Abstract
Conventional antimicrobial strategies have become increasingly ineffective due to the emergence of multidrug resistance among pathogenic microorganisms. The need to overcome these deficiencies has triggered the exploration of alternative treatments and unconventional approaches towards controlling microbial infections. Photodynamic therapy was originally established as an anti-cancer modality and is currently used in the treatment of age related macular degeneration. The concept of photodynamic inactivation requires cell exposure to light energy, typically wavelengths in the visible region that causes the excitation of photosensitizer molecules either exogenous or endogenous, which results in the production of reactive oxygen species. ROS produce cell inactivation and death through modification of intracellular components. The versatile characteristics of PDT prompted its investigation as an anti-infective discovery platform. Advances in understanding of microbial physiology have shed light on a series of pathways, and phenotypes that serve as putative targets for antimicrobial drug discovery. Investigations of these phenotypic elements in concert with PDT have been reported focused on multidrug efflux systems, biofilms, virulence and pathogenesis determinants. In many instances the results are promising but only preliminary and require further investigation. This review discusses the different antimicrobial PDT strategies and highlights the need for highly informative and comprehensive discovery approaches.
Introduction
The 20th century saw the discovery of antibiotics and led to a wide array of successful methods for preventing and controlling infectious diseases. This fostered a mindset that the war against infectious microbes had been won and research efforts were shifted to more pressing matters such as cancer, diabetes, and heart disease. In the 1980s the consensus among pharmaceutical companies was that there were enough antibiotics already on the pharmacy shelf and they began to redirect their research efforts accordingly [1]. Optimism transformed into skepticism, however as outbreaks and epidemics of new, re-emerging, and drug--resistant infections arose. The microorganisms responsible for these infections possessed effective and dynamic pathogenic capabilities and gave rise to the term “superbugs”. Infectious diseases in the twenty-first century continue to be a dangerous threat. Each year over 13 million deaths worldwide are attributed to the emergence of new infectious diseases or to the re-emergence of diseases previously thought to be under control.
Addressing this challenge requires rational as well as unconventional antimicrobial discovery efforts [1]. A prominent player in these efforts is likely to be the light-based technology known as antimicrobial photodynamic inactivation or photodynamic therapy [2], which uses harmless visible light in combination with non-toxic photosensitizers to control infections. Antimicrobial PDT was accidentally discovered over 100 years ago with the observation that Paramecium spp. protozoans stained with acridine orange died upon exposure to bright light[3]Historically PDT has been more prominent in the cancer setting and is currently used for the treatment of age-related macular degeneration.[4] Recent years have seen the migration of PDT research efforts and ophthalmology settings towards being used as a discovery and treatment alternative for localized infections [5].
PDT involves the use of harmless visible light combined with a light-sensitive dye – the photosensitizer – and oxygen present in and around cells. After illumination with the light of the appropriate wavelength, the PS is energized to an excited state that can undergo molecular collisions with oxygen, resulting in the formation of reactive oxygen species (ROS), including singlet oxygen by energy transfer or hydroxyl radicals by electron transfer. The high selectivity of PDT for rapidly growing and thus hyperproliferating malignant cells [6] suggested it should be useful for microbial cell destruction [7]. Studies of antimicrobial PDT have focused on: (i) exploring the photophysical and photochemical properties of the approach (ii) exploring chemical properties to develop more effective and clinically compatible PSs (iii) bypassing the microbial permeability barrier and investing in novel delivery methodologies (iv) preclinical and clinical investigations of PDT applications.
PSs are usually organic aromatic molecules with a high degree of electron delocalization. They contain a central chromophore with auxiliary branches (auxochromes) which add further electron delocalization to the PS and thus alter the absorption spectra [8]. Porphyrins, chlorins, bacteriochlorins, phthalocyanines as well as a plethora of dyes with different molecular frameworks have been proposed as antimicrobial PSs [9,10]. These dyes include halogenated xanthenes (e.g. Rose Bengal (RB)), [11] perylenequinones (e.g. hypericin) [12], phenothiazinium salts, (e.g. toluidine blue O (TBO) and methylene blue (MB)) [13], cationic fullerenes (e.g. derivatives of C60), [14, 15] and psoralens (e.g. furanocoumarins) [16].
In just 20 years antimicrobial PDT has emerged as a discovery and development platform inspiring a proliferation of light-based antimicrobial explorations worldwide. However, the potential for microbial resistance development using PDT remains under-investigated. Studies of resistance have been sporadic but they are rapidly increasing, with recent reports examining key elements of the microbial phenotype. These include multidrug efflux systems, biofilm, spore formation, virulence and pathogenicity determinants. The emerging consensus is that the effectiveness of PDT may be profoundly impacted by all these systems, but the exact mechanisms of these effects remain elusive. This review aims to summarize and provide critical commentary around these aspects of antimicrobial PDT. It also aims to highlight the mechanistic similarities and differences between PDI and conventional antimicrobials. Collecting this diverse information may transform PDI from an alternative discovery platform to a dynamic anti-infective countermeasure.
Efflux and Antimicrobial PDT
Efflux mechanisms are major components of resistance to many classes of antimicrobials as well as chemotherapeutic agents [17]. Efflux results from the activity of membrane transporter proteins, widely known as multidrug efflux systems (MES) [18, 19]. These systems perform essential roles in cellular metabolism and they differ in membrane topology, energy coupling mechanisms, and, most importantly, in substrate specificities [20]. Identifying natural substrates and inhibitors of efflux systems is an active and expanding research topic [21].
Based on their sequence similarity, efflux systems were classified into the following six super-families: ATP-binding cassettes (ABC), major facilitators (MFS), resistance-nodulation cell division (RND), small multidrug resistance family (SMR), multi-antimicrobial extrusion protein family and multidrug endosomal transporters (MET). The first five families were found in microorganisms while the MET family appears to be restricted to higher eukaryotes. Representatives of all groups are expressed in mammalian cells [22]. ABC transporters are the largest super family, containing seven subfamilies designated A to G based on sequence and structural homology [23]. The best-studied families of fungal MES are from Saccharomyces cerevisiae, especially those responsible for pleiotropic drug resistance (PDR). Members of this family are highly conserved and are often responsible for drug resistance among pathogenic fungal species [24, 25].
A challenging clinical scenario involves MES in Pseudomonas aeruginosa [26]. Sequence analysis of the P. aeruginosa genome has revealed the presence of MES from all five super families, with the largest number of predicted pumps, a total of 12, belonging to the RND family [27]. X-ray crystal structures of most transporter families were reported in a variety of organisms [28–36],
Studies into the effects of efflux in antimicrobial PDT have only recently commenced. Structural similarities exist between efflux substrates and a number of PSs, most notably their amphipathic nature. The participation of MES in PS mediated PDT has been observed with ABC mammalian systems. Primary evidence came initially from investigations with porphyrins and the system ABCG2 (or Breast Cancer Resistance Protein BCRP). Transport of phytoporphyrin (phylloerythrin) was blocked by the ABCG2-specific inhibitor fumitremorgin C (FTC) in human embryonic kidney cells transfected with full length human ABCG2 [37, 38]. Serum-dependent export of protoporphyrin IX by ABCG2 in T24 cells was also demonstrated [39]. In a more comprehensive study a series of conjugates of substrate PSs with varying groups attached to different positions on the tetrapyrrole macrocycle were designed. Pyropheophorbides and purpurinimides were found to be substrates for ABCG2 which affected the phototoxic response of a side population of stem cell-like cancer cells to PDT [40]. This was also the case for hypericin and ABG2 and ABCC1 (or multidrug resistance-associated protein 1, MRP1) where both systems affected the outcome of hypericin-mediated PDT in HT-29 adenocarcinoma cells [41]. In these two systems it is clear that MES affect PDT for a variety of PS chemotypes. In contrast, for ABCB1 (P-glycoprotein, P-gp) the evidence for PS substrates is sporadic and contradictory. For example, it has been shown that the multidrug resistance modulator and Cyclosporine A Analogue SDZ-PSC 833 potentiates the photodynamic activity of chlorin e6 independently of P-gp in multidrug resistant human breast adenocarcinoma cells [42]. Furthermore, psoralen inhibits the function of the transporter in the dark [43]. A hypericin-mitoxantrone (MTZ, chemotherapeutic) cocktail plus illumination with blue light potentiates cytotoxicity in bladder and breast cancer cells that -overexpress P-gp [44].
Phenothiazinium dyes MB and TBO are amphipathic cations and physicochemically similar to the antibacterial alkaloid berberine, a well-characterized substrate of MFS efflux systems in Gram-positive bacteria [45, 46]. This raised the possibility that phenothiazinium PSs could also be substrates of microbial efflux systems. Recent experimental evidence indicated that phenothiaziniums were NorA (MFS) substrates in Staphylococcus aureus and possibly MexAB (RND) substrates in P. aeruginosa [47]. This evidence was not supported by a model study using 60 P. aeruginosa clinical isolates overexpressing efflux systems it was demonstrated that antibiotic-resistant P. aeruginosa cells are as susceptible to TBO-mediated PDI as susceptible strains [48].
