (Fig 2G–I), and a decrease in the IL-12/IL-23 subunit p40 (Fig 2J), in the mice receiving Pd1−/− cells, but no difference in the IL-12 specific p70 subunit (Fig 2K) between groups, consistent with a decrease in circulating IL-23. Together these data indicate an increased inflammatory profile in the hearts of mice given PD-1 deficient T cells, with a cytokine profile dominated by IFN-γ and IFNγ related chemokines.
Figure 2. PD-1 regulates pathogenic gene expression in the heart, and influences circulating pathogenic cytokines.
RNA was purified from heart tissue of the mice described in Figure 1 and used for qRT-PCR analysis of Ifng, Tnfa, Cxcl10, Ccl3, Ccl5, and Nos2 (A–F). Serum samples from the time of sacrifice were analyzed using multiplex luminex-bead based cytokine assays for TNFα, IFNγ, IL-10, IL-12p40, and IL-12p70 (G–K). Data are the mean ± S.E.M. N=3 mice. * P<0.05, ** P<0.01 *** P< 0.001
PD-1 deficiency increases OT-1 proliferation, intracardiac inflammatory cytokine production and cytotoxicity
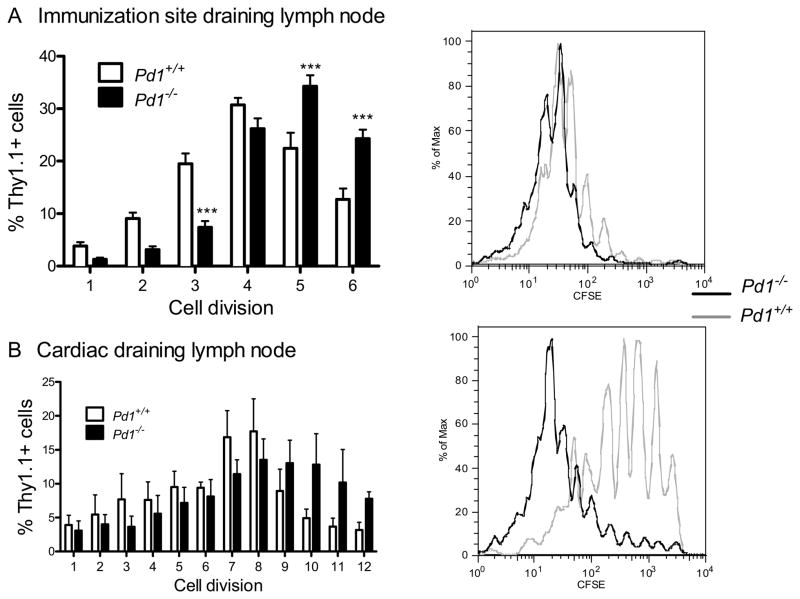
One possible mechanism by which PD-1 deficiency enhanced myocardial inflammation in our model is deregulated T cell proliferative responses to antigen stimuli. We therefore analyzed in vivo proliferation of the adoptively transferred OT-I cells. Naïve CD8+ OT-1+ cells were labeled with the florescent dye CFSE, injected into cMy-mOva mice, which were then immunized with ovalbumin plus CFA and sacrificed 72 hours later. Lymph nodes draining the immunization site or the heart were collected for analysis. In the lymph nodes draining the immunization site, the majority of Pd1+/+ OT-I cells had undergone minimal CFSE-dilution, indicating few cell divisions, whereas a significantly greater percentage of Pd1−/− OT-I cells had undergone multiple cell divisions (Fig 3A). A similar pattern was seen in the cardiac draining lymph node (Fig 3B). Together these data suggest an increased proliferative capacity in T cells lacking PD-1, representing a possible mechanism by which mice receiving PD-1 null T cells experience enhanced disease severity.
Figure 3. PD-1 controls in vivo proliferation of transferred T cells.
