Abstract
The isolation of whey proteins from human and bovine milks followed by profiling of their entire N-glycan repertoire is described. Whey proteins resulting from centrifugation and ethanol precipitation of milk were treated with PNGase F to release protein-bound N-glycans. Once released, N-glycans were analyzed via nano-flow liquid chromatography coupled with quadrupole time-of-flight mass spectrometry following chromatographic separation on a porous graphitized carbon chip. In all, 38 N-glycan compositions were observed in the human milk sample while the bovine milk sample revealed 51 N-glycan compositions. These numbers translate to over a hundred compounds when isomers are considered and point to the complexity of the mixture. High mannose, neutral and sialylated complex/hybrid glycans were observed in both milk sources. Although NeuAc sialylation was observed in both milk samples, the NeuGc residue was only observed in bovine milk and marks a major difference between human and bovine milks. To the best of our knowledge, this study is the first MS based confirmation of NeuGc in milk protein bound glycans as well as the first comprehensive N-glycan profile of bovine milk proteins. Tandem MS was necessary for resolving complications presented by the fact that (NeuGc:Fuc) corresponds to the exact mass of (NeuAc:Hex). Comparison of the relative distribution of the different glycan types in both milk sources was possible via their abundances. While the human milk analysis revealed a 6% high mannose, 57% sialylation and 75% fucosylation distribution, a 10% high mannose, 68% sialylation and 31% fucosylation distribution was observed in the bovine milk analysis. Comparison with the free milk oligosaccharides yielded low sialylation and high fucosylation in human, while high sialylation and low fucosylation are found in bovine. The results suggest that high fucosylation is a general trait in human, while high sialylation and low fucosylation are general features of glycosylation in bovine milk.
Keywords: Human and bovine milk, PNGase F, N-glycans, PGC Chip, nano-LC/Q-TOF MS, Tandem MS, NeuGc
Introduction
Oligosaccharides are amongst the most abundant solid components in human 1–4 and bovine milk 5. These free oligosaccharides perform a number of biological functions that indicate that nutrients are not the only benefits the infants get from their mothers’ milk. As an example, milk oligosaccharides serve as prebiotics to stimulate growth of beneficial intestinal bacteria such as bifidobacteria in neonates.2,4,6,7 Modulation of the postnatal immune system is a beneficial consequence due to the development of a balanced intestinal microbiota.2,7 Additionally, milk oligosaccharides have been reported to bind to certain pathogenic microorganisms thereby limiting their virulence.2,7–9 This behavior lowers the risk of diseases such as diarrhea, meningitis and otitis media in infants.2,7 Extensive analysis of human milk oligosaccharides (HMOs) 10 and bovine milk oligosaccharides (BMOs) 11 reveal structural diversity suggesting that they may contribute to a diversity of functions. Although certain compositional similarities were identified in both milk oligosaccharides, such studies also identified remarkable differences in the structures and relative abundances of the different oligosaccharide types in both milk sources. For example, while high fucosylation and low sialylation were observed as general features of HMOs, BMOs were reported as significantly highly sialylated and almost lacking in fucosylation. Other oligosaccharide sources in milk (such as the protein-bound N-glycans) are yet to be extensively characterized. This study will provide important information to nutritionists, food regulatory agencies, and the dairy industry in general.
Proteins are a vital component in mammalian milk and have been well characterized as a rich source of essential amino acids to infants.12,13 Nonetheless, milk proteins are on average highly glycosylated, a fact inconsistent with a simple role of proteins as sources of digestible and absorbable amino acids. In fact, besides free oligosaccharides; proteins are an additional and potentially significant source of milk oligosaccharides. Indeed, recent proteomics studies in human 12 and bovine milk 13 have shown that milk proteins are extensively glycosylated. Initial predictions suggested that over 70% of human proteins may be glycosylated.14,15 The functions of these oligosaccharide components in glycoconjugates (particularly glycoproteins) are speculated to provide protective features similar to those observed in free oligosaccharides in milk.16–24 In support of this hypothesis, protein-bound glycans have been shown to act as decoys for pathogens 25,26 justifying further research to determine their actual compositions and structures in milk. In other biological milieu, glycans have been implicated to have profound effects on the properties of proteins such as solubility, folding, secretion, antigenicity, immunogenicity, circulatory half-life, resistance to proteolysis and thermal stability.27 Surprisingly, and in contrast to free oligosaccharides in milk, the glycans on milk proteins have not been as well studied.
