Abstract
Neisseria meningitidis inhibits the alternative pathway (AP) of complement utilizing diverse mechanisms, including expression of capsule (select serogroups), Neisserial surface protein A (NspA), factor H binding protein (fHbp) and lipooligosaccharide (LOS) sialylation. The contribution of the latter three molecules in AP regulation in encapsulated meningococci was studied using isogenic mutants. When LOS was unsialylated, deleting NspA alone from group A strain A2594 (low fHbp/high NspA) significantly increased AP-mediated C3 deposition. C3 deposition further increased ~2-fold in a ΔfHbpΔNspA double mutant, indicating cooperative fHbp function. LOS sialylation of A2594 ΔfHbpΔNspA decreased the rate of C3 deposition, revealing AP inhibition by LOS sialic acid. Maximal C3 deposition on group B strain H44/76 (high fHbp/low NspA) occurred when all three molecules were absent; again, LOS sialylation attenuated the AP in the absence of both fHbp and NspA. When H44/76 LOS was unsialylated, both fHbp and NspA independently inhibited the AP. LOS sialylation enhanced binding of fH C-terminal domains 18–20 to C3 fragments deposited on bacteria. Interaction of meningococci with non-human complement is relevant for animal models and vaccine evaluation studies that employ non-human complement. Consistent with their human-specific fH binding, neither fHbp nor NspA regulated the rat AP. However, LOS sialylation inhibited the rat AP and, as with human serum, enhanced binding of rat fH to surface-bound C3. These data highlight the cooperative roles of meningococcal NspA and fHbp in regulating the human AP and broaden the molecular basis for LOS sialylation in AP regulation on meningococci in more than one animal species.
Keywords: complement, alternative pathway, Neisseria meningitidis, factor H, lipooligosaccharide, sialic acid, factor H-binding protein, Neisserial surface protein A
Introduction
The complement system forms an important arm of innate immune defenses against invasive meningococcal disease. The importance of complement in curbing meningococcal disease is illustrated by the observation that individuals with defects in components of the terminal or alternative pathways are at a greatly increased risk of meningococcal infection (1–2). Activation of the complement system on meningococci results in deposition of C3 fragments on the bacterial surface which marks them for phagocytosis. Further activation of the complement cascade may result in direct killing by insertion of the C5b-9 complex. Activation of the complement system occurs through the classical, lectin or alternative pathways and all these pathways ultimately converge at the level of C3 deposition (3). Activation of complement beyond the level of C3 leads to the formation of C5 convertases and subsequent membrane attack complex (C5b-9) insertion into the bacterial membrane that can result in killing of the organism. In most instances, colonization with meningococci is followed by the development of specific antibodies and protective immunity against the homologous and antigenically closely related strains (4–5). Because meningococci possess several mechanisms to subvert complement-dependent killing (6), lack of a protective immune response may lead to development of invasive infection in such individuals.
A unique feature of the alternative pathway (AP) of complement is a positive feedback or amplification loop, where C3b deposited on surfaces participates in the formation of C3 convertase (C3b,Bb), which in turn serves to activate more C3 molecules. Work over the past three decades has revealed several mechanisms by which Neisseria meningitidis inhibits AP activation. The polysialic capsules of group B and group C N. meningitidis limit C3 fragment deposition on the bacterial surface (7–9). Sialylation of meningococcal lipooligosaccharide (LOS) also limits C3 deposition (10–11), although the molecular basis of this observation remains unclear. In recent years, meningococci have been shown to bind to the AP inhibitor, factor H (fH) via two membrane proteins, factor H binding protein (fHbp; previously referred to as Genome-derived Neisserial antigen (GNA) 1870 or LP2086) (12–13) and Neisserial surface protein A (NspA) (14). fH blocks the positive feedback loop of the AP at several steps. It serves as a cofactor for factor I-mediated cleavage of C3b to iC3b, prevents the association of factor B with C3b and causes irreversible dissociation of factor Bb from the C3 convertase, C3b,Bb (reviewed in Ref. (15)).
We recently studied meningococcal bacteremia in a human fH transgenic Wistar rat model (16). Meningococcal strain H44/76 normally does not cause bacteremia following intraperitoneal inoculation into 5–7 day old wild-type Wistar rats but caused bacteremia in the human fH transgenic rat. Interestingly, a “double” mutant that lacked both fHbp and NspA remained virulent in this rat model (16). This finding suggested that additional molecule(s) on the meningococcal surface facilitated bacteremia in a human fH-dependent manner. Lipooligosaccharide (LOS) sialic acid was identified as a candidate molecule evidenced by the observation that a triple fHbp NspA lst (lst encodes lipooligosaccharide sialyltransferase that is required for the addition of sialic acid on to LOS) mutant was avirulent in the human fH transgenic animal (16). The aim of this study was to define the relative contributions of fHbp, NspA and LOS sialic acid in regulating the AP of complement on encapsulated disease-causing isolates of N. meningitidis. In addition, further insights into the molecular basis of AP inhibition by LOS sialylation have been elucidated.
Materials and Methods
Bacterial strains
The wild-type strains used in this study were H44/76 (B:15:P1.7,16:ST-32; Norway, 1976) (17) and A2594 (A:4:1–9:ST-5) (12). The LOS of both strains express the lacto-N-neotetraose substitution from HepI. H44/76 can endogenously sialylate its LOS because it synthesizes 5'-cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA), the donor molecule for sialic acid. In contrast, group A meningococci do not synthesize CMP-NANA, but can sialylate lacto-N-neotetraose expressing LOS when CMP-NANA is added to growth media (described below). Relative to A2594, H44/76 expresses high levels of fHbp, but comparatively low levels of NspA (14). fHbp, NspA and LOS sialyltransferase (lst) deletion mutants (fHbp::erm, nspA::spc and lst::kan, referred to as ΔfHbp, ΔNspA and Δlst, respectively) were created in H44/76 and A2594 as described previously (14).
Bacteria were routinely grown on chocolate agar plates supplemented with IsoVitaleX equivalent at 37°C in an atmosphere enriched with 5% CO2. GC plates supplemented with IsoVitaleX equivalent were used for antibiotic selection. Antibiotics where used at the following concentrations when indicated; 100 μg/ml kanamycin, 5 μg/ml erythromycin and 50 μg/ml spectinomycin. In some experiments, bacteria were grown in gonococcal liquid media supplemented with IsoVitaleX equivalent to which CMP-NANA (Nacalai USA, Inc., San Diego, CA) to a final concentration of 0.02 mM was added.
Sera and complement reagents
Normal human serum (NHS) was obtained from a healthy adult human volunteer and aliquoted and stored at −70 °C till used. To selectively inactivate the classical and lectin complement pathways and isolate the AP as the only active pathway, MgCl2 and EGTA, both to a final concentration of 10 mM, were added to NHS (NHS-Mg/EGTA). Normal rat serum (NRS) was from Complement Technologies, Inc (Tyler, TX).
Antibodies
Anti-fHbp mAb JAR 3 (IgG3) (18) and anti-NspA mAb 14C7 (IgG3) (19) used in Western blotting assays were provided by Dr. Dan M. Granoff (Childrens Hospital Oakland Research Institute, Oakland, CA). Anti-iC3b mAb G-3E (gift from Dr. Kyoko Iida (20)) recognizes a neoepitope on the 68 kD α1′ chain of iC3b. mAb 3F11 (mouse IgM) recognizes the unsialylated lacto-N-neotetraose LOS species (the 3F11 epitope); sialylation of LOS obscures the 3F11 epitope and decreases mAb 3F11 binding (21). FITC-conjugated anti-human C3 was from Biodesign/Meridian Life Science, Inc. (Memphis, TN). Polyclonal goat anti-human fH was prepared by passing goat anti-serum raised against human fH (Complement Technologies) over a human fH-Sepharose 6B. Bound antibody was eluted with 0.1 M glycine pH 3, neutralized immediately with 0.5 M Tris, pH 8, and then spin concentrated and dialyzed against 0.9% NaCl to a concentration of 1 mg/ml. Goat anti-human C3 anti-serum was from Complement Technologies. To block the function of factor I and thereby limit conversion of C3b to iC3b, a mAb that blocks the function of factor I (Quidel Corporation, San Diego, CA; anti-factor I mAb #1, Cat. No. A247) was added to serum at a concentration of 50 μg/ml. Sheep polyclonal anti-human factor B (IgG fraction) was from Cortex Biochem (San Leandro, CA). Polyclonal rabbit anti-mouse fH was provided by Dr. Wen-Chao Song (University of Pennsylvania). Goat anti-mouse C3 and FITC-conjugated goat anti-mouse C3 (MP Biomedicals, Solon, OH) were used to detect rat C3 fragment deposition on bacteria by western blotting and FACS, respectively. Anti-mouse IgG, anti-goat IgG, anti-rabbit IgG and anti-sheep IgG conjugated to alkaline phosphatase were from Sigma (St. Louis, MO).