Naïve WT or PD-1 deficient OT-1+ CD8+ T cells were labeled with CFSE, and then transferred into cMy-mOVA recipients. The recipient mice immunized s.c. 24 hours later with CFA/Ovalbumin, and sacrificed 72 hours later. Immunization site draining lymph nodes (A), and cardiac draining lymph nodes (B), were analyzed for CFSE dilution of Thy1.1+ transferred cells. Data presented represent the mean ± S.E.M. N=3 mice, from one of 3 experiments with similar results. * P<0.05, ** P<0.01 *** P< 0.001
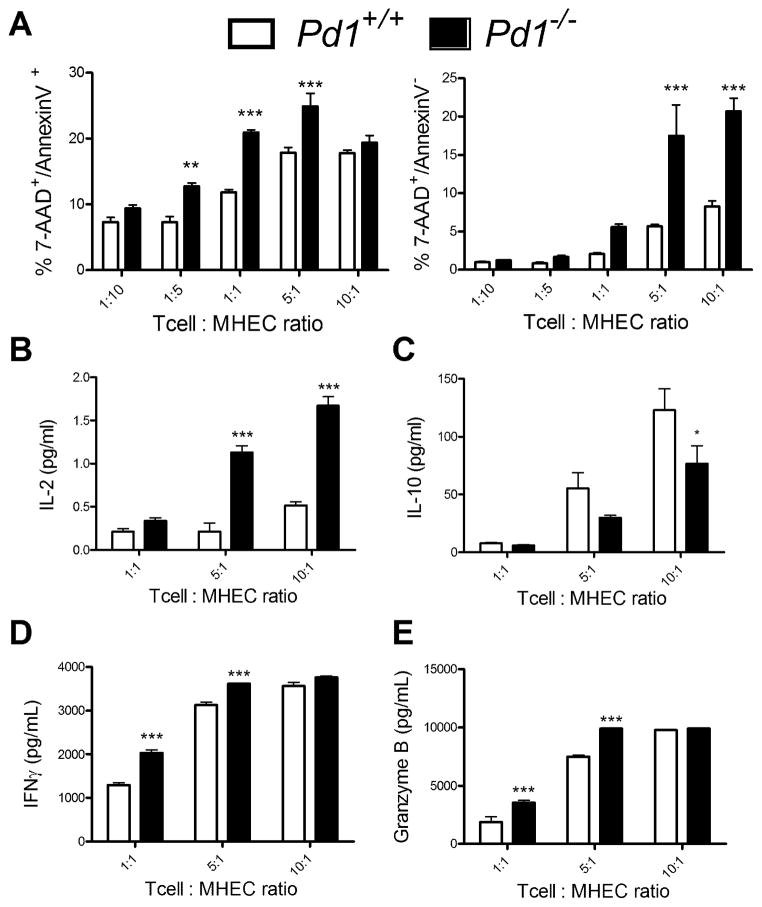
To address the possibility that the enhanced myocardial damage in recipients of PD-1 deficient OT-I cells was related to enhanced cytotoxic activity of those T cells, we performed in vitro killing assays. We chose mouse heart endothelial cells (MHEC) as the target cell as previous data from our lab suggest that the role for PD-L1 in contributing to this model is largely non-hematopoietic (31). Results from these killing assays showed that co-culture of MHEC with Pd1−/− OT-1 CTL and Ova peptide resulted in more apoptotic and dead cells at high T cell:EC ratios than co-culture with Pd1+/+ CTL (Fig. 4A). Supernatants from these assays were evaluated for the presence of cytokines, revealing an increase in IL-2 and a decrease in IL-10 in cultures with Pd1−/− OT-I cells compared to Pd1+/+ T cells (Fig. 4B, 4C). Additionally, supernatants from the cultures with Pd1−/− T cells had more IFNγ and granzyme B compared to supernatants of cultures with Pd1+/+ T cells (Fig. 4D, 4E). Together these data suggest that PD-1 deficient cells are more efficient killers of target cells than WT, providing a mechanism for increased heart damage in the absence of PD-1 on the transferred OT-I cells. Additionally the elevated levels of IL-10 in the supernatants of the Pd1+/+ group suggest that the Pd1+/+ T cells are exhibiting characteristics of exhausted T cells, i.e. secreting anti-inflammatory cytokines, whereas T cells lacking PD-1 are not. In conclusion, CD8+ T cells lacking PD-1 appear to be capable of inducing more target cell death and inflammation than T cells that express PD-1, by secreting more IFNγ and granzyme B, and less IL-10.
Figure 4. PD-1 deficient CTLs are more effective killers of cardiac-derived target cells, in vitro.