Information related to milk N-glycans is typically limited to those associated with the abundant proteins. Lactoferrin, a 78 KDa iron binding glycoprotein; is the most abundant glycoprotein in human milk and also one of the most abundant proteins in bovine milk. Human lactoferrin (h-LF) has been reported to be associated with highly branched complex/hybrid type N-glycans, most of which are highly sialylated and fucosylated.28 However, bovine lactoferrin (b-LF) is reported to be predominantly high mannose-containing.29 Immunoglobulin G (IgG), a 150 KDa protein; is another abundant protein found in both human and bovine milk. In both milk sources, IgG has been reported to be predominantly associated with core-fucosylated bi-antennary N-glycans.30 Secretory immunoglobulin A (sIgA) 31, bile salt-stimulated lipase 32 and milk fat globule membrane proteins such as clusterin 33 are additional examples of abundant proteins in milk whose N-glycans have been characterized. Although information from these studies is useful, they do not present a comprehensive list of the N-glycan repertoire in both human and bovine milks.
N-glycans are complicated by their monosaccharide compositions, branching, linkage and connectivity.34 N-glycans are classified as high mannose, complex and hybrid types depending on their monosaccharide composition and their branching. Individual sites of glycan attachment (glycosites) on a polypeptide can be associated with multiple glycans further complicating the diversity of the N-glycan pool in a protein mixture such as milk. Additionally, unlike with proteomic studies where there is a template, N-glycan analysis requires more rigorous analyses as there are no templates for their structures. Glycomics approaches towards profiling N-glycans from a wide range of biological samples typically employ the release of the glycans via peptide N:glycosidase F (PNGase F) treatment prior to mass spectrometric (MS) analysis.35,36 The use of high performance liquid chromatography (HPLC) coupled to mass spectrometry is becoming the instrument of choice for glycan analysis. LC-MS enables the separation of glycans thus minimizing suppression effects during ionization.37 Furthermore; separation of glycans sharing the same compositions (isomers) is possible during LC-MS analysis when the appropriate stationary phase is used. In general, we find porous graphitized carbon (PGC) to be the best stationary phase for effectively separating native glycans and their isomers.38
An additional complication in the comprehensive characterization of the N-glycans of milk proteins is the presence of N-glycolylneuraminic acid (NeuGc) in bovine milk, which is generally not found in humans. NeuGc prevents determining composition based strictly on accurate mass because combinations of fucose (Fuc) and NeuGc can yield masses equivalent to oligosaccharides containing N-acetylneuraminic acid (NeuAc) and hexose (Hex). For example, the neutral mass 1931.69 Da may correspond to (GlcNAc4:Hex5:NeuAc1), but can also correspond to (GlcNAc4:Hex4:Fuc1:NeuGc1) if NeuGc is present. For this reason, tandem MS is required for this type of analysis.
In this report, the isolation of the non-casein, soluble (whey) proteins from human and bovine milk followed by profiling of their entire N-glycan repertoire is described. The study investigated the sialylation and fucosylation trends in milk N-glycans to determine if they correspond to similar evidence already observed in the free milk oligosaccharides. The results presented here also provide the first tandem MS analysis of NeuGc containing N-glycans. Whey proteins isolated by centrifugation and ethanol precipitation of milk were treated with PNGase F to release protein-bound N-glycans. The released N-glycans were then analyzed via nano-flow liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (nano-LC/Q-TOF MS) following chromatographic separation of the glycans on a micro fluidic chip packed with PGC. While the nano-LC MS analysis afforded the milk glycan profiles, the tandem MS analysis revealed monosaccharide compositions and in some cases monosaccharide connectivities. Glycan MS/MS data were identified via the presence of certain oxonium ions resulting from monosaccharide and disaccharide fragments during the tandem MS analyses. With the use of PGC, isomeric glycans were adequately resolved and compositions verified with tandem MS. The development of this strategy and the results observed afford a better understanding of the types, compositions and diversity of the N-glycan repertoire associated with both human and bovine milk.