Recombinant human fH/Fc fusion proteins
fH domains 5–8 and 18–20 fused to the Fc fragment of murine IgG2a (called fH/Fc) have been described previously (22). These constructs contain contiguous human fH domains (5 through 8 or 18 through 20) fused in frame at their C-terminal ends to the N-terminus of the Fc fragment of murine IgG2a. Briefly, Chinese hamster ovary cells were transfected with each of the fH/Fc constructs using lipofectin (Invitrogen), according to the manufacturer's instructions. Media from transfected cells were collected after a 2-day period, and constructs were purified over a protein G sepharose column. Purified proteins were concentrated using Amicon Ultra 10,000 MWCO (Millipore) and fH/Fc protein concentrations determined using the BCA assay kit (Thermo Scientific Pierce, Rockford, IL).
Western blot analysis of fHbp and NspA expression
Relative levels of fHbp and NspA expression by strains A2594 and H44/76 was assessed by western blot analysis. Serial 2-fold dilutions of bacterial lysates in 4× LDS sample buffer (Invitrogen) were electrophoresed on a 4–12% Bis-Tris gel using MES running buffer (Invitrogen) and transferred by western blotting on to a 0.2 μm PVDF membrane (Millipore, Bedford, MA). Proteins migrating above the 50 kD marker were stained with Coomassie blue (Imperial Protein Stain, Thermo Scientific, Rockford, IL) and served as loading controls. The remainder of the membrane was blocked with PBS-1% dry milk for 1 h at 23 °C. Proteins migrating above 20 kD were probed for fHbp (molecular mass ~29 kD) with mAb JAR 3 and proteins migrating faster than 20 kD on the same blot were probed for NspA (molecular mass ~17 kD) with mAb 14C7 (both mAbs were mouse IgG3 and were used at a concentration of 1 μg/ml in PBS-0.05% Tween 20), followed by anti-mouse IgG conjugated to alkaline phosphatase. Membranes were developed with BCIP®/NBT-Purple Liquid Substrate System (Sigma).
iC3b deposition, fH and fH/Fc fusion protein binding to bacteria by western blotting
Incubation of bacteria with serum results in complement activation and deposition of C3b on the bacteria. C3b deposited on bacteria is converted to iC3b by fH (cofactor) and factor I (enzyme). Almost all the C3b deposited on meningococci is rapidly converted to iC3b, which is the predominant fragment detected, even on meningococcal strains that that lack fHbp, NspA, LOS sialic acid (8). Thus, the amount of iC3b deposited is indicative of the total amount of C3 fragment deposition on meningococci. AP-mediated iC3b deposited on bacteria was assessed by western blotting as described previously (8). Briefly, 108 bacteria suspended in HBSS containing 1 mM MgCl2 and 1 mM EGTA were incubated with NHS-Mg/EGTA (final concentration 25% (v/v)) in a final reaction volume of 80 μl for 10 or 30 min at 37°C. Bacteria were washed twice in HBSS2+ and lysed in 4× LDS sample buffer (Invitrogen) containing 10% 2-ME. Proteins were separated on NuPAGE Novex 4–12% Bis-Tris gradient gels using NuPAGE 3-morpholinopropanesulfonic acid (MOPS) running buffer (Invitrogen). Proteins were transferred to a 0.45 μm PVDF membrane (Millipore) by western blotting. iC3b was detected using mAb G-3E (tissue culture supernatants containing ~20 μg/ml of antibody diluted 1:4 in TBS), followed by anti-mouse IgG conjugated to alkaline phosphatase. Similarly, fH and fH/Fc fusion protein binding was also measured by western blotting. Bacteria were incubated with purified fH or fH/Fc (10 μg/ml) for 15 min at 37 °C, washed twice and pellets were lysed in 4× LDS sample buffer (Invitrogen) without the addition of 2-ME. fH migrates at its native molecular mass (~150 kD) and was detected with polyclonal goat anti-human fH, followed by anti-goat IgG alkaline phosphatase (Sigma). fH/Fc fusion proteins were detected using anti-mouse IgG conjugated to alkaline phosphatase.
Flow cytometry
Human and rat C3 deposition on and fH binding to bacteria were measured using flow cytometry as described previously (12, 23). Samples were prepared as described above for western blotting. Data were collected using a FACSCalibur instrument (Becton Dickinson) and data analysis was performed using the FlowJo data analysis software package (www.treestar.com). Events on the negative control samples (histograms with fluorescence on the x-axis) were gated such that 95% of the bacteria lay to the left of the gate (i.e., negative control samples showed 5% of events as positive). This gate was then applied to all samples and the percentage of positive events for each sample was recorded. Each datum point on the FACS figures represents an average of at least 5 separate observations.
Statistical analysis
Flow cytometry data were analyzed using GraphPad Prism 5 software. Comparisons across multiple groups were performed by ANOVA; P values less than 0.05 were considered significant.
Results
C3 fragment deposition on N. meningitidis that vary in fHbp, NspA and LOS sialic acid expression
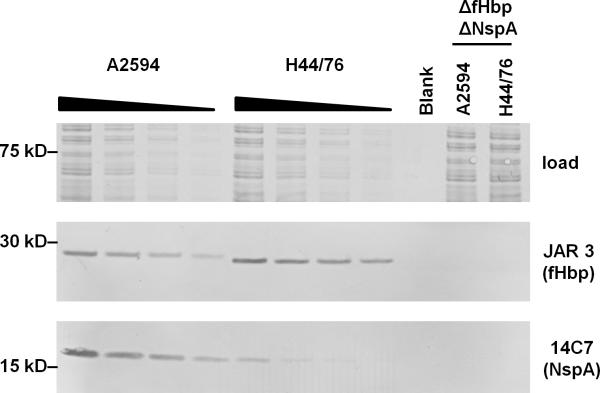
Expression levels of fHbp (24–25) and NspA (26) vary widely across meningococcal isolates. In order to compare the expression levels of fHbp relative to NspA on strains H44/76 and A2594, we used anti-fHbp mAb JAR 3 and anti-NspA mAb 14C7, which both belong to the same subclass (IgG3). Serial 2-fold dilutions of bacterial lysates that were western blotted were probed with the mAbs. As shown in Fig. 1, and as reported previously (14), A2594 expressed more NspA than H44/76, while H44/76 expressed more fHbp than A2594.
Figure 1.
Relative expression levels of fHbp and NspA on strains A2594 and H44/76. Serial 2-fold dilutions of lysates of wild-type strains A2594 and H44/76 were western blotted and probed with mAbs JAR 3 (anti-fHbp; mouse IgG3) and mAb 14C7 (anti-NspA; mouse IgG3). The ΔfHbpΔNspA mutants of both strains served as negative controls. Proteins on the blot that migrated above ~50 kD were stained with Coomassie blue to normalize for protein loading.