Naïve OT-1+ CD8+ T cells were isolated from Pd1−/− or WT mice, and cultured for 5 days in the presence of IL-2 and IL-12 to generate effector cells, and then rested overnight. Confluent mouse heart endothelial cells (MHEC) were pre-treated with IFNγ for 2 h and pulsed with the ovalbumin peptide SIINFKL. The MHEC were then co-cultured with OT-1 effectors at the indicated ratios of effector to target cell ratios for 1 h, and were then analyzed by FACS for Annexin V and 7-AAD staining (A). Supernatants from these assays were analyzed for the presence of IL-2 (B) and IL-10 (C) using multiplex luminex-bead based cytokine assays, and IFNγ (D) and granzyme B (E) by ELISA. Data presented represent the mean ± S.E.M of 3 experiments. * P<0.05, ** P<0.01 *** P< 0.001
PD-1 deficiency augments CD4+ dependent experimental autoimmune myocarditis
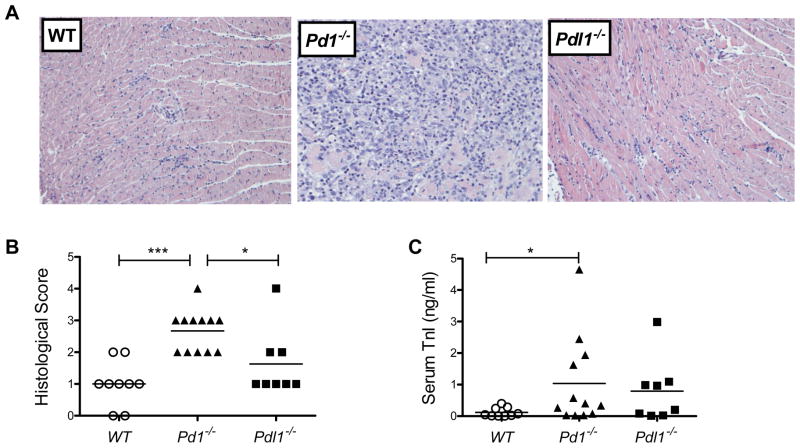
To investigate if PD-1 also plays a regulatory role in a model of autoimmune CD4+ T cell-dependent myocarditis, we used the EAM model in BALB/c mice. In this model, myocardial inflammation is induced in susceptible strains by immunization with a peptide of the mouse α-myosin heavy chain (38). Wild-type, Pd1−/−, and Pdl1−/− BALB/c mice were immunized twice, at day 0 and day 7, and sacrificed at day 21. Histological analysis of the heart revealed a significant increase in the pathology of the Pd1−/− hearts, compared with the WT and Pdl1−/− (Fig. 5A, 5B). Along with increased infiltration into the heart, Pd1−/− mice had significantly elevated circulating troponin levels at day 21, but not day 14 (Fig. 5C). These data show an increased susceptibility to EAM in the absence of PD-1. Analysis of the cardiac draining lymph node showed an overall increase in the cellularity of the node, but no distinguishable differences in the percentage of T cells or specific subsets (data not shown).
Figure 5. PD-1 deficiency exacerbates experimental autoimmune myocarditis.