The observation that ABC transporters and not MFS affect MB-mediated PDI in the pathogenic yeast Candida albicans is perplexing [47, 49]. Furthermore, the structurally related phenothiazines thioridazine and chromazine have been characterized as inhibitors as opposed to substrates of a variety of pathogen efflux systems [50–52]. A recent study identified a sigma factor network responsive to cell-envelope damage by thioridazine in Mycobacterium tuberculosis suggesting roles other than efflux inhibition or anti-mycobacterial activity for the compound [53]. One plausible explanation for this complex behavior comes from the promiscuous substrate specificities of efflux systems. For example, clinically important PDR transporters include C. albicans Cdr1p (CaCdr1p) and CaCdr2p, which are orthologs of S. cerevisiae Pdr5p (ScPdr5p) and mammalian ABCG2 and ABCC1 transporters. Fungal PDR efflux pumps have relatively promiscuous substrate specificities that are thought to be defined primarily by their transmembrane domains. These specificities often partially overlap among family members in a particular organism and thus provide broad-spectrum protection against xenobiotic threat, including that posed by the widely-used and well-tolerated azole and triazole drugs. Both RND and ABC systems expel a wealth of potential substrates. This overlap in substrate specificity highlights the obstacles to blocking pumps efficiently.
The well documented promiscuity may explain the additional scattered reports for the interaction of porphyrins with microbial efflux systems. Porphyrin uptake and efflux seem to be regulated by the TolC system in E coli [54]. In Streptococcus agalactiae, two coregulated efflux transporters modulate intracellular heme and protoporphyrin IX availability [55]. In contrast the PDI pattern of amphipilic protoporphyrin diarginate PPArg in a variety of efflux related S. aureus strains showed no correlation for the PS with MES [56].
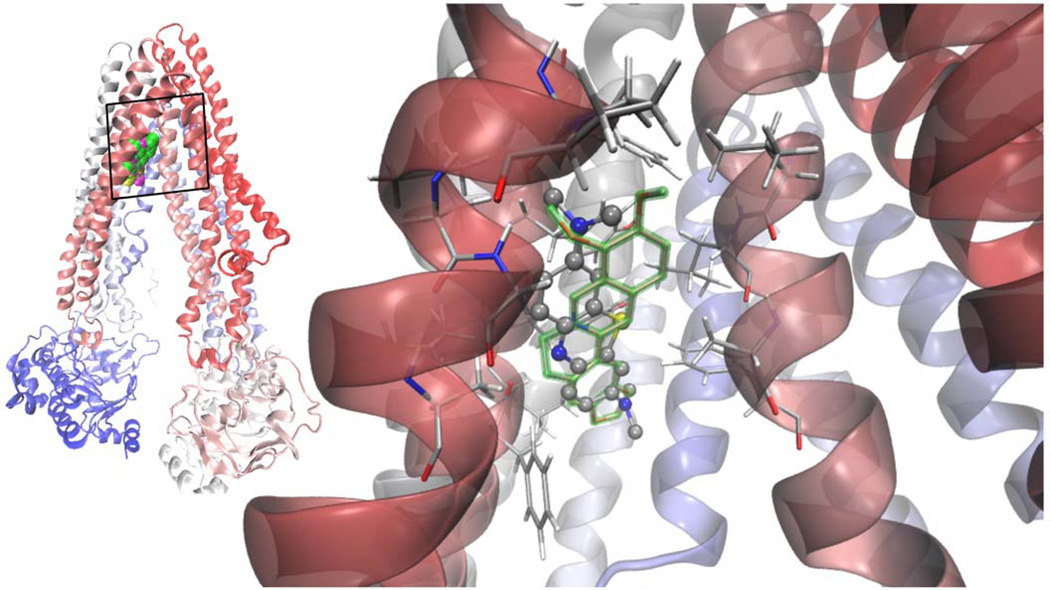
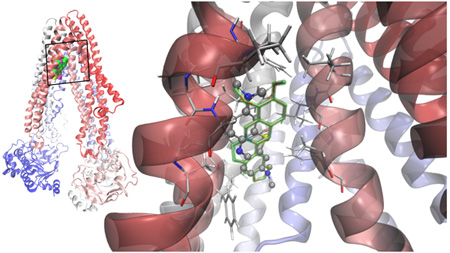
This wealth of contradicting information prompt us to apply a combination of first principles Quantum Mechanics calculations (QM) together with a docking protocol to understand whether the two phenothiazinium dyes MB and TBO are a priori candidates of being substrates of an ABC transporter. We used the ABCB1 mammalIan P-gp as a paradigmatic structural model. Despite its homology with other yeast and bacterial ABC transporters, the mouse P-gp is the only eukaryotic ABC experimental structure [30], crystallized in a drug-binding competent state [57]. We compared the docking properties of phenothiaznium dyes with berberine. The methodology and results are summarized on Figure 2 and Table 2. It was found that MB and berberine have a strong binding affinity with ABCB1 where as TBO showed less affinity. TBO had also a second binding site with less than 1 kcal/mol of difference in energy with respect to the main site. Both dyes share the site with berberine (Figure 2), in their lowest and most populated cluster of conformations. This pocket involves hydrophobic and aromatic residues mainly from TM 4, 5 and 6.
Figure 2.
Left: superimposed Van der Waals (VdW) models of the lowest energy docked structures of berberine [75] TBO (yellow) and MB (magenta) in apex of the inverted "V" depicted by the transmembrane α-helices 4 and 6 as they separate from top to bottom (in this orientation the extracellular side at the top; cartoon representation of the protein colored according to the sequence, from red to blue). Right: MB showed a clear specificity for the site overlapping with berberine, the calculated binding energies being practically indistinguishable. The hydrophobic pocket of negative electrostatic potential is rich in hydrophobic and aromatic residues, mainly form helices 4, 5 and 6.
The structure of the dyes were optimized with the method for fundamental vibrational frequencies B3LYP/6-311+G(d,p level of theory using the gaussian03 [129] package in a model polar solvent (PCM acetonitrile) [130]. The structures were docked onto the whole chamber formed by the twelve Transmembrane Domains (TMs) of the protein at the height to which they span the cell membrane in order to locate their binding sites, using a Lamarckian genetic algorithm implemented in Autodock 4.2.3. Afterwards, the whole protocol was applied in a smaller region comprising their main binding site. Their highly delocalized charge distribution was modeled from the QM results instead of the default program charges [131].
Table 2.
Summary of molecular docking results for MB, TBO compared to berberine.
| Compound | Binding Energy (kcal/mol) |
|---|---|
| Berberine | −5.38 |
| MB | −5.29 |
| TBO | −4.40 |
The use of small molecules known as multidrug efflux pump inhibitors (EPIs) that block MES in combination with conventional antibiotics has been proposed as an approach for circumventing efflux-mediated antimicrobial resistance. Biochemical approaches have yielded a number of promising EPIs in several pathogenic systems [58]. This concept of synergistic action has been exploited in PDT to potentiate the phototoxic action of phenothiazinium PSs [59]. The PDT effect of MB or TBO was substantially enhanced by small molecule EPIs in S. aureus that affect NorA as assessed by both reduction of viable cells and fluorescent dye accumulation. The potentiation is less pronounced against P. aeruginosa with MexAB.
It has been shown that near-infrared light can cause selective photodamage to multidrug resistant pathogens [60]. In a recent study, it has been demonstrated that photodamage of multidrug-resistant Gram-positive and Gram-negative bacteria by near infrared (870 nm/930 nm) light potentiated the action of erythromycin, tetracycline and ciprofloxacin [61]. Although the antibiotics used in this study are MES substrates, and it is therefore reasonable to assume that near infrared light may play role in efflux inhibition, the experimental evidence is weak and this requires further exploration. The potentiation mechanism is hypothetical at this stage and not clearly distinct from PDI, as it may involve an optically mediated mechano-transduction of cellular redox pathways, decreasing DeltaPsi and increasing ROS.
Development of Microbial Resistance to PDT and analogies with bacteriocidal antibiotics
The first step towards addressing microbial resistance development to PDT is understanding the mechanisms and their implications. PDT leads to the production of singlet oxygen and other ROS through a variety of photochemical mechanisms resulting in cell death. This mechanism is by default analogous to bacteriocidal antibiotics but recent reports suggested that the analogy may go much deeper. It has now been demonstrated that the three major classes of bactericidal antibiotics (quinolones, b-lactams and aminoglycosides) regardless of drug-target interaction, stimulate the production of hydroxyl radicals in Gram-negative and Gram-positive bacteria, which ultimately contribute to cell death [62]. In the same report it was observed that bacteriostatic drugs do not produce hydroxyl radicals. The mechanism of hydroxyl radical formation induced by bactericidal antibiotics is the end product of an oxidative damage cellular death pathway involving the tricarboxylic acid cycle, a transient depletion of NADH, destabilization of iron-sulfur clusters, and stimulation of Fenton chemistry. The same group at Boston University employed systems-level approaches and phenotypic analyses to elucidate the pathway by which the aminoglycosides kanamycin and gentamycin ultimately trigger hydroxyl radical formation. In brief, the pathway involves mistranslation and misfolding of membrane proteins and signaling through the envelope stress-response two-component system, with the redox-responsive two-component system playing an associated role [63].