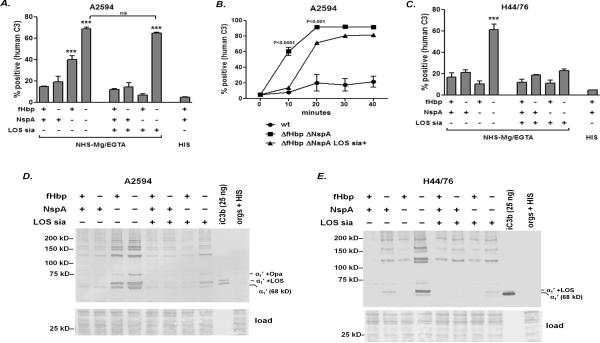
Deposition of human C3 fragments (C3b is initially deposited on bacteria, which is then converted to iC3b by the action of factors H and I) on isogenic mutants of group A strain A2594 (low fHbp, high NspA) and group B strain H44/76 (high fHbp, low NspA) that differed only in expression of fHbp, NspA and LOS sialic acid was measured by flow cytometry. As shown in Fig. 2A, loss of NspA, but not fHbp alone, from unsialylated A2594 resulted in significantly higher C3 deposition compared to the wild-type strain. The mutant that expressed only fHbp bound ~2-fold less C3 when compared to the unsialylated mutant that lacked both fHbp and NspA, thus demonstrating that fHbp could augment the function of NspA in regulating the AP on this strain. When the LOS of A2594 was sialylated, C3 deposition on the ΔNspA mutant decreased significantly. However, LOS sialic acid alone (in the absence of both fHbp and NspA) did not significantly reduce the amount of C3 deposited on strain A2594 at 30 min. Representative histogram tracings are shown in Supplemental Figure 1A. We hypothesized that LOS sialic acid could reduce the rate of C3 deposition on the ΔfHbpΔNspA mutant. As shown in Fig. 2B, LOS sialic acid significantly increased the lag-phase of AP activation. While C3 accumulated rapidly on the unsialylated ΔfHbpΔNspA mutant (>50% of maximal C3 deposition was seen at 10 min and reached maximal levels between 10 and 20 min), the presence of LOS sialic acid significantly delayed C3 accumulation on the bacterial surface. C3 deposition was only marginally above baseline levels at 10 min, following which C3 deposition reached maximal levels between 20 and 30 min. Representative histogram tracings are shown in Supplemental Figure 1B.
Figure 2.
iC3b deposition on strains A2594 and H44/76 and their ΔfHbp, ΔNspA and ΔfHbp ΔNspA mutants that either possess or lack LOS sialic acid. A. Flow cytometry quantifying human C3 deposition on strain A2594 and its mutants. A2594 and its derivatives were grown in media supplemented with 0.02 mM CMP-NANA to sialylate LOS where indicated. Bacteria were incubated with NHS-Mg/EGTA (25% (v/v)) for 30 min at 37 °C. The percentage of positive events relative to organisms incubated with heat-inactivated human serum (HIS) was determined as described in the materials and methods section. Each bar represents the mean (SEM) of at least 5 independent data points. The comparison across groups was performed by 1-way ANOVA. ***, P<0.001 compared to all other groups, except where indicated by `ns' (not significant). B. LOS sialylation of A2594 ΔfHbp ΔNspA reduces the rate of C3 fragment deposition. The wild-type strain (expresses fHbp and NspA, but not LOS sialic acid) was used as a control. Bacteria were incubated with 25% NHS-Mg/EGTA for the time periods indicated on the x-axis. C3 deposition on bacteria was measured by flow cytometry as described in A. Each point represents the mean (SEM) of 5 separate observations. The comparison across groups was carried out by 2-way ANOVA. The P values shown compare ΔfHbp ΔNspA (LOS not sialylated) with its sialylated derivative. C. Human C3 deposition on strain H44/76 and its mutants. The methods and analysis are as described in A. D and E. Western blotting analysis of iC3b deposition on A2594 and H44/76, respectively. Bacteria were incubated with NHS-Mg/EGTA (25% (v/v)) for 30 min at 37 °C. Bacteria were lysed and proteins were separated on a 4–12% Bis-Tris gel, followed by transfer to a PVDF membrane by western blotting. iC3b and iC3b complexes with its meningococcal surface targets were detected with mAb G-3E. The 68 kD α1′ chain of iC3b is indicated as “α1′”. Note that the iC3b α1′ chain in the lane containing purified iC3b in panel A migrates as a doublet (~68- and ~70-kDa bands) likely because a fraction of the α′ chain of C3b was not cleaved at the second site by factor I to release the 2 kD C3f fragment in some lots of purified iC3b, as reported previously (8). Complexes of the α1′ chain of iC3b with LOS or opacity protein (Opa) have previously been characterized (31) and are indicated. The lower section of the blot (proteins migrating faster than ~40 kD) was stained with Coomassie blue (labeled as “load”) and served to illustrate similar loading of bacteria across lanes.
Fig. 2C shows C3 deposition on group B strain H44/76 and its isogenic mutants (see Supplemental Figure 1C for representative histograms). Again, loss of fHbp, NspA and LOS sialic acid resulted in the highest levels of C3 deposition. The presence of fHbp, NspA or LOS sialic acid alone or in combination restricted C3 deposition. A noteworthy observation was that in contrast to A2594, LOS sialic acid alone on H44/76 could limit C3 deposition at 30 min. Previous work has shown that the group B capsule (independent of fHbp, NspA and LOS sialic acid), but not the group A capsule, of N. meningitidis inhibits AP activation and C3 fragment deposition on bacteria (8). Thus, the combined effects of the group B capsule of H44/76 and LOS sialic acid may have served to limit C3 deposition on this mutant.
We also performed western blotting to examine the major targets for C3 fragment deposition on these mutants and also to verify the results obtained above by a second method. Activation of the AP results in C3b deposition on bacteria. C3b binds to surfaces through the thioester in its ~106 kD α′ chain. The α′ chain is linked to the 75 kD β chain by a disulfide bond. iC3b is formed by cleavage of the α′ chain into α1′ (~68 kD) and α2′ (~40 kD) fragments by fH and factor I. In the unreduced state, the α1′ and α2′ fragments remain united through a second disulfide bond. Electrophoresis under reducing conditions results in migration of the β chain (present in both C3b and iC3b) at its calculated mass of ~75 kD, while the 106 kD α′ chain of C3b and the 68 kD α1′ chain of iC3b migrate complexed with their targets; the α2′ chain of iC3b migrates independently at 40 kD. Although the iC3b is covalently linked to its bacterial targets through an ester bond, a proportion of the α1′ chain is released from its targets spontaneously even without treatment with nucleophiles such as methylamine (27–29). Hydrolytic attack by a His residue, located 113 amino acids downstream of the ester-forming Gln residue (30) is responsible for the free α1′ chain seen in the blots in Fig. 2. We have shown previously that almost all the C3b is converted to iC3b even on meningococcal mutants that lack fHbp, NspA and LOS sialic acid (8). Therefore, an anti-iC3b specific mAb was used in the western blotting assays to measure C3 deposition on strains A2594 and H44/76 and their mutant derivatives (Figs. 2D and 2E).
The amount of iC3b deposition paralleled the data obtained above in flow cytometry assays. The major targets for iC3b on the A2594 mutants included LOS and Opa as described previously (31) and were similar across the mutants. Of note, strain H44/76 expresses very low levels of Opa (31). An anti-iC3b-reactive band at ~130 kD (a doublet at this location is seen in the Fig. 2E (lane containing fHbp-, NspA-, LOS sia-)) was also noted and the identity of this target has not been defined.
These results suggested that regulation of AP-mediated C3 fragment deposition on A2594 (low fHbp, high NspA; LOS not sialylated) was mediated primarily by NspA, even when capsule and lacto-N-neotetraose LOS were expressed. A role for fHbp in AP regulation on this strain was evident from the ΔfHbpΔNspA double mutant, which showed higher C3 fragment deposition compared to the ΔNspA single mutant. Growth of these strains in media that contained CMP-NANA to sialylate LOS resulted in a marked decrease in C3 deposition on the ΔNspA mutant and also slowed the rate of C3 deposition on the ΔfHbpΔNspA mutant. While these data confirm the role for fHbp in inhibiting AP-mediated C3 deposition on H44/76 that expresses the group B capsule, they illustrate a key role for NspA in limiting AP activation on this strain.