WT, Pd1−/− or Pdl1−/− BALB/c mice were immunized at day 0 and day 7 with CFA/γ-myosin heavy chain peptide, and sacrificed at day 21. Representative sections of H&E stained sections of heart tissue are shown (A). Sections were scored for histopathology (B), and troponin I levels were measured in serum collected at sacrifice (C). Data are mean ± S.E.M. of 7–10 mice per group. * P<0.05, ** P<0.01 *** P< 0.001
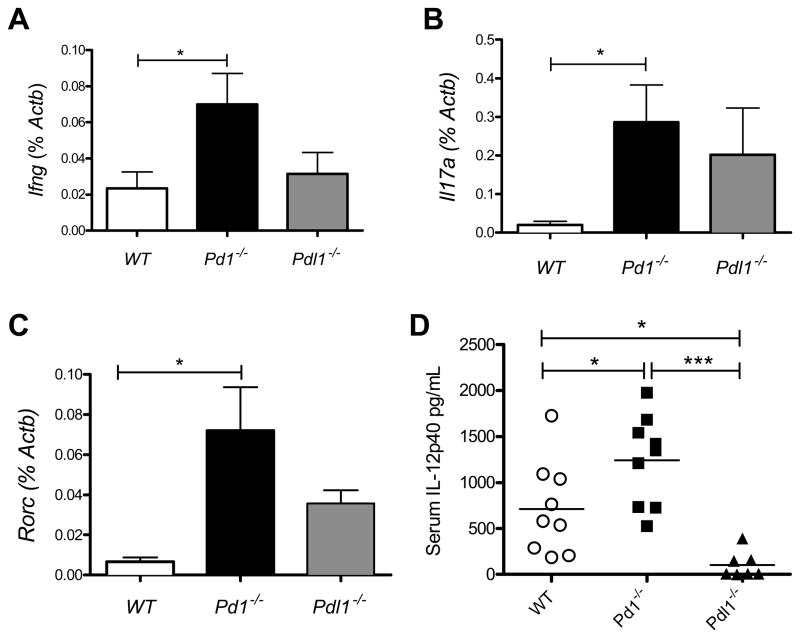
Analysis of mRNA from heart tissue of the EAM mice by RT-PCR revealed an increase in the expression of IL-17A, IFNγ and the transcription factor RORγt in Pd1−/−animals compared to WT (Fig 6A–C). No significant differences were found when comparing Pdl1−/− to either wild type or Pd1−/− animals. Interestingly no differences were seen in the cytokines IL-6 and IL-23, both of which are considered essential for the development of EAM (4, 39, 40). Additionally no differences between groups were found in expression of the chemokines RANTES, MIP1α or MCP1 (data not shown). Circulating cytokine analysis of plasma from these mice revealed significant differences between all three groups in the IL-12/IL-23 subunit p40 at day 14, but not day 21 (Fig. 6D). These data are consistent with previous studies that report the height of Th17 responses in this model to be at day 14 (3). Together this data indicate an increase in expression of proinflammatory genes in the hearts of PD-1 deficient mice with EAM compared to WT mice with EAM, while PD-L1 deficient mice showed an intermediate phenotype that was not significantly different from either WT or PD-1 deficient animals.
Figure 6. PD-1 regulates cardiac inflammatory gene expression in EAM and the release of circulating cytokines.
RNA was isolated from heart tissue of the mice described in Figure 5, sampled at the time of sacrifice, and analyzed by qRT-PCR for expression of Ifng(A), Il17α (B), and Rorc (B). Serum samples from day 14 and day 21 were analyzed using multiplex luminex-bead based cytokine assays for the presence of IL-12p40 (D). Data are the mean ± S.E.M. * P<0.05, ** P<0.01 *** P< 0.001
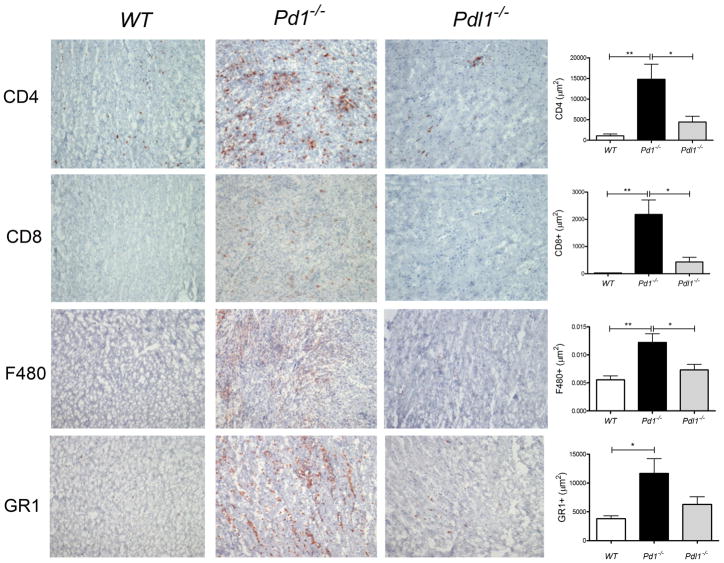
Immunohistochemical analysis of heart tissue from the mice with EAM revealed an increase in CD4+ T cells in the hearts at day 21 in the Pd1−/− group compared to WT and Pdl1−/− groups. Additionally we found more CD8+ T cells in the hearts of both Pd1−/− and Pdl1−/− animals, compared to the WT group; the latter group had virtually no CD8+ T cells. We also found an increase in GR1+ cells in the Pd1−/− group, as well as F4/80+ cells, suggesting that the inflammatory infiltrate was a mix of T cells, macrophages and neutrophils, with neutrophils accounting for the majority of infiltrating cells in the Pd1−/− group (Fig 7). Together these data show an enhanced inflammatory profile in the hearts of mice lacking PD-1, and reveal an important role for the PD-1 pathway in limiting CD8+ T cell responses and secondary neutrophil responses.