Materials and Methods
Materials and Reagents
Graphitized carbon cartridges (GCC) were purchased from Alltech Associated (Deerfield, IL) while C8 cartridges were from Suppelco (Bellefonte, PA). PNGase F was purchased from New England Biolabs (Ipswich, MA). All reagents used were either of analytical grade or better.
Protein Extraction
Prior to N-glycan release from human and bovine milk, the whey proteins were isolated from the other milk components. To achieve this, 0.5 mL of a pool of human milk (obtained from an on-going project in our research group) and 0.5 mL of a pool of bovine milk (obtained from Jersey and Holstein cows) over three months of lactation were individually combined with 0.5 mL of water in 1.5 mL vials and centrifuged at 4°C and 1500 rpm for 1 h. The middle aqueous layer consisting of whey proteins and free oligosaccharides was carefully drawn out from each vial. The top layer consisting of fat and the bottom layer made up of casein and cellular residues were discarded. The whey proteins were then further separated from the free oligosaccharides via ethanol precipitation. To achieve this, two volumes of ethanol was combined with the protein-oligosaccharide mixture, stored at 4 °C for 2 h and centrifuged at 4°C and 1500 rpm for 1 h. Next, free oligosaccharides were carefully drawn out from the tube leaving the whey protein precipitate at the bottom of the tube. The whey proteins were later re-dissolved in 500 μL of a 200 mM ammonium bicarbonate (NH4HCO3) with rigorous agitation.
N-glycan Release
N-glycan release from the whey proteins was achieved by incubating a mixture composed of 100 μL of the whey protein solution, 100 μL of a 200 mM NH4HCO3, 1 μL 1M sodium dodecyl sulphate (SDS) and 2 μL of PNGase F. Incubation was allowed to proceed overnight at 37°C with gentle agitation.
Glycan Purification
To purify the released N-glycans from each milk source, PNGase F and de-glycosylated proteins were first removed from the N-glycan mixture via a first solid phase extraction (SPE) step involving a C8 cartridge as earlier described by our group.38 The eluting N-glycans from the C8 cartridge were then collected and desalted/enriched in a second SPE step involving a graphitized carbon cartridge (GCC) as earlier described by our group.38 The enriched N-glycans were finally eluted from the cartridge with 9mL of 0.05% trifluoroacetic acid (TFA) in 40% ACN solution. The N-glycan mixture was then completely dried in the speed vac prior to MS and MS/MS analyses.
HPLC-Chip/Q-TOF-MS Analysis
After drying, the N-glycans were reconstituted in 20 μL of deionized water from which 2 μL was required each for the MS and MS/MS analyses. The N-glycans were analyzed using an Agilent 1200 series LC system coupled to an Agilent 6520 Q-TOF mass spectrometer (Agilent Technologies, Santa Clara, CA). The HPLC-Chip/Q-TOF system was equipped with a micro well-plate auto sampler (maintained at 6°C by the thermostat), a capillary loading pump for sample enrichment, a nano pump as the analytical pump for sample separation, HPLC-Chip Cube, and the Agilent 6520 Q-TOF MS detector. The tandem mass spectra of the N-glycans were acquired in a data-dependent manner following LC separation on the microfluidic chip. The microfluidic chip consisted of a 9 × 0.075 mm i.d. enrichment column and a 150 × 0.075 mm i.d. analytical column, both packed with 5 μm porous graphitized carbon (PGC) as the stationary phase. A PGC chip was used as glycan populations can be