Thus, all three molecules (fHbp, NspA and LOS sialic acid) contribute to restricting C3 deposition on encapsulated meningococci and highlight redundancy in complement regulatory mechanisms. The relative roles of fHbp and NspA in limiting C3 fragment deposition through the AP likely correlate with their expression levels.
LOS sialylation and surface-bound C3 fragments cooperatively enhance fH binding
Having confirmed the importance of LOS sialylation in AP inhibition on meningococci, we next investigated the molecular basis of this observation. Earlier work has shown that while sialic acid on sheep erythrocytes does not affect the affinity of factor B for C3b, surface-bound C3b enhances the affinity of fH (32). We hypothesized that in an analogous manner, meningococcal LOS sialylation would enhance human fH binding to bacteria when C3 fragments were deposited on the bacterial surface. In contrast, the ability of factor B to bind to C3b deposited on bacteria would not be affected by LOS sialylation.
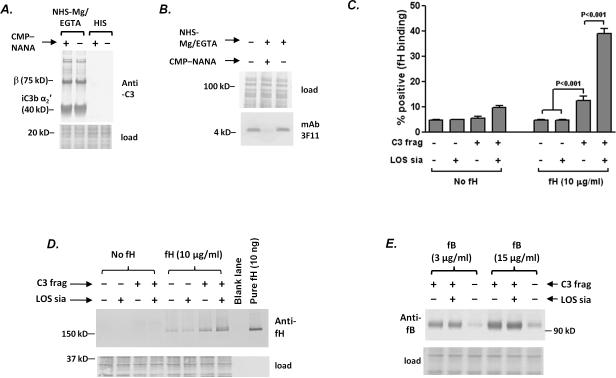
We first incubated A2594 ΔfHbpΔNspA with NHS-Mg/EGTA to allow for C3 fragment deposition and then grew one half of the bacteria in gonococcal growth media with CMP-NANA for 1 h to sialylate LOS and grew the other half in media without CMP-NANA to generate organisms that lacked LOS sialylation. Sialylation of bacteria after C3 fragment deposition ensured that similar amounts of C3 were deposited and that C3 deposition occurred at similar sites on both sialylated and unsialylated bacteria (Fig. 3A). Control reactions included bacteria incubated with heat-inactivated serum (HIS) or with buffer alone. Loss of mAb 3F11 binding (Fig. 3B) confirmed that bacteria that had been incubated with serum and then grown in CMP-NANA-containing media, incorporated sialic acid onto their LOS. Bacteria (coated with C3 fragments with or without LOS sialic acid) were then incubated with purified human fH (10 μg/ml) and bound fH was detected by flow cytometry (Fig. 3C) and western blotting (Fig. 3D). The highest levels of fH binding were seen to sialylated bacteria on which C3 fragments were deposited. Approximately 3-fold lower levels (as measured by the % of positive events) of fH bound to unsialylated bacteria coated with C3 fragments; this level of fH binding was significant when compared with fH binding to bacteria (with or without LOS sialic acid) that did not possess C3 fragments on their surface (Fig. 3C). Representative histograms are shown in Supplemental Figure 2. It is noteworthy that the higher sensitivity of western blotting (Fig. 3D) revealed low amounts of fH binding to sialylated and unsialylated bacteria even in the absence of bacteria-bound C3 fragments, which raises the possibility that meningococci may possess an acceptor site(s) for human fH in addition to fHbp and NspA. Controls without added human fH (bars or lanes marked “No fH”) were performed to ensure that significant amounts of residual fH bound to bacteria were not carried over from the initial serum incubation step.
Figure 3.
LOS sialylation enhances human fH binding to C3 fragments deposited on meningococci, but does not affect factor B interactions with surface-bound C3b. A. C3 deposition (detected with goat polyclonal anti-human C3) on A2594 ΔfHbpΔNspA bacteria that were incubated with NHS-Mg/EGTA (20% (v/v) and subsequently grown in the presence or absence of CMP-NANA. Bacteria incubated with heat inactivated serum (HIS) were used as controls. Proteins below the 40 kD marker were stained with Coomassie blue (“load”) and served as a loading control. B. LOS sialylation following growth of bacteria in NHS-Mg/EGTA. Sialylation of the LOS A2594 ΔfHbpΔNspA incubated with NHS-Mg/EGTA and then grown in the presence of CMP-NANA was confirmed by western blotting with mAb 3F11 that recognizes the unsialylated lacto-N-neotetraose LOS; mAb 3F11 does not recognize sialylated LOS. Proteins above the 50 kD marker were stained with Coomassie blue served as a loading control. C. LOS sialylation augments binding of full length fH to meningococci coated with C3 fragments. Aliquots of bacteria that were incubated with NHS-Mg/EGTA or HIS (controls) were grown either in the presence or absence of CMP-NANA, followed by incubation with purified human fH (10 μg/ml) or buffer alone (no fH). fH bound to bacteria was detected by flow cytometry using affinity-isolated polyclonal goat anti-human fH. The percentage of positive events in each sample was measured relative to control reaction (bacteria without C3, LOS sialic acid or added fH). Each bar represents the mean (SEM) of 5 independent observations. Comparisons across groups was made using 1-way ANOVA. D. Western blotting to demonstrate that LOS sialylation augments binding of full length factor H to meningococci coated with C3 fragments. Bacteria (A2594 ΔfHbpΔNspA) were coated with C3 fragments and incubated with human fH, as in C. Controls included bacteria that lacked C3 and/or added human fH. Bacteria were washed, lysed and proteins separated on a 4–12% Bis-Tris gel followed by western blotting. Proteins below the 50 kD marker were stained with Coomassie blue to measure bacterial lysate loading. This experiment was repeated thrice separately with similar results. E. LOS sialylation does not affect factor B binding to C3 fragments deposited on meningococci. Strain A2594 ΔfHbpΔNspA was incubated with NHS-Mg/EGTA (20%) that contained an anti-factor I mAb to decrease conversion of C3b to iC3b and then grown in media that either contained or lacked CMP-NANA as in C. Organisms incubated with heat-inactivated serum (HIS) served as a control. Bacteria were then incubated with purified factor B (fB) at concentrations of 3 μg/ml or 15 μg/ml and factor B bound to bacteria was measured by western blotting..
In the next experiment we sought to determine whether LOS sialic acid affected the interaction between factor B and bacteria-bound C3b. Strain A2594 ΔfHbpΔNspA was incubated with NHS-Mg/EGTA containing an anti-factor I mAb that blocked the function of factor I in order to limit conversion of C3b to iC3b. Bacteria were sialylated after C3 fragment deposition, as described above. The presence of intact C3b on the bacterial surface was confirmed by western blotting, demonstrating that anti-factor I reduced intensity of the 40 kD α2′ chain of iC3b and enhanced appearance of the 106 kD α′ C3b fragment (data not shown). Consistent with earlier work (32), binding of factor B to surface bound C3b was not affected by LOS sialylation at the two concentrations of factor B tested (3 and 15 μg/ml) (Fig. 3E). These data suggest that LOS sialic acid on meningococci bearing C3 fragments selectively enhances the binding of fH, but does not affect the interaction between factor B and C3b.
LOS inhibits the AP of nonhuman complement
Previous studies have shown that binding of fH to meningococcal fHbp and NspA is restricted to humans, which could contribute to the species-specificity of meningococcal infection. Group B strain H44/76 is rapidly cleared from the bloodstream of wild-type infant rats (23). Pre-incubation of bacteria from this strain with human fH enhanced bacteremia and decreased rat C3 deposition (23). A recent study demonstrated that H44/76 causes bacteremia in human fH transgenic rats (16). Interestingly, meningococci lacking expression of both fHbp and NspA (H44/76 ΔfHbpΔNspA) also caused bacteremia in human fH transgenic rats and survived in wild-type infant rat serum supplemented with human fH (33). In contrast, a fully encapsulated ΔfHbpΔNspAΔlst mutant that was unable to sialylate LOS or bind human fH via fHbp or NspA did not cause bacteremia.