Figure 7. PD-1 deficiency results in increased inflammatory infiltrates EAM.
Immunohistochemical analysis was performed in heart sections of the mice described in Figures 5 and 6 for CD4, CD8, F480 (macrophages), and Ly6G (neutrophils). Total positive staining area was quantified using ImagePro softwareJ. Error bar represent the mean ± S.E.M. * P<0.05, ** P<0.01 *** P< 0.001
Discussion
The data reported here provide new insight into the role of PD-1 in regulating pathogenic T cell responses in the heart in vivo. While a significant body of work has been devoted to the study of the PD-1/PD-L1 pathway in many auto-immune diseases, limited work has focused on the role of PD-1 on heart antigen specific T cells. Here we report enhanced cardiac pathology in the absence of PD-1 in two distinct models of myocarditis.
Previous results from our laboratory have demonstrated a role for the PD-1 ligand PD-L1 in endothelial-T cell interactions, both in vitro (41) (41) and in vivo (31). Specifically, in our CD8 adoptive transfer model, recipient mice lacking PD-L1 and PD-L2 on non-hematopoietic cells exhibited enhanced disease pathology and an increase in neutrophilic infiltrate, in comparison to recipients that express PD-L1 and PD-L2. Previous studies have also indicated that in addition to PD-L1 interactions with PD-1, PD-L1 can also bind directly to B7-1 on T cells to dampen immune responses (42). Our work addresses an important unresolved question of whether PD-1 is a relevant receptor by which PD-L1 or PD-L2 protects against disease in the heart. Similarly to our previous results, here we find that mice receiving PD-1 deficient T cells enhanced pathology, increased clinical markers of myocyte death, and enhanced inflammation, with severe neutrophil infiltration in the heart. This indicates that PD-1 on the T cells is an important receptor for PD-L1 in regulating myocyte damage. It does remain possible that the enhanced myocarditis we previously found in PD-L1 deficient cMy-mOva mice may in part reflect the absence of PD-L1:B7-1 interactions.
Previous studies from our laboratory using our CD8+ T cell-mediated myocarditis model have utilized effector CD8+ T cells that were derived in vitro, and then transferred into the cMy-mOVA mouse. This study, while complementing previous results from our laboratory indicating the importance of PD-L1 in dampening T cell responses in the heart, was performed using naïve CD8+ T cells, and therefore represents a new adaptation of this model. When we compared myocarditis induced by transfer of in vitro activated Pd1−/− vs. Pd1+/+ effector OT-1 cells, we observed no significant difference (data not shown). Therefore, our results are consistent with a regulatory role for PD-1 during the initial activation of cardiac antigen-specific T cells, as well as their effector responses.
PD-1 is a known marker of T cell exhaustion, and targeting the PD-1 pathway has been an active area of interest for clinical development. Here we provide two mechanisms by which PD-1 controls T cell functionality. First we report that naive CD8+ T cells lacking PD-1 exhibit an enhanced capacity for proliferation in vivo upon encountering target antigen in the lymph node. Additionally we show that PD-1 plays a direct role in the effector function of T cells in vitro, by demonstrating a heightened killing capacity of mature CD8+ T cells in response to cardiac endothelial cells presenting a target antigen. Additionally we saw an increase in the CTL effector molecules IFNγ and Granzyme B in these assays, as well as a decrease in the anti-inflammatory cytokine IL-10. We observed less IL-10 production by Pd1−/− T cells in response to target cells expressing antigen, but also an increase in circulating IL-10 in the serum mice receiving Pd1−/− T cells. Many cell types, including macrophages, can secrete IL-10 and the observed discrepancy in this study is likely due to non-T cell sources of IL-10 in response to inflammation. Together this data supports the notion that PD-1 on CD8+ T cells controls both proliferative capacity and effector functions which together impact immune pathology in the heart.