sufficiently separated on a graphitized carbon stationary phase. Both pumps used binary solvent: A, 3.0% ACN/water (v/v) with 0.1% formic acid; B, 90% ACN/water (v/v) with 0.1% formic acid. A flow rate of 4 μL/min of solvent A was used for sample loading with 2 μL injection volume. A nano pump gradient was delivered at 0.4 μL/min using (A) 0.1% formic acid in 3.0% ACN/water (v/v) and (B) 0.1% formic acid in 90.0% ACN /water (v/v). Samples were eluted with 0% B (0.00–2.50 min); 0 to 16% B (2.50–20.00 min); 16 to 44% B (20.00–30.00 min); 44 to 100% B (30.00–35.00 min) and 100% B (35.00–45.00 min). The drying gas temperature was set at 325 °C with a flow rate of 4 L/min (2 L of filtered nitrogen gas and 2 L of filtered dry grade compressed air). MS and MS/MS spectra were acquired in the positive ionization mode with an acquisition rate of 0.63 spectra per second. Also, while the MS data were acquired over a mass range of 500–3000 m/z, the MS/MS data were acquired over 100–3000 m/z mass range. Mass calibration was enabled using reference masses of m/z 922.010 and 1521.972 (ESI-TOF Tuning Mix G1969-85000, Agilent Technologies, Santa Clara, CA). Data analysis was performed on Agilent MassHunter (Agilent Technologies Inc.). For the MS/MS analysis, N-glycans were subjected to collision induced fragmentation with nitrogen as the collision gas using a series of collision energies that were dependent on the m/z (mass to charge) values of the different N-glycans. The collision energies correspond to voltages (Vcollision) that were based on the equation: Vcollision = m/z (1.8 /100 Da) Volts – 2.4 Volts; where the slope and offset of the voltages were set at (1.8/100 Da) and (− 2.4) respectively. The preferred charge states were set at +2, +3, >+3 and unknown.
Glycan Assignment and Data Processing
Common to all three glycan types is a chitobiose or trimannosyl core corresponding to the pentasaccharide (GlcNAc2:Man3). High mannose glycans are unique in that they typically contain two to six mannose (Man) residues in addition to their trimannosyl core. Complex type glycans are characteristic for containing non-mannose residues such as Fuc, galactose (Gal), N-acetyl glucosamine (GlcNAc) and sialic acid beyond their trimannosyl core. As the name implies; hybrid N-glycans contain features common to both the high mannose and complex type glycans. Putative structures can be assigned based on mass (composition) and established biological rules. In this research, the N-glycan mass list was established from the MS data using an in-house software “oligosaccharide calculator” written in Igor.10 All glycan assignments were made within a specified tolerance level (≤20 ppm) while the data from the tandem MS analysis of the corresponding glycans were investigated to ensure correlation with the assignments made. To rapidly identify tandem MS data belonging to glycan structures, the product ion spectra were automatically sorted by the presence of carbohydrate-specific oxonium fragment ions using “find by auto MS/MS” (a feature in the Agilent MassHunter software). Diagnostic ions include m/z 163.06 [Hex+H]+1, m/z 204.09 [HexNAc+H]+1, m/z 366.14 [HexNAc+Hex+H]+1, m/z 512.20 [HexNAc+Hex+Fuc + H]+1, m/z 292.10 [NeuAc+H]+1, m/z 274.10 [NeuAc–H2O+H]+1, m/z 308.10 [NeuGc+H]+1 and m/z 290.10 [NeuGc–H2O+H]+1.37 The N-glycan compositions were confirmed by the presence of B-type and Y-type ions derived from the sequence of glycan fragmentations in the product ion spectra.