As three of the main resistant components to antimicrobials (permeability barrier, multidrug efflux systems and drug availability) have been extensively explored, the focus had naturally shifted to the remaining two, target and pathway modification. The non-selective nature of PDT and the lack of specific molecular targets for the ROS produced during PDT means that it is unlikely that a specific microbial resistance pathway could develop. Reports discussing the potential for microbes to develop resistance to PDT are scattered and provided no clear conclusions. In a study of routine stress exposure followed by re growth, the photosensitizer 5,10,15-tris(1-methylpyridinium-4-yl)-20-(pentafluorophenyl)-porphyrin triiodide (Tri-Py(+)-Me-PF) was employed against Vibrio fischeri and E. coli model cells. After ten cycles of partial inactivation followed by regrowth, neither of the bacteria developed resistance to the photodynamic process [64]. Giulianai et al subjected P. aeruginosa, S. aureus and C. albicans to 20 consecutive PDI treatments with cationic Zn(II) phthalocyanine RLP068/Cl, but did not find any resistant mutants [65]. Only S. aureus showed increased MIC in dark conditions, but even in this case, the susceptibility of the mutated bacteria to PDI was not affected by their MIC increase.
Up regulation of the key oxidative stress enzyme superoxide dismutase has been observed following protoporphyrin-mediated PDT in S. aureus and RB-mediated PDT in S. mutans. This correlated with induction of GroEL, the bacterial heat shock protein responsible for refolding denatured proteins and stabilizing lipid membranes during stress [66]. Expression of the bacterial heat shock protein Dnak was also increased after sub-lethal PDI stress [67]. In the same study, heat pre-treatment (a positive up regulator) prior to PDI for E. coli and Enterococcus faecalis conferred stress tolerance, increasing E. coli cell viability by 2log10 and E. faecalis cell viability by 4log10. PDI with RB in the yeast S. cerevisae demonstrated a role for Yap1p and Skn7p in defense against singlet oxygen insult [68].
Efflux Pump Inhibitor-Photosensitizer Hybrids and their Potential use in PDT
The interaction between efflux systems and some but not all the molecular classes of PS was documented. The potentiation of PS mediated PDI by EPIs is emphatically demonstrated at least for Gram-positive bacteria and fungi. As MES also play a role in invasion, adherence and colonization by microbial cells, a PDT-EPI based combination approach may in some cases reduce bacterial virulence in vivo. A major obstacle, however, may arise from the fact that efflux systems manipulation could cause unexpected toxicities due to the multitude of physiological roles MES may play in human cells. In this context, efforts directed at specifically inhibiting efflux pumps operating only in prokaryotes may offer a greater chance of therapeutic success. Interestingly, it has been shown that target bacteria respond to clinical challenge with EPIs by developing resistance mutations that decrease the efficacy of the EPI [69, 70]. Recently it was demonstrated that reserpine can select multidrug resistant Strepotococcus pneumoniae strains [71].
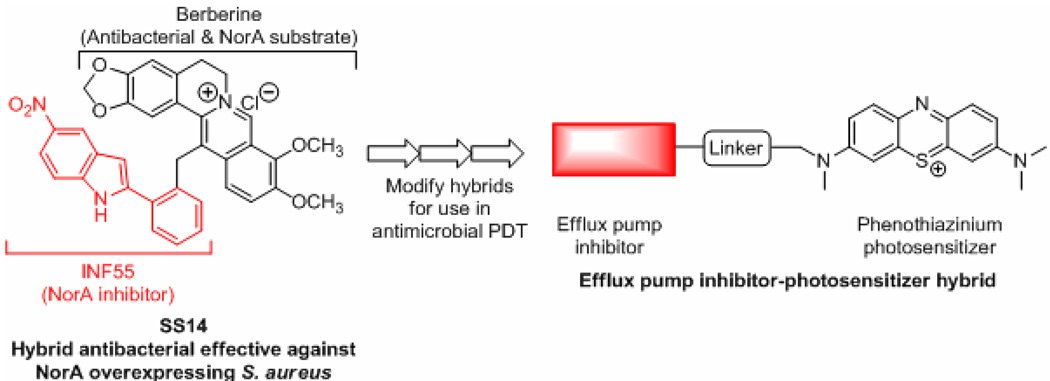
The threat of cross-resistance to various different antibiotics elevates the complexity of EPI discovery ventures. Addressing this requires that efflux substrates and inhibitors be clearly differentiated, particularly with respect to PS for use in antimicrobial PDT. It also demands rational approaches that simultaneously address both the photochemical mechanistic aspects of PDT and efflux phenotypic variations. A reported strategy of “dual antimicrobial action” targeting NorA in Gram-positives may serve as a useful precedent for addressing these issues. A hybrid compound (SS14) created by fusing the plant antimicrobial berberine to the synthetic NorA EPI INF55 was found to be an effective antimicrobial against S. aureus, including mutant strains that over express NorA [72]. MIC’s for SS14 against S. aureus were 2–16 times lower than berberine and combinations of berberine with INF55. The hybrid rapidly accumulated in bacterial cells and showed higher efficacy than vancomycin in a Caenorhabditis elegans model of enterococcal infection [72]. Analogs of SS14 exhibited similar antimicrobial activities [73, 74] suggesting that significant structural changes can be made to these hybrids without adversely affecting their ability to block MES or their antibacterial activity. Such hybrids are predicted to have an advantage over separate compound administration in terms of synchronous or near synchronous delivery of both agents to the appropriate bacterial target sites.
The physicochemical similarites between berberine and the phenothiazinium photosensitizers MB and TBO (i.e. both amphipatic cations) and evidence that they are overlapping substrates for various efflux pumps (e.g. NorA [47,59]) suggested that an analogous strategy might be successfully applied in antimicrobial PDT. For example, hybrids which link EPIs to phenothiaziniums may decrease efflux of the PS leading to increased microbial cell killing upon illumination Hybrids of this type are currently under investigation in our laboratories (Figure 3).
Figure 3.
Extension of the hybrid antimicrobial approach [72, 74] to compounds for use in antimicrobial PDT
Biofilm Inactivation
Chronic infections are most often associated with the formation of biofilms, [75, 76]. The dense protective environment of biofilms along with the significant differences in properties compared to free-floating or planktonic bacteria of the same species have been implicated to confer biofilm bacteria with as much as 1000-fold higher resistance to detergents, antiseptics and antibiotics [77]. The eradication of microbial biofilms remains a key challenge in the antimicrobial discovery arena and new discoveries are required to address a number of clinical conditions. PDT studies have been explored to some degree as an alternative treatment for several recalcitrant infections. For example, PDT has been used to target dental plaques, [78] periodontitis, [79] gingivitis, endodontics, [80] osteomyelitis [81], infections in cystic fibrosis, [82] infections of permanent indwelling devices such as joint prostheses and heart valves and implants [83] and oral candidiasis [84]. Peri-implantitis involves the biofilm colonization, of implant surfaces and may lead to patient infection and damage to the implant surface. Dörtbudak et al. used TBO PDT to successfully decontaminate implants with bacterial colonization in 15 patients, leading to the reduction in bacterial counts by approximately 2 log10,[85].
It is important to compare PDI with conventional antibiotics both in terms of mechanism and efficacy when targeting biofilms. Biofilms generally do not restrict penetration of antibiotics [86], but they do form a barrier to the larger components of the immune system [87–89]. There is a wealth of literature describing PDT-based anti-biofilm strategies which focuses mostly on the use of different PSs against a variety of microbial species [90]. In contrast there are only a limited number of studies exploring the effects of PDT on phenotypic biofilm elements (e.g. adhesins). Moreover, there is no consensus as to which is the most reliable model for evaluating PDT efficacy against biofilms. The majority of published reports use methodologies where biofilms are grown in/on plastic or silicon microtiter plates and surfaces. These bioassays have been repetitively criticized for lack of robustness and occasionally yield inconsistent results. A very recent report [91] discussed the impact of PDT on the viability of Streptococcus mutans cells in an artificial biofilm model that used sterile chambered cover glasses, to form a salivary pellicle layer. PDT using phenothiazine chloride and red laser gave a significant reduction of biofilm bacterial viability as measured by Live-Dead assay. PDT studies with clinically relevant multi-species biofilms are virtually non-existent with the notable exception being studies of dental plaque-derived biofilms and endodontic polymicrobial infections in vitro [92, 93].