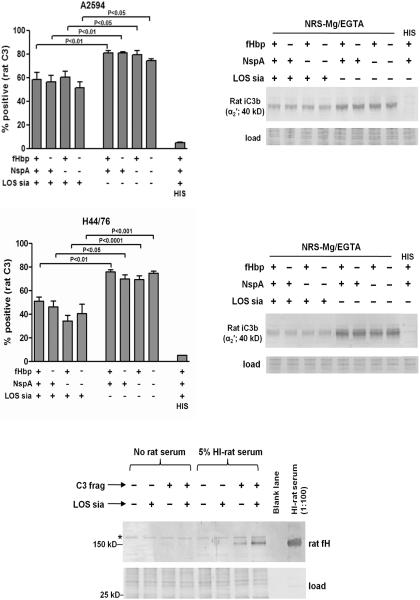
In the present study, we have demonstrated a decrease in AP-dependent human C3 fragment deposition mediated by meningococcal LOS sialic acid. We next asked whether this was also restricted to humans. Rat serum was used in the following species specificity experiments because of its relevance to the results described above on the effect of human fH on bacteremia in the transgenic infant rat model of meningococcal bacteremia (16). Strains A2594 and H44/76 and their ΔfHbp and/or ΔNspA mutants that differed in sialylation of their LOS were incubated with Mg/EGTA-treated adult rat serum and rat C3 fragment deposition on bacteria was measured by flow cytometry (Figs. 4A and 4C) and western blotting (Figs. 4B and 4D) under reducing conditions. In both strains, LOS sialylation of each of the mutants significantly decreased C3 deposition when compared to the corresponding mutant that lacked LOS sialic acid (Figs. 4A and 4C; see Supplemental Figure 3 for representative histograms). Consistent with the ability of meningococcal fHbp and NspA to bind only to human, but not to rat fH, the presence of either of these molecules did not affect AP-dependent rat C3 deposition on bacteria.
Figure 4.
LOS sialylation, but not fHbp or NspA, regulates the rat AP. A and C. Flow cytometry to show that LOS sialylation inhibits deposition of rat C3 fragments on A2594 (panel A) and H44/76 (panel C) and their mutant derivatives. Bacteria were incubated with Mg/EGTA-treated rat serum (20% (v/v)) for 30 min at 37 °C and rat C3 fragments deposited on bacteria were detected with FITC-conjugated anti-mouse C3. Each bar represents the mean (SEM) of at least 5 independent observations. Statistical analysis was performed using 1-way ANOVA. B and D. Western blotting to show that LOS sialic acid decreases rat C3 deposition on A2594 (panel B) and H44/76 (panel D). Bacteria were treated with normal rat serum (NRS) (20% (v/v)) containing 10 mM Mg and 10 mM Mg EGTA as described for A and C, washed and western blotted as described in Fig 2C and 2D. Rat C3 fragment reactive bands were detected using goat anti-mouse C3 followed by anti-goat IgG conjugated to alkaline phosphatase. Proteins that migrated faster than ~30 kD were stained with Coomassie blue and served as loading controls. E. LOS sialic acid enhances binding of rat fH to rat C3 fragments deposited on meningococci. Strain A2594 ΔfHbp ΔNspA was incubated with Mg/EGTA-treated normal rat serum to deposit C3 fragments (C3 frag “+”); heat-inactivated rat serum served as controls (C3 frag “−”). This was followed by growth in media that contained CMP-NANA (LOS sia “+”) or that lacked CMP-NANA (LOS sia “−”). Bacteria were then incubated in heat-inactivated rat serum (“5% HI-rat serum”) as a source of rat fH, or with HBSS (“No rat serum”). Bacteria-bound rat fH was detected by western blotting with polyclonal rabbit anti-mouse fH. The western blotting experiments were performed twice with similar results.
The polyclonal anti-mouse C3 antibody preferentially detects the ~40 kD α2′ fragment of rat iC3b, which is also part of the 105 kD α′ chain C3b. Thus, rat iC3b will migrate independently as a 40 kD band, while the ~105 kD α′ chain of deposited C3b will migrate covalently complexed with its target. Fig. 4B and Fig. 4D show deposition of rat C3 fragments on the mutants of A2594 and H44/76, respectively. The only prominent band detected was the ~40 kD α2′ fragment of iC3b which suggested that, as with human serum, most of the rat C3b deposited on meningococci was also converted to iC3b. In accordance with the flow cytometry data presented above, expression of fHbp and/or NspA did not affect deposition of rat C3 fragments on bacteria. However, absence of LOS sialic acid resulted in a uniform increase in rat C3 deposition on the wild-type strain and the three mutants of both A2594 and H44/76.
We next asked whether LOS sialic acid enhanced binding of rat fH (as supplied in 5% HI rat serum) to rat C3 fragments “pre-deposited” on meningococci as described above (Figs. 3C and 3D). As expected, binding of rat fH to meningococci in the absence of C3 deposition was not detected. Similar to observations with the human complement system (Fig. 3D), LOS sialylation increased the interactions between rat fH and rat C3 fragments deposited on strain A2594 ΔfHbpΔNspA (Fig. 4E). A meningococcal band that non-specifically reacted with polyclonal anti-mouse fH and migrated just above the rat fH band is indicated by the asterisk.
The LOS sialic acid–fH–C3 fragment interaction selectively involves the C-terminal domains of fH
A recent study by Kajander et al (34) proposed a model where the simultaneous binding of surface-bound C3 fragments to fH SCR domain 19 and glycosaminoglycans to fH SCR domain 20 contributed to AP inhibition and rendered a surface a complement non-activator.
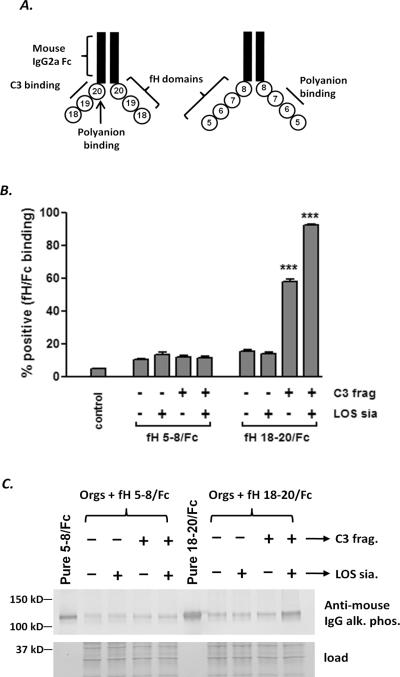
To assess whether the C-terminal domains of fH selectively interact with C3 fragments deposited on meningococci and LOS sialic acid, strain A2594 ΔfHbpΔNspA coated with C3 fragments and then sialylated as described above was incubated with a construct that contained human fH domains 18–20 fused to mouse IgG Fc (18–20/Fc). We also used a second construct that contains fH SCRs 5–8 fused to mouse IgG Fc (5–8/Fc). This construct binds to both fHbp and NspA (14, 35) and further, fH domains 7–8 contain a heparin binding site (36–37) and can interact weakly with C3b (37). Schematics of the recombinant fH/Fc fusion molecules used in this experiment and the ligands for the fH domains are shown in Fig. 5A. Consistent with the model proposed recently (34) and evidenced in Fig. 5B, the presence of bacteria-bound C3 fragments enhanced binding of fH 18–20/Fc, which further increased when LOS was also sialylated; binding of fH 5–8/Fc was not affected by LOS sialylation in the mutant strain A2594 ΔfHbpΔNspA (see Supplemental Figure 4 for representative histograms). The synergy between LOS sialic acid and C3 fragments in enhancing binding of the fusion protein containing the three C-terminal fH domains was also confirmed by western blotting (Fig. 5C). Taken together, these data suggest that LOS sialic acid and C3b on the meningococcal surface enhance fH binding through its C-terminal domains and may contribute to AP regulation by LOS sialylation.
Figure 5.