Results and Discussions
LC-MS Analysis of the Human and Bovine Milk N-glycome
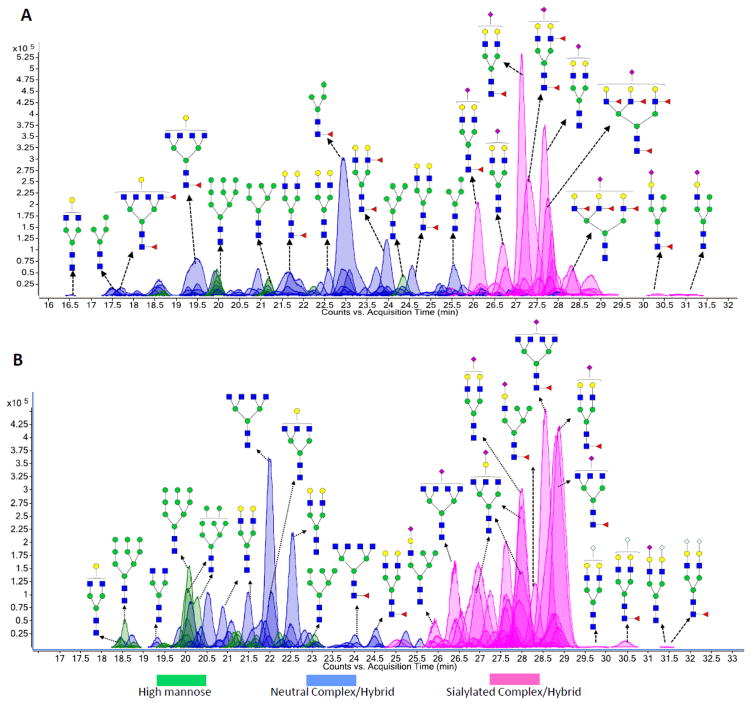
Separation of native oligosaccharides into isomeric species is most effectively performed with porous graphitized carbon (PGC). Previous studies from this laboratory have discussed the effectiveness and the characteristics of the nanoLC separation employing PGC.38 The capability to separate isomers is essential during tandem MS analysis when unique compositions of individual glycans are determined and structural information is obtained, and where interference from other glycan species can affect the analyses. Figure 1A and 1B are representative of the extracted compound chromatograms (ECCs) of the N-glycans from human and bovine milk, respectively. It must be stated that the glycan structures shown in both chromatograms (Figure 1A and 1B) are only putative structures. However, more detailed glycan structures detailing monosaccharide connectivities obtained by MS/MS analysis are described below. Analysis of the accurately measured glycan masses corresponding to each peak in both chromatograms reveals a complicated glycan pool composed of high mannose glycans (peaks shaded in green), neutral complex/hybrid glycans (peaks shaded in blue) and sialylated complex/hybrid glycans (peaks shaded in pink). The human milk N-glycan pool (Figure 1A) corresponds to 38 N-glycan compositions while 51 N-glycan compositions were observed in the bovine milk N-glycan pool (Figure 1B). These numbers translate to over a hundred compounds when isomers are considered and point to the complexity of the mixture. However, they also point to a certain simplicity as the number of glycans is not infinitely large at least over five orders of magnitude observed by LC MS. Table 1 shows a list of N-glycans represented in both chromatograms with their m/z values, neutral masses, monosaccharide compositions, glycan types and their milk source.
Figure 1.
(A) Extracted compound chromatogram (ECC) showing the elution profile of human milk N-glycans via nano-LC/MS. (B) Extracted compound chromatogram (ECC) showing the elution profile of bovine milk N-glycans via nano-LC/MS. Green circles (
 ), yellow circles (
), yellow circles (
 ), blue squares (
), blue squares (
 ), red triangles (
), red triangles (
 ), purple diamonds (
), purple diamonds (
 ) and grey diamonds (
) and grey diamonds (
 ) represent mannose, galactose, GlcNAc, fucose, NeuAc and NeuGc residues, respectively.
) represent mannose, galactose, GlcNAc, fucose, NeuAc and NeuGc residues, respectively.
Table 1.
Comprehensive list of human and bovine milk N-glycans detailing glycan masses, monosaccharide compositions, glycan types and glycan sources.