By using isogenic pairs of wild-type and transposon mutants of Staphylococcus epidermidis and S. aureus deficient in capsular polysaccharide and slime production it has been shown that the cationic PSs pL-c(e6) and MB can overcome the protective effect of extracellular slime and stationary bacterial growth to PDI [94]. TBO has a substantial impact on PDI of staphylococcal biofilms which it decreases cell numbers (5log10 after irradiation with red light), disrupts biofilm architecture and damages bacterial cell membranes [95]. PDI with merocyanine 540 has a comparable effect on the viability of biofilms from Gram-positive pathogens when 400 J/cm2 green light is used [96, 97]. Tri-meso (N-methyl-pyridyl), meso (N-tetradecyl-pyridyl) porphine (C14) has a significantly greater PDI effect in eradicating S. epidermidis biofilms compared with the parent tetra-substituted N-methyl-pyridyl-porphine (C1) [98]. TBO mediated PDI affects Streptococcus mutans biofilms in different stages of maturity (4 log10 with red light for mature biofilms) [99] as well as mature S. sobrinus and S. sanguinis biofilms [100]. Erythrosine was found to inactivate S, mutans biofilms better than MB and protoporphyrin with the effect enhanced to 2 log10 by light fractionation [101, 102]. Erythrosine is also more potent than MB against Aggregatibacter actinomycetemcomitans biofilms as are the anionic PSs RB and TBO [103, 104]. PDT with 5-ALA and TMP at different concentrations can inactivate P. aeruginosa biofilms [105, 106].
Although the PDT results in many systems seem promising they don’t address the central problem. The paradox of chronic biofilm infections is that they are often unresponsive to antimicrobial therapy even when caused by a pathogen that is not resistant to the antimicrobial agent. The simple explanation is that the agent fails to effectively reach at least some cells in vivo, resulting in a relapsing infection [107]. As light delivery is location specific and dependent this has been implied in a few PDT explorations. For example Helicobacter pylori, a Gram-negative spiral bacterium that forms biofilms on the gastric mucosae, naturally accumulates porphyrins, which may then act as endogenous PSs [108]. In H. pylori infected patients the application of 405 nm endoscopic light alone is capable of reducing CFU counts by about 90% [109].
The bulk of cells in biofilms are actually highly susceptible to killing by antimicrobials and it is indeed only a small fraction of cells known as persisters that remain alive following antimicrobial treatments [110]. Persisters represent a subpopulation of cells that spontaneously go into a dormant, non-dividing state. When a population is treated with a bactericidal antibiotic, regular cells die but the persisters survive. According to the persister cell model of chronic relapsing infections, antimicrobial agents working in concert with the immune system are able to eliminate all regular and persister cells from the bloodstream, along with regular cells from the biofilm [111]. The only remaining live cells are then persisters present in biofilms and it is these persisters that repopulate causing the infection to relapse once the level of the bactericidal agent drops. This model seems to be more realistic and is partially supported by PDT explorations. For example, optimal light dosimetry was required to simultaneously maximize bacterial killing and allowed neutrophil accumulation into the infected site when for Photofrin-mediated PDT with red light was tested in a murine MRSA bacterial arthritis model [112]. Additionally, PDT employed a three dimethylpyrrolidinium functionalized C60-fullerene in a mouse wound P. aeruginosa biofilm infection did not enhance survival but when the PDT was combined with a suboptimal dose of tobramycin a synergistic therapeutic effect was observed, with 60% of mice surviving compared to 20% with tobramycin alone [113]. PDT with the cationic porphyrin, tetra-substituted N-methyl-pyridyl-porphine (TMP) resulted in almost complete eradication of staphylococcal biofilms when they were exposed to vancomycin or subjected to the phagocytic action of whole blood [114, 115].
PDI inter-relationship with the “microbial phenotype”
The broad-spectrum activity and what appears to be non-specific action of antimicrobial PDI should be explored deeper in alignment with the complexity of the microbial phenotype. There is no documented evidence whether or not PDI can disrupt sophisticated microbial defensive lines. We have to take into account that PDI is able to eradicate microorganisms without producing resistant isolates, both in planktonic and biofilm forms. This is in concert with the potential of localized photooxidative stress to inactivate virulence factors in the absence of any documented conventional resistance mechanism. PDI with the phenothiazinium MB inhibits in a dose -dependent manner the biological activities of the proteinaceous virulence factors V8 protease, alpha-haemolysin and sphingomyelinase in S. aureus TBO has a similar effect in the two key bacterial virulence factors in both E. coli and P. aeruginosa, lipopolysaccharide (LPS) and proteases [116, 117]. A single antimicrobial PDT treatment in vitro inactivated protease activity and resulted in a 4-log10 reduction in the viability of P. gingivalis. Dose and time-of-exposure experiments revealed that protease inactivation occurred at lower concentrations of PS and less time of light exposure. Also, antimicrobial PDT treatment has been shown to functionally inactivate IL-1 beta and TNF-alpha [118].
A series of pathways, components and phenotypes that may serve as potential alternative and attractive targets for antimicrobial drug discovery are under investigation. One alternative approach is targeting the bacterial communication system (quorum sensing, QS) with emphasis the signal molecules that bacteria produce and detect and thereby coordinate their behavior in a cell-density dependent manner (quorum sensing inhibitors, QSI [119]. There is no evidence for the effect of PDI to QS although the action of PDT with a QSI seems like an attractive has been a plausible combinatorial alternative. In some bacteria QS and RND efflux pump expression are linked. For example, the extracellular autoinducer concentration was significantly reduced when BpeAB-OprM in Burkholderia pseudomallei and MexAB-OprM in P. aeruginosa were knocked out [120] suggesting that inhibition of these efflux pumps could be useful therapeutically. Recent studies have dissected intercellular interaction at the molecular level through analysis of both synthetic and natural microbial populations. These approaches have revealed novel molecular mechanisms that stabilize cooperation among cells and define new roles of population structure for the evolution of cooperative interactions. This knowledge of interaction parameters is changing the view of microbial processes, with emphasis on pathogenesis and antibiotic resistance, and suggests new ways to fight infection by exploiting social interaction [121].
Conclusion
PDI is not a conventional drug discovery platform as it is usually understood, since three elements (PS, visible light and oxygen) are essential for successful deployment. Many of the elements of the bacterial phenotype may come to play an important role as PDI evolves. A set of technical challenges will have to be met using sophisticated tools and approaches to address complex biological questions regarding resistance mechanisms, biofilm inactivation and persister cell formation. There are no validated examples of biofilm photoinactivation or PDT being used in reliable polymicrobial infection models. Minimal information exists for the design of host-pathogen studies exploring the ability of PDT to interfere with virulence determinants. One example is a report of a host-parasite model to assess intracellular targeting specificity of novel phthalocyanines against Leishmania parasites infecting macrophages and dendritic cells [122].
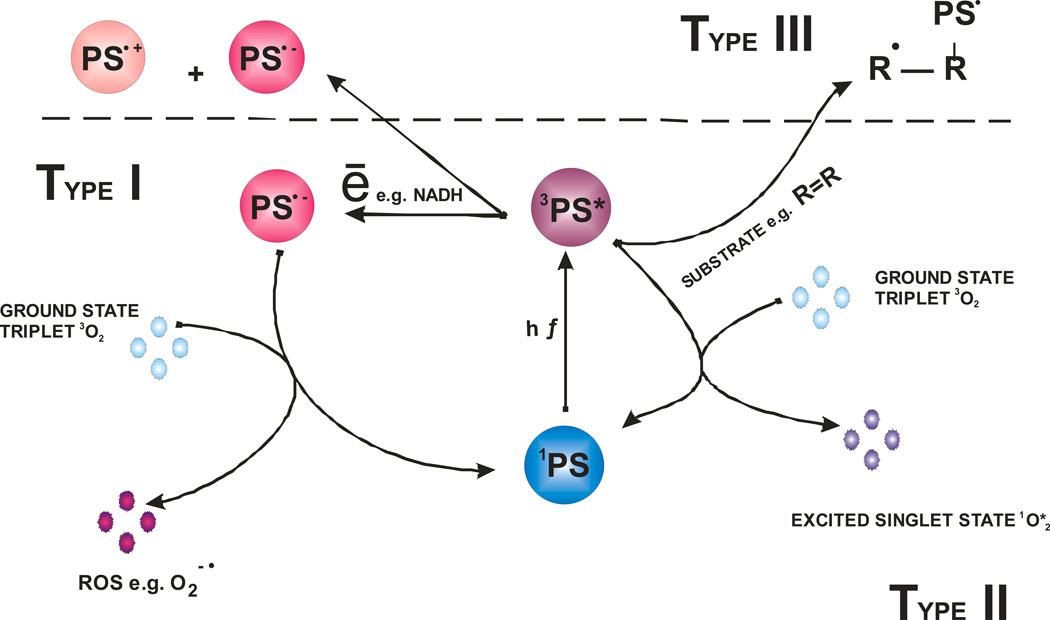
Figure 1.
Schematic illustration of photodynamic acion. The PS initially absorbs a photon that excites it to the first excited singlet state and this can relax to the more long lived triplet state. This triplet PS can interact with molecular oxygen in two pathways, type I and type II, leading to the formation of reactive oxygen species (ROS) and singlet oxygen respectively. In the absence of oxygen the PS may interact with a substrate (R) in a pathway known as Type III.
Table 1.