Our data indicate that there is more cardiac myocyte death and more myocardial inflammation when naïve Pd1−/− CD8+ T cells are transferred compared to Pd1+/+. One possible interpretation of this data is that the increased inflammation reflects only a secondary response to increased cytotoxicity of the Pd1−/− T cells with more myocyte death. However, we also saw enhanced proliferation and IFN-γ production by Pd1−/− T cells, and we did not see increased cardiac inflammation when in vitro Pd1−/− CD8+ effector cells were transferred (not shown). Together, these data argue for a more complex effect of PD-1 in regulating several aspects of the CD8+ T cell response, including proliferation during the in vivo priming phase, the inflammatory functions of the effector CTL that emerge, and their cytotoxic functions.
Because a significant body of in vivo work has previously been focused on PD-1 on CD8+ T cells, we investigated the role of PD-1 on CD4+ T cell mediated inflammatory disease. For this purpose, we chose the CD4+ T cell dependent EAM model, and also showed that PD-1 exerts a profound regulatory influence on T cell mediated inflammation in the heart. Our data show enhanced disease severity in the hearts of Pd1−/− mice, with increased cardiac myocyte damage. Again, this model addresses the importance of PD-1 in the initiation of T cell responses, and adds significantly to the breadth of knowledge on how PD-1 controls not only CD8+ T cell response, but also CD4+ T cell responses. Thus by utilizing these two complementary models we are extending the understanding of the PD-1 pathway to a wider range of T cell mediated heart pathologies. This study represents some of the first work on T cell co-inhibition in the EAM model. One previous report showed a role for CTLA-4 in modulating Th17 responses in EAM (25). Additionally, the lack of PD-1 in this model unmasked a role for CD8+ T cells that is not usually seen in EAM. This corresponds to a recent finding from our laboratory that identified a robust CD8+ T cell response in atherosclerotic lesions in mice lacking PD-1 (14).
The basis for the distinct myocarditic response in PD-1 deficient and PD-L1 deficient mice in EAM is not clear. Mice lacking PD-1 showed enhanced disease severity and increased circulating troponins compared to WT mice. Interestingly PD-L1 deficient mice showed an intermediate phenotype in regard to histology, troponin, and inflammatory gene expression, as well in immunohistochemical analysis of heart infiltrating cells. Together these results leave open the possibility that unlike the CD8-mediated myocarditis model, where PD-L1 is the PD-1 ligand responsible for modulating disease severity, in EAM there may be overlapping roles for PD-L1 and PD-L2, in regards to antigen presentation.
In summary, our studies provide evidence that PD-1 is important for protecting the heart against T cell mediated injury. Our findings also highlight the mechanisms by which T cells mediated heart pathology can be enhanced, by increased proliferation and enhanced killing capacity. Additionally we find that the PD-1 on T cells may contribute to the recruitment of other heart infiltrating cells, specifically macrophages and neutrophils. Importantly this work compares two models in distinct genetic backgrounds of mice, with distinct pathologies, broadening the applicability of these observations.
Acknowledgments
The authors acknowledge Dr. Petr Jarolim, Brigham and Women’s Hospital Department of Pathology for assistance with the serum troponin assays and Dr. James Lederer, Brigham and Women’s Hospital Department of Surgery for assistance with flow-based cytokine bead assays.
Footnotes
This work was supported by NIH grants RO1 HL36028 and P50 HL56985 (AHL), and P01 AI56299 (AHS).