| m/z | z | m | Glycan Composition
|
Glycan Type | Milk Source | ||||
|---|---|---|---|---|---|---|---|---|---|
| Hexose | HexNAc | Fucose | NeuAc | NeuGc | |||||
| 1235.452 | 1 | 1234.447 | 5 | 2 | - | - | - | High mannose | Human and Bovine |
| 699.288 | 2 | 1396.563 | 6 | 2 | - | - | - | Human and Bovine | |
| 780.316 | 2 | 1558.617 | 7 | 2 | - | - | - | Human and Bovine | |
| 861.338 | 2 | 1720.660 | 8 | 2 | - | - | - | Human and Bovine | |
| 942.332 | 2 | 1882.650 | 9 | 2 | - | - | - | Human and Bovine | |
| 529.207 | 2 | 1056.400 | 3 | 2 | 1 | - | - | Neutral Complex/Hybrid | Human |
| 537.204 | 2 | 1072.381 | 4 | 2 | - | - | - | Human | |
| 610.235 | 2 | 1218.456 | 4 | 2 | 1 | - | - | Human | |
| 659.250 | 2 | 1316.485 | 3 | 4 | - | - | - | Bovine | |
| 719.810 | 2 | 1437.606 | 5 | 3 | - | - | - | Human and Bovine | |
| 732.284 | 2 | 1462.554 | 3 | 4 | 1 | - | - | Bovine | |
| 740.278 | 2 | 1478.541 | 4 | 4 | - | - | - | Human and Bovine | |
| 792.804 | 2 | 1583.593 | 5 | 3 | 1 | - | - | Human | |
| 800.825 | 2 | 1599.635 | 6 | 3 | - | - | - | Bovine | |
| 813.309 | 2 | 1624.604 | 4 | 4 | 1 | - | - | Human and Bovine | |
| 821.308 | 2 | 1640.602 | 5 | 4 | - | - | - | Human and Bovine | |
| 841.820 | 2 | 1681.626 | 4 | 5 | - | - | - | Bovine | |
| 862.335 | 2 | 1722.656 | 3 | 6 | - | - | - | Bovine | |
| 865.831 | 2 | 1729.648 | 5 | 3 | 2 | - | - | Human | |
| 881.823 | 2 | 1761.632 | 7 | 3 | - | - | - | Bovine | |
| 894.336 | 2 | 1786.657 | 5 | 4 | 1 | - | - | Human and Bovine | |
| 902.339 | 2 | 1802.664 | 6 | 4 | - | - | - | Bovine | |
| 913.022 | 3 | 2736.044 | 8 | 7 | - | - | - | Human | |
| 914.867 | 2 | 1827.720 | 4 | 5 | 1 | - | - | Bovine | |
| 935.361 | 2 | 1868.708 | 3 | 6 | 1 | - | - | Bovine | |
| 937.348 | 2 | 2809.022 | 7 | 6 | 3 | - | - | Bovine | |
| 967.357 | 2 | 1932.700 | 5 | 4 | 2 | - | - | Human and Bovine | |
| 975.353 | 2 | 1948.692 | 6 | 4 | 1 | - | - | Human and Bovine | |
| 995.903 | 2 | 1989.791 | 5 | 5 | 1 | - | - | Bovine | |
| 1003.883 | 2 | 2005.752 | 6 | 5 | - | - | - | Bovine | |
| 1008.378 | 2 | 2014.741 | 3 | 6 | 2 | - | - | Human | |
| 1016.377 | 2 | 2030.740 | 4 | 6 | 1 | - | - | Human | |
| 1040.384 | 2 | 2078.753 | 5 | 4 | 3 | - | - | Human and Bovine | |
| 1048.382 | 2 | 2094.750 | 6 | 4 | 2 | - | - | Human and Bovine | |
| 1076.910 | 2 | 2151.806 | 6 | 5 | 1 | - | - | Bovine | |
| 1081.411 | 2 | 2160.807 | 3 | 6 | 3 | - | - | Bovine | |
| 1089.408 | 2 | 2176.802 | 4 | 6 | 2 | - | - | Human | |
| 1109.922 | 2 | 2217.829 | 3 | 7 | 2 | - | - | Human | |
| 1121.439 | 2 | 2240.863 | 6 | 4 | 3 | - | - | Human and Bovine | |
| 1182.925 | 2 | 2363.835 | 3 | 7 | 3 | - | - | Human | |
| 1251.453 | 2 | 2500.892 | 6 | 6 | 2 | - | - | Human | |
| 804.805 | 2 | 1607.595 | 3 | 4 | - | 1 | - | Sialylated Complex/Hybrid | Bovine |
| 857.331 | 2 | 1712.648 | 4 | 3 | 1 | 1 | - | Human | |
| 865.323 | 2 | 1728.631 | 5 | 3 | - | 1 | - | Human and Bovine | |
| 871.667 | 3 | 2611.979 | 3 | 6 | - | 2 | 1 | Bovine | |
| 885.832 | 2 | 1769.650 | 4 | 4 | - | 1 | - | Bovine | |
| 896.342 | 3 | 2686.005 | 3 | 7 | 1 | - | 2 | Bovine | |