Combinatorial antimicrobials based on PDT and representatives of the dual antimicrobial platform
| PDT- combinations |
Synergist | Microorganism(s) | Target(s) | Reference |
|---|---|---|---|---|
| Polycationic conjugates of chlorin | visible light | S. aureus, E. coli, P. aeruginosa, C. albicans | non specific reactive oxygen species (ROS) | [123] |
| Methylene Blue (MB), Tolouidine Blue (TBO) | visible light, EPIs | S. aureus, P. aeruginosa, C. albicans | ROS | [49, 59] |
| TBO | visible light, EPIs | E. faecalis biofilms | ROS | [124] |
| functionalized C60-fullerene-tombramycin | visible light | P. aeruginosa biofilms | ROS | [113] |
| Dual Action Antimicrobials | ||||
| Chitosan-silver nanoparticle | - | P aeruginosa, P. mirabilis, A. baumannii | membrane | [125] |
| Berberine-Indole Derivatives | - | S. aureus, E. faecalis, E. faecium, B. anthracis, B. cereus | DNA and membrane | [72, 126, 127] |
| Oxazolidinone-Quinolone | - | S. aureus, E. faecalis, E. faecium E. coli, H. pylori | DNA gyrase and topoisomerase IV | [128] |
Acknowledgments
George P Tegos is supported by the NIH (grant 5U54MH084690-02). Research conducted in the Hamblin Laboratory was supported by NIH (RO1 AI050875 to MRH) and US Air Force MFEL Program (FA9550-04-1-0079). TD was partially supported by a Bullock-Wellman Fellowship Award and an Airlift Research Foundation Extremity Trauma Research Grant (grant 109421).
Biographies
D. Mariano A. Vera PhD Dr. Vera obtained his PhD degree in 2001 at the Universidad Nacional de Córdoba (Argentina), with Prof. Adriana B. Pierini as advisor, studying electron transfer processes in organic reactions by means of computational simulations. He had a stay at the SISSA in Trieste (Italy) in 2004 under the advisory of Prof. Paolo Carloni and he continued the simulations of biological systems up today, alternating with ab initio calculations on systems of interest in Photochemistry, Photophysics and charge transfer systems. He entered the researcher carrier (CONICET) in 2005. He started a new group of Molecular Modeling in Mar del Plata (Argentina) in 2010 as Adjunct Researcher and staff Assistant Professor at the Universidad Nacional de Mar del Plata (Argentina).
Mark Haynes PhD Dr Haynes earned his bachelor’s degree from McGill University and his PhD from The Department of Microbiology and Immunology at The University of Miami’s School of Medicine. His work has focused on the biology of autoimmune diseases and the role particular immune cells play in disease development or resolution. His current work involves studying lymphocyte signaling, protein-protein interactions, and the involvement of antibody recognition in autoimmune disease. He is currently the Immunology Project Leader in the University of New Mexico Center for Molecular Discovery (UNMCMD)
Anthony Ball B.S., NRCM: Mr. Ball holds a Bachelor of Sciences and graduate research from Northeastern University; where he was trained as a Molecular Microbiologist at the Antimicrobial Discovery Center under supervisor Prof. Kim Lewis. His research and scientific interests focus on multidrug efflux systems, non-traditional discovery strategies including prodrugs, anti-infectives, dual action antimicrobials, and photodynamic inactivation of microorganisms. He is currently a Study Director at Toxikon Inc., (Bedford, MA) a pre-clinical bioanalytical research organization. He has published 6 peer-reviewed articles, 1 book chapter, 2 conference proceedings and serves as a Reviewer for Photochemistry and Photobiology.
Tianhong Dai, Ph.D. Dr Dai is an Instructor of Dermatology at the Harvard Medical School. His research interests are primarily focused on the development of light-based techniques for wound infections. Specific areas of interest include photodynamic therapy, ultraviolet-C light therapy, blue light therapy, animal models of wound infections, in-vivo bioluminescence imaging technique to assess the extent of infection.
Dr. Dai obtained his Ph.D. in mechanical engineering in 1995 in China and performed postgraduate work in this area for about 7 years. In 2002, Dr. Dai switched his career to biomedical research by joining the Department of Bioengineering at Rice University. During his time at Rice, Dr. Dai managed 4 1st-authored peer-reviewed basic research papers and co-authored another one. In addition, Dr. Dai collaborated with physicians in clinical work and co-authored 5 clinical research papers. In 2006, Dr. Dai joined the Massachusetts General Hospital. During the past 5 years, Dr. Dai has been involved in several projects, including UVC inactivation of dermatophytes, low-level light treatment of arthritis, and antimicrobial photodynamic therapy of burn infections. He has published 12 1st-authored peer-reviewed papers and co-authored 17. Dr. Dai’s research has been supported by the Bullock-Wellman Fellowship, the Airlift Research Foundation, and the Orthopaedic Trauma Association.
Christos K Astrakas B.S.M.D. Dr Astrakas holds a Medical degree and a Bachelor of Sciences in area of Molecular Biology and Genetics. He was trained as an undergraduate student in Rahme Lab, Department of Surgery, Massachusetts General Hospital, Harvard Medical School and he acquired there his Diploma Thesis. His research and scientific interests focus on skin infections, antibiotics, photodynamic inactivation of microorganisms, applications of photodynamic therapy (PDT) and pathogenesis of nosocomial microorganisms with emphasis in Pseudomonas aeruginosa. He has published 2 peer-reviewed articles, participated in over 25 medical conferences and serves as a Reviewer for Photochemistry and Photobiology.
Michael J Kelso PhD. Dr Kelso graduated in 1996 from the University of Wollongong (Australia) with a B. Medicinal Chemistry (Hons I) and received his PhD (Peptidomimetics) in 2002 from the Institute for Molecular Bioscience (University of Queensland, Australia). After a one year postdoctoral stay in the laboratory of Professor Claudio Palomo (Universidad del Pais Vasco, Spain) studying catalytic asymmetric synthesis he moved to The Scripps Research Institute (CA, USA) where he studied medicinal chemistry as a CJ Martin Postdoctoral Research Fellow under Professor Dale Boger. Upon returning to Australia in 2006 he completed his CJ Martin Fellowship under Professor John Bremner at the University of Wollongong where in 2008 he was appointed to the faculty as a lecturer in chemistry and as senior lecturer in 2010. His current research focuses on the design, synthesis and biological evaluation of dual- and multi-action antimicrobial and anti-cancer hybrid drugs and prodrugs.
Michael R Hamblin Ph.D. is a Principal Investigator at the Wellman Center for Photomedicine at Massachusetts General Hospital, an Associate Professor of Dermatology at Harvard Medical School and is a member of the affiliated faculty of the Harvard-MIT Division of Health Science and Technology. He was trained as a synthetic organic chemist and received his PhD from Trent University in England. His research interests lie in the areas of photodynamic therapy (PDT) for infections, cancer, and heart disease and in low-level light therapy (LLLT) for wound healing, arthritis, traumatic brain injury and hair regrowth. His research program is supported by NIH, CDMRP and CIMIT among other funding agencies. He has published 158 peer-reviewed articles, over 150 conference proceedings, book chapters and International abstracts and holds 8 patents. He is Associate Editor for 3 journals and serves on NIH Study Sections. For the past seven years Dr Hamblin has chaired an annual conference at SPIE Photonics West entitled "Mechanisms for low level light therapy" and he has co-edited the six proceedings volumes together with two other textbooks.
George P Tegos Ph.D. is an Assistant Professor at the Department of Pathology School of Medicine at the University of New Mexico affiliated with the Center of Molecular Discovery and a Visiting Scientist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Harvard Medical School. He was trained as a molecular microbiologist and received his PhD from University of Ioannina Greece. He completed postdoctoral fellowships in Molecular Microbiology (Antimicrobial Discovery Center at Northeastern University, 2001–2003) and Translational therapeutics in Dermatology (Wellman Center for Photomedicine, Harvard Medical School at Massachusetts General Hospital, 2003–2006).His research interests lies in the areas of drug discovery and development of antimicrobial strategies with emphasis in photodynamic therapy (PDT) for infections, multidrug efflux systems as well as virulence and microbial pathogenesis His research program is supported by NIH, DOD (DTRA). He has published 50 peer-reviewed articles, over 50 conference proceedings, book chapters and International abstracts and holds 3 patents. He is Associate Editor for 2 journals. He was recently chaired a session for antifungal drug discovery and repurposing in the 10th Annual Meeting South Central Medical Mycology He is the co-editor of the book “Antimicrobial Drug Discovery; Emerging Strategies” (CABI , Molecular and Cellular Microbiology series)
Footnotes
This paper is part of the Symposium-in-Print on “Antimicrobial Photodynamic Therapy and Photoinactivation”.