Meningococcal LOS sialylation selectively enhances the interaction of the C-terminal domains of human fH with C3 fragments on the bacterial surface. A. Schematic of two fH/Fc fusion proteins used in this study. Contiguous domains of human fH were fused to murine IgG2a Fc. The locations of the C3d binding regions (domains 19–20) and polyanion binding sites (domains 6, 7 and 20) in each of the molecules are indicated. B. LOS sialylation selectively enhances binding of fH 18–20/Fc to C3b/iC3b-coated meningococci. Bacteria (A2594 ΔfHbpΔNspA) were incubated with NHS-Mg/EGTA (20% (v/v)), sialylated as described in Fig. 3 and then incubated with either fH 5–8/Fc or fH 18–20/Fc (10 μg/ml of each protein). Bacteria incubated with HIS were used as controls. Bound fH/Fc was detected with anti-mouse IgG conjugated to FITC. The percentage of positive events was determined relatie to bacteria incubated with HIS alone. Each bar represents the mean (SEM) of 5 separate observations and data sets were compared by 1-way ANOVA. C. Western blotting to show that LOS sialic acid and C3 fragments together cooperate to enhance the interaction of the C-terminus of human fH with meningococci. Samples were prepared as in B, washed and western blotted and bound fH/Fc was detected with anti-mouse IgG conjugated to alkaline phosphatase. This experiment was performed twice with similar results.
Discussion
AP regulation on meningococci is a complex process that is modulated by several redundant mechanisms. The expression of groups B or C capsular polysaccharides (7–9), LOS sialic acid (10, 38), fHbp (12, 39–40) and NspA (14, 39, 41) all contribute to decreasing C3 fragment deposition on meningococci and enhance the organisms ability to resist killing by complement. In this study we have shown how LOS sialic acid, fHbp and NspA interact cooperatively to mitigate deposition of C3 fragments through the AP on encapsulated meningococci that express the lacto-N-neotetraose LOS species.
The role of meningococcal fHbp in complement regulation has received considerable attention in recent years because this molecule is a key component of two group B meningococcal vaccines that are currently undergoing pre-clinical trials in humans (42–43). fHbp binds to fH with affinity in the nM range (13, 44–45) and although different fHbp molecules bind to human fH with affinities that vary as much as 50-fold (45), these differences in affinity do not appear to contribute to bacterial survival in serum or blood (44–45). Rather, the amount of fHbp expressed by a strain may correlate directly with its contribution to serum resistance (39, 45). Similarly and consistent with recently published data (14, 39), the current results show that the amount of NspA expressed may also contribute to the extent of AP regulation by a strain. The high levels of NspA expressed by A2594 (Fig. 1) plays a key role in limiting AP activation on this strain (Fig. 2). Evidence for the importance of fHbp and NspA in complement evasion is further supported by the observation that the genes that encode both proteins are upregulated when bacteria are exposed to blood (39, 46), where they encounter high levels of complement.
A redundancy of complement inhibition mechanisms may also permit some strains to cause disease in the absence of one of these variables – for example, a recent study has identified strains from patients with invasive meningococcal infection that lack fHbp expression (47). Further, Welsch et al (48) have provided evidence for fHbp-independent survival of select meningococcal strains in whole blood. Loss of fHbp from strains such as NZ98/254 and 4243 has minimal impact on their survival in whole human blood, in contrast deleting fHbp from other strains, such as MC58 and H44/76, adversely affects bacterial survival in serum, as well as, whole blood (45, 48).
We previously showed that binding of fH to NspA on intact meningococci by flow cytometry was best observed when either the capsule was deleted and/or when the HepI glycan extensions of LOS were truncated (for example, when the L8 LOS immunotype was expressed) (14). However, the function of NspA in inhibiting the human AP is readily evident on encapsulated strains that express the lacto-N- neotetraose LOS species (Fig. 2). Consistent with this, a wild-type encapsulated meningococcal strain called 95N477 (B:2a:P1.2, cpx 11, ST 475) was recently shown to rely on NspA expression for survival in a whole blood bactericidal assay (39). Indeed, the current work shows that wild-type strain A2594 relies largely on NspA, more so than fHbp, for regulation of the AP even when capsule is expressed and when LOS elaborates unsialylated lacto-N-neotetraose species from HepI. Giuntini et al (41) showed that anti-fHbp mAbs that blocked fH binding to H44/76 were more bactericidal against H44/76 ΔNspA than against the wild-type parent strain, which underscores the role of NspA even in strains that express low levels of this protein. fH binding assays using flow cytometry likely underestimate the amount of fH bound to bacteria, perhaps because the number of fluorophores per bacterium on low fHbp expressers lies below the threshold of detection and/or because binding may in some cases be of lower affinity. Using the western blotting assays described in this study, we have readily detected fH binding to strains such as 2996 and 4243 that are low fHbp expressers and show barely detectable binding to fH by flow cytometry (data not shown). It is noteworthy that western blotting also reveals binding of pure fH to the A2594 ΔfHbpΔNspA double mutant (Fig. 3D). We have observed similar binding of fH to corresponding double mutants of strains H44/76, 2996, 4243 and NZ98/254 (data not shown). Recently, the ΔHbpΔNspA double mutant of group B strain H44/76 was shown to cause bacteremia in human fH transgenic rats (16); further, the addition of human fH to wildtype rat serum permitted survival of the double mutant in a bactericidal assay (16). Collectively the data indicate the presence of additional ligand(s) for fH that are distinct from fHbp and NspA on meningococci and contribute to evasion of innate immunity.
The data presented here demonstrate an important role for LOS sialylation in inhibition of the AP of complement. The molecular basis for complement regulation by LOS sialic acid on Neisseriae is multi-faceted and complex. In studies where all pathways of complement are intact, LOS sialylation has been shown to limit classical pathway activation (49–51). One study has suggested that LOS sialic acid may limit the binding of antibodies, in this instance the binding of specific monoclonal antibodies to gonococcal porin (52). LOS sialic acid may interfere with binding of C1q on the gonococcal surface (51). LOS is one of the bacterial targets for C3 and C4 (31, 53) and it is likely that the addition of a sialic acid residue may obscure targets for these complement components either on LOS itself or on other proximate molecules (54). Sialylation of the lacto-N-neotetraose LOS species appears to modulate binding of fH to `non-LOS' structures. As examples, LOS sialylation on gonococci increases the association of fH to gonococcal porin (55), while LOS sialylation on meningococci increases fH binding to NspA (14). Neisserial LOS sialylation also inhibits opsonophagocytosis by polymorphonuclear leukocytes (56–57). The importance of LOS sialylation in meningococcal pathogenesis is illustrated by the observation that most strains recovered from the bloodstream or CSF express the sialylated lacto-N- neotetraose LOS species; in contrast, carriage isolates often elaborate LOS immunotypes with more truncated glycan extensions from HepI (58).
Sialylation of host cells serves to limit activation of the AP (59–61), thereby limiting complement-mediated damage to host tissue. fH plays a key role in “self-nonself” discrimination (60, 62) and a recent study by Kajander and colleagues has provided mechanistic insights for this function. They proposed a model where the C-terminal domains 19 and 20 of fH interacted with surface-associated C3 fragments and glycosaminoglycans, respectively and facilitated regulation of the AP. In addition to Neisseria, other gram-negative bacteria such as Haemophilus influenzae and Campylobacter jejuni express LOS molecules that can be modified by sialic acid (63–64). Sialylated LOSs of these bacteria mimic gangliosides elaborated by their human hosts and contribute to limiting complement activation (63–64). Whether sialic acid on other bacterial species also enhances the interaction between fH and surface-bound C3 fragments remains to be determined.
Strain H44/76 does not cause disease in wild-type infant Wistar rats. Given that fHbp and NspA do not bind to rat fH, this suggests that regulation of the AP by LOS sialic acid alone in this context is insufficient for virulence (23). Further attenuation of the AP as occurs in the human fH transgenic rat permitted virulence when LOS was sialylated, even in the absence of fHbp and NspA expression, or in the Δlst mutant where LOS was not sialylated but human fH could interact with fHbp and NspA (16). Only when fHbp, NspA and LOS sialic acid were all deleted did the strain become avirulent in the human fH transgenic rat (16). For reasons not fully understood, meningococcal strains vary widely in their ability to cause bacteremia in rats. As an example, strain 4243 can disseminate into the bloodstream of wild-type infant Wistar rats following intraperitoneal inoculation (18, 65). The mechanisms of complement regulation by such strains may merit further investigation.