| 912.685 | 3 | 2735.032 | 6 | 5 | 3 | 1 | - | Human | |
| 938.352 | 2 | 1874.690 | 5 | 3 | 1 | 1 | - | Human | |
| 946.353 | 2 | 1890.691 | 6 | 3 | 0 | 1 | - | Human and Bovine | |
| 958.857 | 2 | 1915.700 | 4 | 4 | 1 | 1 | - | Bovine | |
| 961.371 | 3 | 2881.091 | 6 | 5 | 4 | 1 | - | Human | |
| 966.863 | 2 | 1931.711 | 5 | 4 | 0 | 1 | - | Human and Bovine | |
| 974.853 | 2 | 1947.692 | 5 | 4 | - | - | 1 | Bovine | |
| 987.369 | 2 | 1972.724 | 4 | 5 | - | 1 | - | Bovine | |
| 995.348 | 2 | 1988.682 | 4 | 5 | - | - | 1 | Bovine | |
| 1007.880 | 2 | 2013.746 | 3 | 6 | - | 1 | - | Bovine | |
| 1019.373 | 2 | 2036.732 | 6 | 3 | 1 | 1 | - | Bovine | |
| 1039.883 | 2 | 2077.751 | 5 | 4 | 1 | 1 | - | Human and Bovine | |
| 1047.880 | 2 | 2093.745 | 5 | 4 | 1 | - | 1 | Bovine | |
| 1060.4073 | 2 | 2118.800 | 4 | 5 | 1 | 1 | - | Human and Bovine | |
| 1068.379 | 2 | 2134.743 | 5 | 5 | - | 1 | - | Bovine | |
| 1112.430 | 2 | 2222.845 | 5 | 4 | - | 2 | - | Human and Bovine | |
| 1112.922 | 2 | 2223.830 | 5 | 4 | 2 | 1 | - | Human | |
| 1120.399 | 2 | 2238.784 | 5 | 4 | - | 1 | 1 | Bovine | |
| 1128.400 | 2 | 2254.785 | 5 | 4 | - | - | 2 | Bovine | |
| 1185.952 | 2 | 2369.890 | 5 | 4 | 3 | 1 | - | Human | |
| 1201.425 | 2 | 2400.836 | 5 | 4 | 1 | - | 2 | Bovine | |
| 1201.425 | 2 | 2400.836 | 6 | 4 | - | 1 | 1 | Bovine | |
The two chromatograms (Figure 1A and 1B) reveal an elution pattern mainly starting with the high mannose followed by neutral complex/hybrid glycans and finally the sialylated glycans. This elution profile is consistent with previous observations suggesting that sialylated glycans have a higher affinity for graphitized carbon than the neutral glycans.38,39,40 For the high mannose glycans, the larger species (GlcNAc2:Man8 and GlcNAc2:Man9) typically have earlier retention times than the smaller species (GlcNAc2:Man5 and GlcNAc2:Man6). In both chromatograms, sialylated N-glycans were observed containing the NeuAc residue. However, the bovine milk glycan chromatogram (Figure 1B) revealed NeuGc-containing sialylated species as well. The NeuGc-containing species were confirmed by the tandem MS experiments described below. This marks the significant difference between human and bovine milk sources. To our best knowledge, this study also represents the first MS based confirmation of NeuGc in milk glycoproteins.
Characterization of Human and Bovine Milk N-glycans via Tandem MS Analysis
As MS analysis does not always yield unequivocal glycan compositions, tandem MS is often necessary for verification. Tandem MS or specifically collision-induced dissociation (CID) provides fragments that yield glycan sequence thus revealing monosaccharide compositions and connectivities. Hence, to obtain structural information for each peak in both chromatograms (Figure 1A and 1B), tandem MS was performed. Glycans were subjected to collision energies that were dependent on their m/z values. It should be noted that glycan ions were observed as mono-protonated [M+H]+1, di-protonated [M+2H]+2 or tri-protonated [M+3H]+3 species.