References
- 1.Rose NR, Beisel KW, Herskowitz A, Neu N, Wolfgram LJ, Alvarez FL, Traystman MD, Craig SW. Cardiac myosin and autoimmune myocarditis. Ciba Found Symp. 1987;129:3–24. doi: 10.1002/9780470513484.ch2. [DOI] [PubMed] [Google Scholar]
- 2.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 3.Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 4.Sonderegger I, Rohn TA, Kurrer MO, Iezzi G, Zou Y, Kastelein RA, Bachmann MF, Kopf M. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol. 2006;36:2849–2856. doi: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- 5.Afanasyeva M, Georgakopoulos D, Rose NR. Autoimmune myocarditis: cellular mediators of cardiac dysfunction. Autoimmun Rev. 2004;3:476–486. doi: 10.1016/j.autrev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Gebhard JR, Perry CM, Harkins S, Lane T, Mena I, Asensio VC, Campbell IL, Whitton JL. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am J Pathol. 1998;153:417–428. doi: 10.1016/S0002-9440(10)65585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henke A, Huber S, Stelzner A, Whitton JL. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber S. T cells in coxsackievirus-induced myocarditis. Viral Immunol. 2004;17:152–164. doi: 10.1089/0882824041310667. [DOI] [PubMed] [Google Scholar]
- 9.Grabie N, Delfs MW, Westrich JR, Love VA, Stavrakis G, Ahmad F, Seidman CE, Seidman JG, Lichtman AH. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Invest. 2003;111:671–680. doi: 10.1172/JCI16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taqueti VR, Grabie N, Colvin R, Pang H, Jarolim P, Luster AD, Glimcher LH, Lichtman AH. T-bet controls pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J Immunol. 2006;177:5890–5901. doi: 10.4049/jimmunol.177.9.5890. [DOI] [PubMed] [Google Scholar]
- 11.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nature reviews Immunology. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 14.Bu DX, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, Jarolim P, Freeman GJ, Sharpe AH, Lichtman AH. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vac Biol. 2011;31:1100–1107. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa Y, Mandelbrot DA, Libby P, Sharpe AH, Mitchell RN. Association of B7-1 co-stimulation with the development of graft arterial disease. Studies using mice lacking B7-1, B7-2, or B7-1/B7-2. Am J Pathol. 2000;157:473–484. doi: 10.1016/S0002-9440(10)64559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KS, Denton MD, Chandraker A, Knoflach A, Milord R, Waaga AM, Turka LA, Russell ME, Peach R, Sayegh MH. CD28-B7-mediated T cell costimulation in chronic cardiac allograft rejection: differential role of B7-1 in initiation versus progression of graft arteriosclerosis. Am J Pathol. 2001;158:977–986. doi: 10.1016/S0002-9440(10)64044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter LL, Leach MW, Azoitei ML, Cui J, Pelker JW, Jussif J, Benoit S, Ireland G, Luxenberg D, Askew GR, Milarski KL, Groves C, Brown T, Carito BA, Percival K, Carreno BM, Collins M, Marusic S. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;182:124–134. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Critical care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J Virol. 2010;84:2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topalian SL, Weiner GJ, Pardoll DM. Cancer Immunotherapy Comes of Age. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seko Y, Yagita H, Okumura K, Azuma M, Nagai R. Roles of programmed death-1 (PD-1)/PD-1 ligands pathway in the development of murine acute myocarditis caused by coxsackievirus B3. Cardiovasc Res. 2007;75:158–167. doi: 10.1016/j.cardiores.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Ying H, Yang L, Qiao G, Li Z, Zhang L, Yin F, Xie D, Zhang J. Cutting edge: CTLA-4--B7 interaction suppresses Th17 cell differentiation. J Immunol. 2010;185:1375–1378. doi: 10.4049/jimmunol.0903369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181:2513–2521. doi: 10.4049/jimmunol.181.4.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, I, Okazaki M, Yoshida T, Chikuma S, Kato Y, Nakaki F, Hiai H, Honjo T, Okazaki T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22:443–452. doi: 10.1093/intimm/dxq026. [DOI] [PubMed] [Google Scholar]
- 28.Koga N, Suzuki J, Kosuge H, Haraguchi G, Onai Y, Futamatsu H, Maejima Y, Gotoh R, Saiki H, Tsushima F, Azuma M, Isobe M. Blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Ather Thromb Vasc Bio. 2004;24:2057–2062. doi: 10.1161/01.ATV.0000145015.23656.e4. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 31.Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 32.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 33.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 34.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nature immunology. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedke T, Pretsch L, Karakhanova S, Enk AH, Mahnke K. Endothelial cells augment the suppressive function of CD4+ CD25+ Foxp3+ regulatory T cells: involvement of programmed death-1 and IL-10. J Immunol. 2010;184:5562–5570. doi: 10.4049/jimmunol.0902458. [DOI] [PubMed] [Google Scholar]
- 37.Grabie N, Hsieh DT, Buono C, Westrich JR, Allen JA, Pang H, Stavrakis G, Lichtman AH. Neutrophils sustain pathogenic CD8+ T cell responses in the heart. Am J Path. 2003;163:2413–2420. doi: 10.1016/S0002-9440(10)63596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 39.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107:320–325. doi: 10.1161/01.cir.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 40.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 42.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]