References
- 1.Binder S, Levitt AM, Sacks JJ, Hughes JM. Emerging infectious diseases: public health issues for the 21st century. Science. 1999;284:1311–1313. doi: 10.1126/science.284.5418.1311. [DOI] [PubMed] [Google Scholar]
- 2.Hamblin M, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raab O. Ueber diewirkung fluoreszierender stoffe auf infusori. Z. Biol. 1900;39:524–536. [Google Scholar]
- 4.Mitton D, Ackroyd R. A brief overview of photodynamic therapy in Europe. Photodiagnosis. Photodyn. Ther. 2008;5:103–111. doi: 10.1016/j.pdpdt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 5.St Denis TG, Dai T, Izikson L, Astrakas C, Anderson RR, Hamblin MR, Tegos GP. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2:1–12. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt DW. Rostaporfin (Miravant Medical Technologies) IDrugs. 2002;5:180–186. [PubMed] [Google Scholar]
- 7.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 8.Wainwright M, Byrne MN, Gattrell MA. Phenothiazinium-based photobactericidal materials. J Photochem Photobiol. B. 2006;84:227–230. doi: 10.1016/j.jphotobiol.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, Huang YY, Mroz P, Tegos GP, Zhiyentayev T, Sharma SK, Lu Z, Balasubramanian T, Krayer M, Ruzié C, Yang E, Kee HL, Kirmaier C, Diers JR, Bocian DF, Holten D, Lindsey JS, Hamblin MR. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob. Agents Chemother. 2010;54:3834–3841. doi: 10.1128/AAC.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn. Photodynam. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schafer M, Schmitz C, Facius R, Horneck G, Milow B, Funken KH, Ortner J. Systematic study of parameters influencing the action of Rose Bengal with visible light on bacterial cells: comparison between the biological effect and singlet-oxygen production. Photochem. Photobiol. 2000;71:514–523. doi: 10.1562/0031-8655(2000)071<0514:ssopit>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Kubin A, Wierrani F, Jindra RH, Loew HG, Grunberger W, Ebermann R, Alth G. Antagonistic effects of combination photosensitization by hypericin, meso-tetrahydroxyphenylchlorin (mTHPC) and photofrin II on Staphylococcus aureus. Drugs Exp. Clin. Res. 1999;25:13–21. [PubMed] [Google Scholar]
- 13.Harris F, Chatfield LK, Phoenix DA. Phenothiazinium based photosensitisers--photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr. Drug Targets. 2005;6:615–627. doi: 10.2174/1389450054545962. [DOI] [PubMed] [Google Scholar]
- 14.Tegos G, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 2005;12:1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Terakawa M, Zhiyentayev T, Huang YY, Sawayama Y, Jahnke A, Tegos GP, Wharton T, Hamblin MR. Innovative cationic fullerenes as broad-spectrum light-activated antimicrobials. Nanomedicine. 2010;6:442–452. doi: 10.1016/j.nano.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher W, Jr Partridge WP, Dees C, Wachter EA. Simultaneous two-photon activation of type-I photodynamic therapy agents. Photochem. Photobiol. 1997;66:141–155. doi: 10.1111/j.1751-1097.1997.tb08636.x. [DOI] [PubMed] [Google Scholar]
- 17.Alekshun M, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Piddock L. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon R, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009;2:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsen IT, Chen J, Nelson KE, Saier MHJ. Comparative genomics of microbial drug efflux systems. In: Lewis K, editor. Microbial Multidrug Efflux. Norfolk: Horizon Press; 2002. pp. 5–21. [Google Scholar]
- 21.Tegos GP. In: Substrates and Inhibitors of microbial efflux pumps; redifine the role of plant antimicrobials. Rai M, Carpinellla C, editors. Cambridge: Cambridge University Press; 2006. pp. 45–59. [Google Scholar]
- 22.Ren Q, Paulsen IT. Comparative analyses of fundamental differences in membrane transport capabilities in prokaryotes and eukaryotes. PLoS Comput. Biol. 2005;3:e27. doi: 10.1371/journal.pcbi.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 24.Cannon RD, Lamping E, Holmes AR, Niimi P, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009;2:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paumi CM, Chuck M, Snider J, Stagljar I, Michaelis S. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol. Mol. Biol. Rev. 2009;73:577–593. doi: 10.1128/MMBR.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lister P, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry R, Tolentino E, Westbrock-Wadman S, Yuan Y. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 28.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science. 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and Mechanism of the Glycerol-3-Phosphate Transporter from Escherichia Coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 30.Aller S, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell P, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 32.Sennhauser G, Bukowska MA, Briand C, Grütter MG. Crystal structure of the multidrug exporter MexB from Pseudomonas aeruginosa. J Mol Biol. 2009;389:134–145. doi: 10.1016/j.jmb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Phan GBH, Lascombe MB, Benas P, Rety S, Picard M, Ducruix A, Etchebest C, Broutin I. Structural and dynamical insights into the opening mechanism of P. aeruginosa OprM channel. Structure. 2010;18:507–517. doi: 10.1016/j.str.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Akama H, Matsuura T, Kashiwagi S, Yoneyama H, Narita S, Tsukihara T, Nakagawa A, Nakae T. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 2004;279:25939–25942. doi: 10.1074/jbc.C400164200. [DOI] [PubMed] [Google Scholar]
- 35.Yum S, Xu Y, Piao S, Sim SH, Kim HM, Jo WS, Kim KJ, Kweon HS, Jeong MH, Jeon H, Lee K, Ha NC. Crystal structure of the periplasmic component of a tripartite macrolide-specific efflux pump. J Mol Biol. 2009;387:1286–1297. doi: 10.1016/j.jmb.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 36.He X, Szewczyk P, Karyakin A, Evin M, Hong WX, Zhang Q, Chang G. Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature. 2010;467:991–994. doi: 10.1038/nature09408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robey R, Steadman K, Polgar O, Bates SE. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol. Ther. 2005;4:187–194. [PubMed] [Google Scholar]
- 38.Robey R, Fetsch PA, Polgar O, Dean SE, Bates M. The livestock photosensitizer, phytoporphyrin (phylloerythrin), is a substrate of the ATP-binding cassette transporter ABCG2. Res. Vet. Sci. 2006;81:345–349. doi: 10.1016/j.rvsc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Ogino TKH, Munetomo K, Fujita H, Yamamoto M, Utsumi T, Inoue K, Shuin T, Sasaki J, Inoue M, Utsumi K. Serum-dependent export of protoporphyrin IX by ATP-binding cassette transporter G2 in T24 cells. Mol. Cell. Biochem. 2011 doi: 10.1007/s11010-011-0980-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Morgan JJJ, Zheng X, Pandey SK, Pandey RK. Substrate Affinity of Photosensitizers Derived from Chlorophyll-a: The ABCG2 Transporter Affects the Phototoxic Response of Side Population Stem Cell-like Cancer Cells to Photodynamic Therapy. Mol. Pharm. 2010 doi: 10.1021/mp100154j. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jendzelovský R, Mikes J, Koval J, Soucek K, Procházková J, Kello M, Sacková V, Hofmanová J, Kozubík A, Fedorocko P. Drug efflux transporters, MRP1 and BCRP, affect the outcome of hypericin-mediated photodynamic therapy in HT-29 adenocarcinoma cells. Photochem. Photobiol. Sci. 2009;8:1716–1723. doi: 10.1039/b9pp00086k. [DOI] [PubMed] [Google Scholar]
- 42.Merlin J, Gautier H, Barberi-Heyob M, Teiten MH, Guillemin F. The multidrug resistance modulator SDZ-PSC 833 potentiates the photodynamic activity of chlorin e6 independently of P-glycoprotein in multidrug resistant human breast adenocarcinoma cells. Int. J. Oncol. 2003;22:733–739. [PubMed] [Google Scholar]
- 43.Wang E, Casciano CN, Clement RP, Johnson WW. Inhibition of P-glycoprotein transport function by grapefruit juice psoralen. Pharm. Res. 2001;18:432–438. doi: 10.1023/a:1011089924099. [DOI] [PubMed] [Google Scholar]
- 44.Adigbli D, Wilson DG, Farooqui N, Sousi E, Risley P, Taylor I, Macrobert AJ, Loizidou M. Photochemical internalisation of chemotherapy potentiates killing of multidrug-resistant breast and bladder cancer cells. Br. J. Cancer. 2007;97:502–512. doi: 10.1038/sj.bjc.6603895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stermitz F, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5'-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci USA. 2000;97:1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents. Chemother. 2002;46:3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tegos G, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob. Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tseng SP, Teng LJ, Chen CT, Lo TH, Hung WC, Chen HJ, Hsueh PR, Tsai JC. Toluidine blue O photodynamic inactivation on multidrug-resistant Pseudomonas aeruginosa. Lasers Surg. Med. 2009;41:391–397. doi: 10.1002/lsm.20765. [DOI] [PubMed] [Google Scholar]
