Abstract
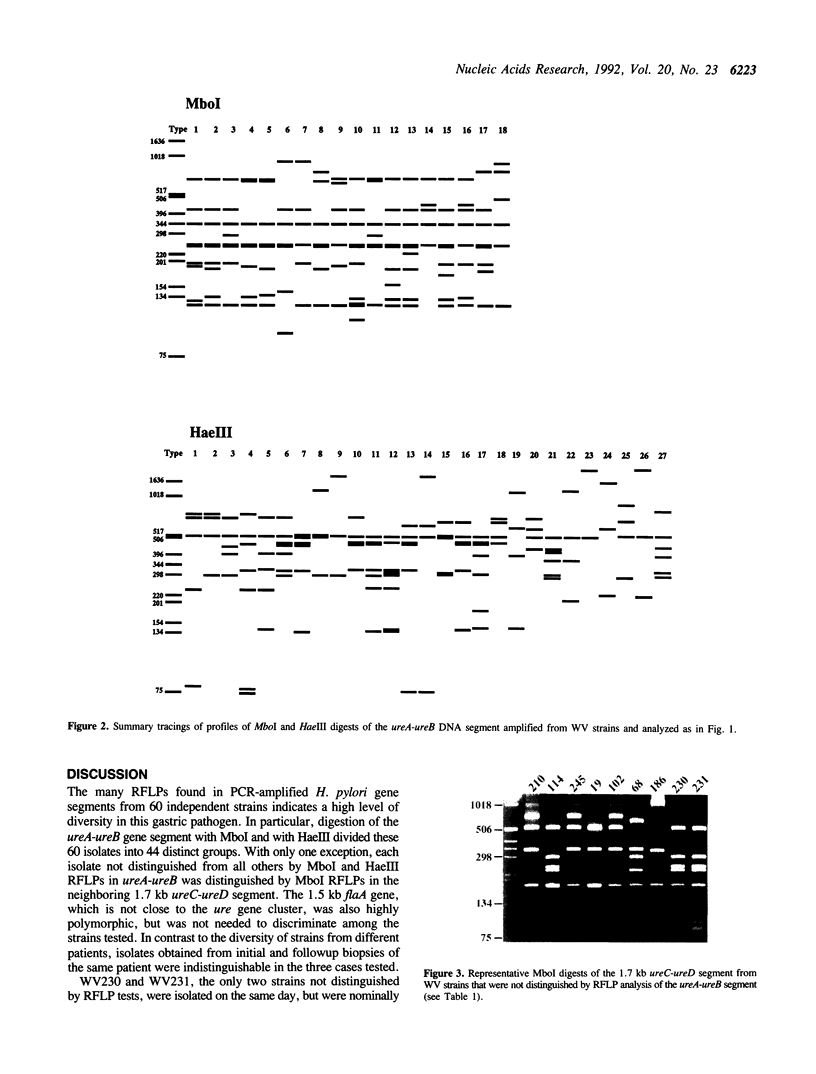
DNA sequence diversity among 60 independent isolates of the gastric pathogen Helicobacter pylori was assessed by testing for restriction fragment length polymorphisms (RFLPs) in several PCR-amplified gene segments. 18 Mbol and 27 HaeIII RFLPs were found in the 2.4 kb ureA-ureB (urease) segment from the 60 strains; this identified 44 separate groups, with each group containing one to four isolates. With one exception, each isolate not distinguished from the others by RFLPs in ureA-ureB was distinguished by Mbol digestion of the neighboring 1.7 kb ureC-ureD segment. The 1.5 kb flaA (flagellin) gene, which is not close to ure gene cluster, was also highly polymorphic. In contrast, isolates from initial and followup biopsies yielded identical restriction patterns in each of the three cases tested. The potential of this method for detecting population heterogeneity was tested by mixing DNAs from different strains before amplification: the arrays of restriction fragments obtained indicated co-amplification from both genomes in each of the five pairwise combinations tested. These results show that H. pylori is a very diverse species, that indicate PCR-based RFLP tests are almost as sensitive as arbitrary primer PCR (RAPD) tests, and suggest that such RFLP tests will be useful for direct analysis of H. pylori in biopsy and gastric juice specimens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATWOOD K. C., SCHNEIDER L. K., RYAN F. J. Selective mechanisms in bacteria. Cold Spring Harb Symp Quant Biol. 1951;16:345–355. doi: 10.1101/sqb.1951.016.01.026. [DOI] [PubMed] [Google Scholar]
- Akopyanz N., Bukanov N. O., Westblom T. U., Kresovich S., Berg D. E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992 Oct 11;20(19):5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori: its role in disease. Clin Infect Dis. 1992 Sep;15(3):386–391. doi: 10.1093/clind/15.3.386. [DOI] [PubMed] [Google Scholar]
- Clayton C. L., Kleanthous H., Coates P. J., Morgan D. D., Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992 Jan;30(1):192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxall P. A., Hu L. T., Mobley H. L. Use of polymerase chain reaction-amplified Helicobacter pylori urease structural genes for differentiation of isolates. J Clin Microbiol. 1992 Mar;30(3):739–741. doi: 10.1128/jcm.30.3.739-741.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar M., Tyszkiewicz T., Wadström T., O'Toole P. W. Rapid detection of Helicobacter pylori in gastric biopsy material by polymerase chain reaction. J Clin Microbiol. 1992 Jan;30(1):54–58. doi: 10.1128/jcm.30.1.54-58.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B. R., Kersulyte D., Brikun I., Berg C. M., Berg D. E. Direct and crossover PCR amplification to facilitate Tn5supF-based sequencing of lambda phage clones. Nucleic Acids Res. 1991 Nov 25;19(22):6177–6182. doi: 10.1093/nar/19.22.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne A., Cussac V., Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991 Mar;173(6):1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Oudbier J. H., Tytgat G. N. Patient-to-patient transmission of Campylobacter pylori infection by fiberoptic gastroduodenoscopy and biopsy. J Infect Dis. 1990 Mar;161(3):507–511. doi: 10.1093/infdis/161.3.507. [DOI] [PubMed] [Google Scholar]
- Leying H., Suerbaum S., Geis G., Haas R. Cloning and genetic characterization of a Helicobacter pylori flagellin gene. Mol Microbiol. 1992 Oct;6(19):2863–2874. doi: 10.1111/j.1365-2958.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Oudbier J. H., Langenberg W., Rauws E. A., Bruin-Mosch C. Genotypical variation of Campylobacter pylori from gastric mucosa. J Clin Microbiol. 1990 Mar;28(3):559–565. doi: 10.1128/jcm.28.3.559-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Bickley J., Costas M., Morgan D. R. Genomic variation in Helicobacter pylori: application to identification of strains. Scand J Gastroenterol Suppl. 1991;181:43–50. doi: 10.3109/00365529109093207. [DOI] [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee W., Lambert J., Smallwood R., Schembri M., Ross B. C., Dwyer B. Ribotyping of Helicobacter pylori from clinical specimens. J Clin Microbiol. 1992 Jun;30(6):1562–1567. doi: 10.1128/jcm.30.6.1562-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]