In conclusion, the current study has shed further light on the molecular basis of AP regulation on meningococci and provides a more detailed understanding of how these bacteria escape an important arm of innate immune defense. Elucidation of interdependent and redundant mechanisms of complement evasion will allow a better understanding of how escape variants may be selected under immune pressure, which may be an important consideration as newer vaccines targeting antigens such as fHbp are tested in humans.
Supplementary Material
Acknowledgements
We thank Dr. Ulrich Vogel, Universität Würzburg, Würzburg, Germany for providing strains A2594, H44/76 and H44/76 Δlst. We thank Dr. Wen-Chao Song (University of Pennsylvania) for providing polyclonal rabbit anti-mouse fH and Dr. Sunita Gulati (University of Massachusetts) for affinity purification of goat anti-human fH. We thank Dr. Dan M. Granoff (Childrens Hospital Oakland Research Institute, Oakland, CA), Dr. Jutamas Shaughnessy and Dr. Peter A. Rice (both at University of Massachusetts, Worcester, MA) for critically reading the manuscript and for helpful discussions. We thank Anthonia Beluchukwu and Joana Pedrosa for technical assistance.
This work was supported by National Institutes of Health grants AI054544, AI084048 and AI32725.
Abbreviations used in this paper
- LOS
lipooligosaccharide
- fH
factor H
- fHbp
factor H binding protein
- NspA
Neisserial surface protein A
- NHS
normal human serum
- HIS
heat-inactivated serum
- HBSS
Hanks Balanced Salt Solution
- lst
lipooligosaccharide sialyltransferase
- CMP-NANA
5' cytidinemonophospho-N-acetylneuraminic acid
- NRS
normal rat serum
- AP
alternative pathway
References
- 1.Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Res. 1993;12:295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- 2.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 2007;15:233–240. doi: 10.1016/j.tim.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis GA, Vedros NA. Sialic acid of group B Neisseria meningitidisregulates alternative complement pathway activation. Infect Immun. 1987;55:174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ram S, Lewis LA, Agarwal S. Meningococcal group W-135 and Y capsular polysaccharides paradoxically enhance activation of the alternative pathway of complement. J Biol Chem. 2011;286:8297–8307. doi: 10.1074/jbc.M110.184838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uria MJ, Zhang Q, Li Y, Chan A, Exley RM, Gollan B, Chan H, Feavers I, Yarwood A, Abad R, Borrow R, Fleck RA, Mulloy B, Vazquez JA, Tang CM. A generic mechanism in Neisseria meningitidis for enhanced resistance against bactericidal antibodies. J Exp Med. 2008;205:1423–1434. doi: 10.1084/jem.20072577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exley RM, Shaw J, Mowe E, Sun YH, West NP, Williamson M, Botto M, Smith H, Tang CM. Available carbon source influences the resistance of Neisseria meningitidis against complement. J Exp Med. 2005;201:1637–1645. doi: 10.1084/jem.20041548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel U, Claus H, Heinze G, Frosch M. Functional characterization of an isogenic meningococcal alpha-2,3- sialyltransferase mutant: the role of lipooligosaccharide sialylation for serum resistance in serogroup B meningococci. Med Microbiol Immunol (Berl) 1997;186:159–166. doi: 10.1007/s004300050059. [DOI] [PubMed] [Google Scholar]
- 12.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009 doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement. PLoS Pathog. 2010;6:e1001027. doi: 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira VP, Pangburn MK, Cortes C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 2010;47:2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vu DM, Shaughnessy J, Lewis LA, Ram S, Rice PA, Granoff DM. Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis. Infect Immun. 2011 doi: 10.1128/IAI.05604-11. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frasch C, Zollinger W, Poolman J. Proposed schema for identification of serotypes of Neisseria meningitidis. In: Schoolnik G, editor. The Pathogenic Neisseria. American Society for Microbioogy; Washington, D.C.: 1985. pp. 519–524. [Google Scholar]
- 18.Welsch JA, Rossi R, Comanducci M, Granoff DM. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol. 2004;172:5606–5615. doi: 10.4049/jimmunol.172.9.5606. [DOI] [PubMed] [Google Scholar]
- 19.Hou VC, Moe GR, Raad Z, Wuorimaa T, Granoff DM. Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies. Infect Immun. 2003;71:6844–6849. doi: 10.1128/IAI.71.12.6844-6849.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iida K, Mitomo K, Fujita T, Tamura N. Characterization of three monoclonal antibodies against C3 with selective specificities. Immunology. 1987;62:413–417. [PMC free article] [PubMed] [Google Scholar]
- 21.Apicella MA, Bennett KM, Hermerath CA, Roberts DE. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981;34:751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngampasutadol J, Ram S, Gulati S, Agarwal S, Li C, Visintin A, Monks B, Madico G, Rice PA. Human Factor H Interacts Selectively with Neisseria gonorrhoeae and Results in Species-Specific Complement Evasion. J Immunol. 2008;180:3426–3435. doi: 10.4049/jimmunol.180.5.3426. [DOI] [PubMed] [Google Scholar]
- 23.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77:764–769. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–799. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeil LK, Murphy E, Zhao XJ, Guttmann S, Harris S, Scott A, Tan C, Mack M, Dasilva I, Alexander K, Jiang HQ, Zhu D, Mininni T, Zlotnick GW, Hoiseth SK, Jones TR, Pride M, Jansen KU, Anderson A. Detection of LP2086 on the cell surface of Neisseria meningitidis and its accessibility in the presence of serogroup B capsular polysaccharide. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 26.Moe GR, Tan S, Granoff DM. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect Immun. 1999;67:5664–5675. doi: 10.1128/iai.67.11.5664-5675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahu A, Pangburn MK. Tyrosine is a potential site for covalent attachment of activated complement component C3. Mol Immunol. 1995;32:711–716. doi: 10.1016/0161-5890(95)98933-f. [DOI] [PubMed] [Google Scholar]
- 28.Venkatesh YP, Levine RP. The esterase-like activity of covalently bound human third complement protein. Mol Immunol. 1988;25:821–828. doi: 10.1016/0161-5890(88)90118-6. [DOI] [PubMed] [Google Scholar]
- 29.Venkatesh YP, Minich TM, Law SK, Levine RP. Natural release of covalently bound C3b from cell surfaces and the study of this phenomenon in the fluid-phase system. J Immunol. 1984;132:1435–1439. [PubMed] [Google Scholar]
- 30.Law SK, Dodds AW. The internal thioester and the covalent binding properties of the complement proteins C3 and C4. Protein Sci. 1997;6:263–274. doi: 10.1002/pro.5560060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis LA, Ram S, Prasad A, Gulati S, Getzlaff S, Blom AM, Vogel U, Rice PA. Defining targets for complement components C4b and C3b on the pathogenic neisseriae. Infect Immun. 2008;76:339–350. doi: 10.1128/IAI.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazatchkine MD, Fearon DT, Austen KF. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol. 1979;122:75–81. [PubMed] [Google Scholar]