- 49.Prates R, Kato IT, Ribeiro MS, Tegos GP, Hamblin MR. Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J. Antimicrob. Chemother. 2011;66:1525–1532. doi: 10.1093/jac/dkr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabatini S, Kaatz GW, Rossolini GM, Brandini D, Fravolini A. From phenothiazine to 3-phenyl-1,4-benzothiazine derivatives as inhibitors of the Staphylococcus aureus NorA multidrug efflux pump. J. Med. Chem. 2008;51:4321–4330. doi: 10.1021/jm701623q. [DOI] [PubMed] [Google Scholar]
- 51.Amaral L, Martins M, Viveiros M, Molnar J, Kristiansen JE. Promising therapy of XDR-TB/MDR-TB with thioridazine an inhibitor of bacterial efflux pumps. Curr. Drug Targets. 2008;9:816–819. doi: 10.2174/138945008785747798. [DOI] [PubMed] [Google Scholar]
- 52.Bailey A, Paulsen IT, Piddock LJ. RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob. Agents Chemother. 2008;10:3604–3611. doi: 10.1128/AAC.00661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dutta N, Mehra S, Kaushal D. A Mycobacterium tuberculosis sigma factor network responds to cell-envelope damage by the promising anti-mycobacterial thioridazine. PLoS One. 2010;5:e10069. doi: 10.1371/journal.pone.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tatsumi R, Wachi M. TolC-dependent exclusion of porphyrins in Escherichia coli. J. Bacteriol. 2008;190:6228–6233. doi: 10.1128/JB.00595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez A, Lechardeur D, Derré-Bobillot A, Couvé E, Gaudu P, Gruss A. Two coregulated efflux transporters modulate intracellular heme and protoporphyrin IX availability in Streptococcus agalactiae. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000860. e1000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grinholc M, Zawacka-Pankau J, Gwizdek-Wisniewska A, Bielawski KP. Evaluation of the role of the pharmacological inhibition of Staphylococcus aureus multidrug resistance pumps and the variable levels of the uptake of the sensitizer in the strain-dependent response of Staphylococcus aureus to PPArg(2)-based photodynamic inactivation. Photochem. Photobiol. 2010;86:1118–1126. doi: 10.1111/j.1751-1097.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 57.Gutmann D, Ward A, Urbatsch IL, Chang G, van Veen HW. Understanding polyspecificity of multidrug ABC transporters: closing in on the gaps in ABCB1. Trends in Biochemical Sciences. 2010;35:36–42. doi: 10.1016/j.tibs.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tegos G, Haynes M, Strouse JJ, MM Khan, MMT, Bologa CG, Oprea TI, Sklar LA. Microbial Efflux Inhibition; tactics & strategies. Current Pharmaceutical Design. 2011;17:1291–1302. doi: 10.2174/138161211795703726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tegos G, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob. Agents Chemother. 2008;52:3202–3209. doi: 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bornstein E, Hermans W, Gridley S, Manni J. Near-infrared photoinactivation of bacteria and fungi at physiologic temperatures. Photochem Photobiol. 2009;85:1364–1374. doi: 10.1111/j.1751-1097.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 61.Bornstein E, Gridley S, Wengender P, Robbins A. Photodamage to multidrug-resistant gram-positive and gram-negative bacteria by 870 nm/930 nm light potentiates erythromycin, tetracycline and ciprofloxacin. Photochem. Photobiol. 2010;86:617–627. doi: 10.1111/j.1751-1097.2010.00725.x. [DOI] [PubMed] [Google Scholar]
- 62.Kohanski M, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 63.Kohanski M, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tavares A, Carvalho CM, Faustino MA, Neves MG, Tomé JP, Tomé AC, Cavaleiro JA, Cunha A, Gomes NC, Alves E, Almeida A. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L, Roncucci G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob Agents Chemother. 2010;54:637–642. doi: 10.1128/AAC.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakonieczna J, Michta E, Rybicka M, Grinholc M, Gwizdek-Wisniewska A, Bielawski KP. Superoxide dismutase is upregulated in Staphylococcus aureus following protoporphyrin-mediated photodynamic inactivation and does not directly influence the response to photodynamic treatment. BMC Microbiol. 2010;10:323. doi: 10.1186/1471-2180-10-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.St. Denis TG, Dai T, Izikson A, Astrakas C, Anderson RR, Hamblin MR, Tegos GP. All you need is light Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2:1–12. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brombacher K, Fischer BB, Rüfenacht K, Eggen RI. The role of Yap1p and Skn7p-mediated oxidative stress response in the defence of Saccharomyces cerevisiae against singlet oxygen. Yeast. 2006;23:741–750. doi: 10.1002/yea.1392. [DOI] [PubMed] [Google Scholar]
- 69.Klyachko K, Schuldiner S, Neyfakh AA. Mutations affecting substrate specificity of the Bacillus subtilis multidrug transporter. BMR J. Bacteriol. 1997;179:2189–2193. doi: 10.1128/jb.179.7.2189-2193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed M, Borsch C, Neyfakh A, Schuldner S. Mutants of Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensive alkaloid reserpine. J Biol. Chem. 1993;268:11086–11089. [PubMed] [Google Scholar]
- 71.Garvey M, Piddock LJ. The efflux pump inhibitor reserpine selects multidrug-resistant Streptococcus pneumoniae strains that overexpress the ABC transporters PatA and PatB. Antimicrob. Agents Chemother. 2008;52:1677–1685. doi: 10.1128/AAC.01644-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ball A, Casadei G, Samosorn S, Bremner JB, Ausubel FM, Moy TI, Lewis K. Conjugating berberine to a multidrug efflux pump inhibitor creates an effective antimicrobial. ACS Chem. Biol. 2006;1:594–600. doi: 10.1021/cb600238x. [DOI] [PubMed] [Google Scholar]
- 73.Samosorn S, Tanwirat B, Muhamad N, Casadei G, Tomkiewicz D, Lewis K, Suksamrarn A, Prammananan T, Gornall KC, Beck JL, Bremner JB. Antibacterial activity of berberine-NorA pump inhibitor hybrids with a methylene ether linking group. Bioorg. Med. Chem. 2009;17:3866–3872. doi: 10.1016/j.bmc.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomkiewicz D, Casadei G, Larkins-Ford J, Moy TI, Garner J, Bremner JB, Ausubel FM, Lewis K, Kelso MJ. Berberine-INF55 (5-nitro-2-phenylindole) hybrid antimicrobials: effects of varying the relative orientation of the berberine and INF55 components. Antimicrob. Agents Chemother. 2010;54:3219–3224. doi: 10.1128/AAC.01715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 76.Del Pozo J, Patel R. The challenge of treating biofilm-associated bacterial infections. Clinical Pharmacol. Ther. 2007;82:204–209. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 77.Lewis K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fontana C, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, Boussios CI, Kent R, Goodson JM, Tanner AC, Soukos NC. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res. 2009;44:751–759. doi: 10.1111/j.1600-0765.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raghavendra M, Koregol A, Bhola S. Photodynamic therapy: a targeted therapy in periodontics. Aust. Dent. J. 2009;54(Suppl 1):S102–S109. doi: 10.1111/j.1834-7819.2009.01148.x. [DOI] [PubMed] [Google Scholar]
- 80.Soukos N, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S, Foschi F, Doucette S, Bammann LL, Fontana CR, Doukas AG, Stashenko PP. Photodynamic therapy for endodontic disinfection. J. Endod. 2006;32:979–984. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 81.Bisland S, Chien C, Wilson BC, Burch S. Pre-clinical in vitro and in vivo studies to examine the potential use of photodynamic therapy in the treatment of osteomyelitis. Photochem. Photobiol. Sci. 2006;5:31–38. doi: 10.1039/b507082a. [DOI] [PubMed] [Google Scholar]
- 82.Donnelly R, McCarron PA, Cassidy CM, Elborn JS, Tunney MM. Delivery of photosensitisers and light through mucus: investigations into the potential use of photodynamic therapy for treatment of Pseudomonas aeruginosa cystic fibrosis pulmonary infection. J. Control Release. 2007;117:217–226. doi: 10.1016/j.jconrel.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 83.Schuckert K, Jopp S, Muller U. De novo grown bone on exposed implant surfaces using photodynamic therapy and recombinant human bone morphogenetic protein-2: case report. Implant Dent. 2006;15:361–365. doi: 10.1097/01.id.0000247856.09740.3f. [DOI] [PubMed] [Google Scholar]
- 84.Donnelly R, McCarron PA, Tunney MM, David Woolfson A. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J Photochem. Photobiol. B. 2007;86:59–69. doi: 10.1016/j.jphotobiol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 85.Dortbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin. Oral Implants Res. 2001;12:104–108. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- 86.Walters MC, 3rd, Roe F, Bugnicourt M, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003;47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leid JG, Shirtliff ME, Costerton JW, Stoodley AP. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect. Immun. 2002;70:6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jesaitis A, Franklin MJ, Berglund D, Sasaki M, Lord CI, Bleazard JB, Duffy JE, Beyenal H, Lewandowski Z. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 2003;171:4329–4339. doi: 10.4049/jimmunol.171.8.4329. [DOI] [PubMed] [Google Scholar]
- 89.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 90.Biel M. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol Biol. 2010;635:175–194. doi: 10.1007/978-1-60761-697-9_13. [DOI] [PubMed] [Google Scholar]
- 91.Schneider M, Kirfel G, Berthold M, Frentzen M, Krause F, Braun A. The impact of antimicrobial photodynamic therapy in an artificial biofilm model. Lasers Med. Sci. 2011 doi: 10.1007/s10103-011-0998-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 92.Fontana C, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, Boussios CI, Kent R, Goodson JM, Tanner AC, Soukos NC. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J. Periodontal Res. 2009;44:751–759. doi: 10.1111/j.1600-0765.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fimple J, Fontana CR, Foschi F, Ruggiero K, Song X, Pagonis TC, Tanner AC, Kent R, Doukas AG, Stashenko PP, Soukos NC. Photodynamic treatment of endodontic polymicrobial infection in vitro. J. Endod. 2008;34:728–734. doi: 10.1016/j.joen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gad F, Zahra T, Hasan T, Hamblin MR. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob. Agents Chemother. 2004;48:2173–2178. doi: 10.1128/AAC.48.6.2173-2178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma M, Visai L, Bragheri F, Cristiani I, Gupta PK, Speziale P. Toluidine blue-mediated photodynamic effects on staphylococcal biofilms. Antimicrob. Agents Chemother. 2008;52:299–305. doi: 10.1128/AAC.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin H, Chen CT, Huang CT. Use of merocyanine 540 for photodynamic inactivation of Staphylococcus aureus planktonic and biofilm cells. Appl. Environ. Microbiol. 2004;70:6453–6458. doi: 10.1128/AEM.70.11.6453-6458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sbarra M, Di Poto A, Arciola CR, Saino E, Sharma M, Bragheri F, Cristiani I, Speziale P, Visai L. Photodynamic action of merocyanine 540 on Staphylococcus epidermidis biofilms. Int. J. Artif. Organs. 2008;31:848–857. doi: 10.1177/039139880803100914. [DOI] [PubMed] [Google Scholar]
- 98.Saino E, Sbarra MS, Arciola CR, Scavone M, Bloise N, Nikolov P, Ricchelli F, Visai L. Photodynamic action of Tri-meso (N-methyl-pyridyl), meso (N-tetradecyl-pyridyl) porphine on Staphylococcus epidermidis biofilms grown on Ti6Al4V alloy. Int. J. Artif. Organs. 2010;33:636–645. doi: 10.1177/039139881003300909. [DOI] [PubMed] [Google Scholar]
- 99.Zanin I, Gonçalves RB, Junior AB, Hope CK, Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J. Antimicrob. Chemother. 2005;56:324–330. doi: 10.1093/jac/dki232. [DOI] [PubMed] [Google Scholar]
- 100.Zanin I, Lobo MM, Rodrigues LK, Pimenta LA, Höfling JF, Gonçalves RB. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur. J. Oral Sci. 2006;114:64–69. doi: 10.1111/j.1600-0722.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 101.Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J. Antimicrob. Chemother. 2006;57:680–684. doi: 10.1093/jac/dkl021. [DOI] [PubMed] [Google Scholar]
- 102.Metcalf D, Robinson C, Devine D, Wood S. Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J. Antimicrob. Chemother. 2006;58:190–192. doi: 10.1093/jac/dkl205. [DOI] [PubMed] [Google Scholar]
- 103.Goulart Rde C, Bolean M, Paulino Tde P, Thedei G, Jr, Souza SL, Tedesco AC, Ciancaglini P. Photodynamic therapy in planktonic and biofilm cultures of Aggregatibacter actinomycetemcomitans. Photomed. Laser Surg. 2010;28(1):S53–S60. doi: 10.1089/pho.2009.2591. [DOI] [PubMed] [Google Scholar]
- 104.Nastri L, Donnarumma G, Porzio C, De Gregorio V, Tufano MA, Caruso F, Mazza C, Serpico R. Effects of toluidine blue-mediated photodynamic therapy on periopathogens and periodontal biofilm: in vitro evaluation. Int. J. Immunopathol. Pharmacol. 2010;23:1125–1132. doi: 10.1177/039463201002300416. [DOI] [PubMed] [Google Scholar]
- 105.Lee C, Lee CJ, Chen CT, Huang CT. delta-Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures. J. Photochem. Photobiol. B. 2004;75:21–25. doi: 10.1016/j.jphotobiol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 106.Collins T, Markus EA, Hassett DJ, Robinson JB. The effect of a cationic porphyrin on Pseudomonas aeruginosa biofilms. Curr. Microbiol. 2006;61:411–416. doi: 10.1007/s00284-010-9629-y. [DOI] [PubMed] [Google Scholar]
- 107.Pflumm M. Caught on film. Nature Medicine. 2011;17:650–653. doi: 10.1038/nm0611-650. [DOI] [PubMed] [Google Scholar]
- 108.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob. Agents Chemother. 2005;49:2822–2827. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR. Helicobacter pylori in patients can be killed by visible light. Lasers Surg. Med. 2005;36:260–265. doi: 10.1002/lsm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 2001;183:6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewis K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tanaka M, Kinoshita M, Yoshihara Y, Shinomiya N, Seki S, Nemoto K, Hamblin MR, Morimoto Y. Photodynamic therapy using intra-articular Photofrin for murine MRSA arthritis: biphasic light dose response for neutrophil-mediated antibacterial effect. Lasers Surg. Med. 2011;43:221–229. doi: 10.1002/lsm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu Z, Dai T, Huang L, Kurup DB, Tegos GP, Jahnke A, Wharton T, Hamblin MR. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (Lond) 2010;5:1525–1533. doi: 10.2217/nnm.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P. The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials. 2009;30:3158–3166. doi: 10.1016/j.biomaterials.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 115.Sbarra M, Arciola CR, Di Poto A, Saino E, Rohde H, Speziale P, Visai L. The photodynamic effect of tetra-substituted N-methyl-pyridyl-porphine combined with the action of vancomycin or host defense mechanisms disrupts Staphylococcus epidermidis biofilms. Int. J. Artif. Organs. 2009;32:574–583. doi: 10.1177/039139880903200906. [DOI] [PubMed] [Google Scholar]
- 116.Tubby S, Wilson M, Nair SP. Inactivation of staphylococcal virulence factors using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:211. doi: 10.1186/1471-2180-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kömerik N, Wilson M, Poole S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem. Photobiol. 2000;72:676–680. doi: 10.1562/0031-8655(2000)072<0676:teopao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 118.Braham P, Herron C, Street C, Darveau R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J. Periodontol. 2009;80:1790–1798. doi: 10.1902/jop.2009.090214. [DOI] [PubMed] [Google Scholar]
- 119.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 120.Pearson JP, Van Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xavier J. Social interaction in synthetic and natural microbial communities. Molecular Systems Biology. 2011;7:483. doi: 10.1038/msb.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dutta S, Ongarora BG, Li H, Vicente Mda G, Kolli BK, Chang KP. Intracellular targeting specificity of novel phthalocyanines assessed in a host-parasite model for developing potential photodynamic medicine. PLoS One. 2011;6:e20786. doi: 10.1371/journal.pone.0020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tegos G, Anbe M, Yang C, Demidova TN, Satti M, Mroz P, Janjua S, Gad F, Hamblin MR. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–1410. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kishen A, Upadya M, Tegos GP, Hamblin MR. Efflux Pump Inhibitor Potentiates Antimicrobial Photodynamic Inactivation of Enterococcus faecalis Biofilm. Photochem Photobiol. 2010;86:1343–1349. doi: 10.1111/j.1751-1097.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang L, Dai T, Xuan Y, Tegos GP, Hamblin MR. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: efficacy against bacterial burn infections. Antimicrob. Agents Chemother. 2011;55:3432–3438. doi: 10.1128/AAC.01803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bremner J, Kelso MJ. Synthesis of Berberine-Efflux Pump Inhibitor Hybrid Antibacterials. Synth. Commun. 2010;40:3561–3568. doi: 10.1080/00397910903457415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ambrus JI, Kelso MJ, Bremner JB, Ball AR, Casadei G, Lewis K. Structure-activity relationships of 2-aryl-1H-indole inhibitors of the NorA efflux pump in Staphylococcus aureus. Bioorg Med. Chem. Lett. 2008;18:4294–4297. doi: 10.1016/j.bmcl.2008.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hubschwerien C, Specklin J, Baseschilin D, Borer Y, Haefeli S, Sigwalt C, Schroeder S, Locher HH. Structure-activity relationship in the oxazolidinone-quinolone hybrid series: Influence of the central spacer on the antibacterial activity and the mode of action. Bioorganic Medicinal Chem. Lett. 2003;13:4229–4233. doi: 10.1016/j.bmcl.2003.07.028. [DOI] [PubMed] [Google Scholar]
- 129.Frisch MJTGW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.02. Wallingford CT: Gaussian, Inc.; 2004. 2004. [Google Scholar]
- 130.Mennucci B, Tomasi J. Continuum solvation models: a new approach to the problem of solute’s charge distribution and cavity boundaries. Journal of Chemical Physics. 1997;106:5151–5158. [Google Scholar]
- 131.Bayly C, Cieplak P, Cornell W, Kollman PA. A Well-behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges: the RESP model. J. Phys. Chem. 1993;97:10269–10280. [Google Scholar]