- 33.Vu DM, Shaughnessy J, Lewis LA, Ram S, Rice PA, Granoff DM. Enhanced Bacteremia in Human Factor H Transgenic Rats Infected by Neisseria meningitidis. Infect Immun. 2011 doi: 10.1128/IAI.05604-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kajander T, Lehtinen MJ, Hyvarinen S, Bhattacharjee A, Leung E, Isenman DE, Meri S, Goldman A, Jokiranta TS. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc Natl Acad Sci U S A. 2011;108:2897–2902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaughnessy J, Lewis LA, Jarva H, Ram S. Functional comparison of the binding of factor H short consensus repeat 6 (SCR 6) to factor H binding protein from Neisseria meningitidis and the binding of factor H SCR 18 to 20 to Neisseria gonorrhoeae porin. Infect Immun. 2009;77:2094–2103. doi: 10.1128/IAI.01561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prosser BE, Johnson S, Roversi P, Herbert AP, Blaum BS, Tyrrell J, Jowitt TA, Clark SJ, Tarelli E, Uhrin D, Barlow PN, Sim RB, Day AJ, Lea SM. Structural basis for complement factor H linked age-related macular degeneration. J Exp Med. 2007;204:2277–2283. doi: 10.1084/jem.20071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt CQ, Herbert AP, Kavanagh D, Gandy C, Fenton CJ, Blaum BS, Lyon M, Uhrin D, Barlow PN. A new map of glycosaminoglycan and C3b binding sites on factor H. J Immunol. 2008;181:2610–2619. doi: 10.4049/jimmunol.181.4.2610. [DOI] [PubMed] [Google Scholar]
- 38.Vogel U, Hammerschmidt S, Frosch M. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med Microbiol Immunol (Berl) 1996;185:81–87. doi: 10.1007/s004300050018. [DOI] [PubMed] [Google Scholar]
- 39.Echenique-Rivera H, Muzzi A, Del Tordello E, Seib KL, Francois P, Rappuoli R, Pizza M, Serruto D. Transcriptome analysis of Neisseria meningitidis in human whole blood and mutagenesis studies identify virulence factors involved in blood survival. PLoS Pathog. 2011;7:e1002027. doi: 10.1371/journal.ppat.1002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seib KL, Serruto D, Oriente F, Delany I, Adu-Bobie J, Veggi D, Arico B, Rappuoli R, Pizza M. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect Immun. 2009;77:292–299. doi: 10.1128/IAI.01071-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giuntini S, Reason DC, Granoff DM. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun. 2011;79:3751–3759. doi: 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, Kieninger D, Prymula R, Dull P, Ypma E, Toneatto D, Kimura A, Pollard AJ. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307:573–582. doi: 10.1001/jama.2012.85. [DOI] [PubMed] [Google Scholar]
- 43.Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, Scott AA, Alexander K, Mason K, Miller L, DaSilva I, Mack M, Zhao XJ, Pride MW, Andrew L, Murphy E, Hagen M, French R, Arora A, Jones TR, Jansen KU, Zlotnick GW, Anderson AS. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28:6086–6093. doi: 10.1016/j.vaccine.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 44.Dunphy KY, Beernink PT, Brogioni B, Granoff DM. Effect of factor H-binding protein sequence variation on factor H binding and survival of Neisseria meningitidis in human blood. Infect Immun. 2011;79:353–359. doi: 10.1128/IAI.00849-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seib KL, Brunelli B, Brogioni B, Palumbo E, Bambini S, Muzzi A, DiMarcello F, Marchi S, van der Ende A, Arico B, Savino S, Scarselli M, Comanducci M, Rappuoli R, Giuliani MM, Pizza M. Characterization of diverse subvariants of the meningococcal factor H (fH) binding protein for their ability to bind fH, to mediate serum resistance, and to induce bactericidal antibodies. Infect Immun. 2011;79:970–981. doi: 10.1128/IAI.00891-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph B, Dondrup M, Schwarz R, Neuweger H, Jaenicke S, Goesmann A, Schneiker-Bekel S, Becker A, Frosch M, Schoen C. Differential genome expression of serogroup B meningococci under in vivo mimicking conditions. 17th International Pathogenic Neisseria conference; Banff, Alberta, Canada. 2010. p. 48. [Google Scholar]
- 47.Lucidarme J, Tan L, Exley RM, Findlow J, Borrow R, Tang CM. Characterization of Neisseria meningitidis isolates that do not express the virulence factor and vaccine antigen factor H binding protein. Clin Vaccine Immunol. 2011;18:1002–1014. doi: 10.1128/CVI.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welsch JA, Ram S, Koeberling O, Granoff DM. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis. 2008;197:1053–1061. doi: 10.1086/528994. [DOI] [PubMed] [Google Scholar]
- 49.McQuillen DP, Gulati S, Ram S, Turner AK, Jani DB, Heeren TC, Rice PA. Complement processing and immunoglobulin binding to Neisseria gonorrhoeae determined in vitro simulates in vivo effects. J Infect Dis. 1999;179:124–135. doi: 10.1086/314545. [DOI] [PubMed] [Google Scholar]
- 50.Ram S, Cox AD, Wright JC, Vogel U, Getzlaff S, Boden R, Li J, Plested JS, Meri S, Gulati S, Stein DC, Richards JC, Moxon ER, Rice PA. Neisserial lipooligosaccharide is a target for complement component C4b: Inner core phosphoethanolamine residues define C4b linkage specificity. J Biol Chem. 2003;278:50853–50862. doi: 10.1074/jbc.M308364200. [DOI] [PubMed] [Google Scholar]
- 51.Zaleski A, Densen P. Sialylation of LOS inhibits gonococcal killing primarily through an effect on classical pathway activation. In: Zollinger WD, Frasch CE, Deal CD, editors. Abstracts of the Tenth International Pathogenic Neisseria Conference; Baltimore, MD: National Institutes of Health; 1996. p. 114. [Google Scholar]
- 52.Elkins C, Carbonetti NH, Varela VA, Stirewalt D, Klapper DG, Sparling PF. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol Microbiol. 1992;6:2617–2628. doi: 10.1111/j.1365-2958.1992.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 53.Edwards JL, Apicella MA. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell Microbiol. 2002;4:585–598. doi: 10.1046/j.1462-5822.2002.00212.x. [DOI] [PubMed] [Google Scholar]
- 54.Estabrook MM, Griffiss JM, Jarvis GA. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65:4436–4444. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madico G, Ngampasutadol J, Gulati S, Vogel U, Rice PA, Ram S. Factor H Binding and Function in Sialylated Pathogenic Neisseriae is Influenced by Gonococcal, but Not Meningococcal, Porin. J Immunol. 2007;178:4489–4497. doi: 10.4049/jimmunol.178.7.4489. [DOI] [PubMed] [Google Scholar]
- 56.Estabrook MM, Christopher NC, Griffiss JM, Baker CJ, Mandrell RE. Sialylation and human neutrophil killing of group C Neisseria meningitidis. J Infect Dis. 1992;166:1079–1088. doi: 10.1093/infdis/166.5.1079. [DOI] [PubMed] [Google Scholar]
- 57.Kim JJ, Zhou D, Mandrell RE, Griffiss JM. Effect of exogenous sialylation of the lipooligosaccharide of Neisseria gonorrhoeae on opsonophagocytosis. Infect Immun. 1992;60:4439–4442. doi: 10.1128/iai.60.10.4439-4442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones DM, Borrow R, Fox AJ, Gray S, Cartwright KA, Poolman JT. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb Pathog. 1992;13:219–224. doi: 10.1016/0882-4010(92)90022-g. [DOI] [PubMed] [Google Scholar]
- 59.Fearon DT. Regulation by membrane sialic acid of beta1H-dependent decay- dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978;75:1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc. Natl. Acad. Sci. U S A. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pangburn MK, Muller-Eberhard HJ. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978;75:2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pangburn MK. Cutting edge: localization of the host recognition functions of complement factor H at the carboxyl-terminal: implications for hemolytic uremic syndrome. J Immunol. 2002;169:4702–4706. doi: 10.4049/jimmunol.169.9.4702. [DOI] [PubMed] [Google Scholar]
- 63.Mandrell RE, Apicella MA. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402. doi: 10.1016/S0171-2985(11)80352-9. [DOI] [PubMed] [Google Scholar]
- 64.Moran AP, Prendergast MM, Appelmelk BJ. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol. 1996;16:105–115. doi: 10.1111/j.1574-695X.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 65.Granoff DM, Morgan A, Welsch JA. Persistence of group C anticapsular antibodies two to three years after immunization with an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J. 2005;24:132–136. doi: 10.1097/01.inf.0000151035.64356.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.