Tandem MS analyses of glycans primarily result in B- and Y-type fragment ions.37 Under our optimized conditions, glycan CID spectra share a group of common diagnostic fragment ions. They include small ions corresponding to m/z 163.06 Da [Hex+H)]+1, m/z 204.09 Da [HexNAc+H]+1 and m/z 366.14 Da [HexNAc+Hex+H]+1. A common LC-MS/MS analysis generates thousands of spectra but not all are glycans. These small ions are important as diagnostic peaks that allow rapid selection of glycan MS/MS spectra. Those that are selected are deconvoluted using the Agilent Mass Hunter software. The deconvoluted tandem spectrum reveals neutral masses of the fragment ions and allows for easier assignment of the peaks in the spectrum. In addition to the common diagnostic ions observed in the CID spectra, unique fragment ions are observed for specific glycan types. As discussed in greater detail below, there are fragment ions that distinguish high mannose from fucosylated (complex) as well as sialylated (complex) type glycans.
LC-MS/MS Analysis of High Mannose N-glycans
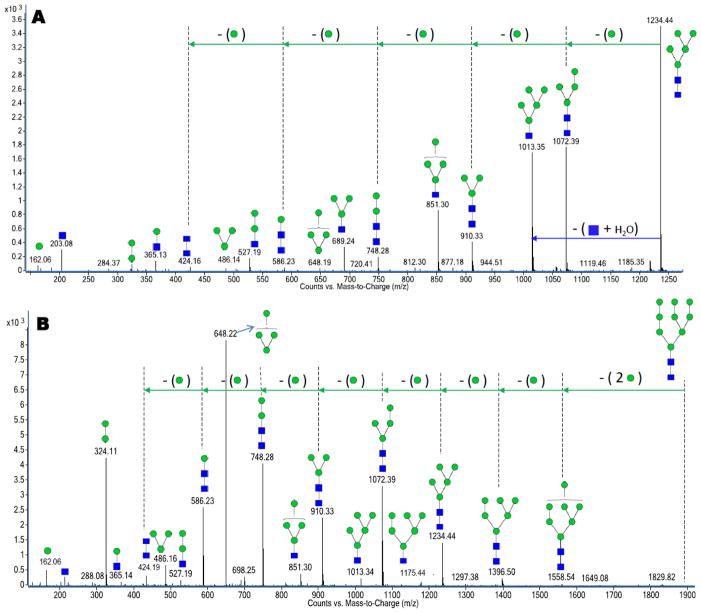
Figure 2A and 2B are representative examples of two high mannose N-glycans with m/z 1235.45 assigned as [GlcNAc2+Man5+H]+1 and m/z 942.33 assigned as [GlcNAc2+Man9+2H]+2, respectively. Both the human and bovine milk N-glycan profiles contained all five possible mannose products (Table 1). These glycans probably originate from lactoferrin in bovine milk (b-LF) and human immunoglobulin M (IgM).29,41 The two proteins are abundant in the respective fluids and have previously been shown to contain strong abundances of high mannose glycans.
Figure 2.
Deconvoluted CID data for (A) a high mannose N-glycan with m/z 1235.45 corresponding in mass to [GlcNAc2+Man5+H]+1. (B) a high mannose N-glycan with m/z 942.33 corresponding in mass to [GlcNAc2+Man9+2H]+2. Both glycans represented in Figures 2A and 2B were observed in human and bovine milk.
The deconvoluted CID spectrum yields extensive fragmentation from the loss of one or two residues from the precursor ion to the monosaccharide and disaccharide fragments. Both large mass fragments and small fragments yield important structural information. Tandem MS data of high mannose N-glycans routinely contain fragment ions composed of sequential mannose losses. This results in a 162 Da difference between adjacent peaks in the deconvoluted MS/MS data. The spectra also include B-type ions that correspond to 162 Da (Man1), 324 Da (Man2), 486 Da (Man3) and 648 Da (Man4) peaks. Other B-type ions with an additional GlcNAc residue attached are also observed in the CID data. This set of fragment ions is due to the cleavage between the chitobiose core. They include fragment ions corresponding to 365 Da (GlcNAc1:Man1), 527 Da (GlcNAc1:Man2), 689 Da (GlcNAc1:Man3), 851 Da (GlcNAc1:Man4) and 1013 Da (GlcNAc1:Man5) in the deconvoluted CID spectra. Apart from these B-type fragment ions, high mannose N-glycans also contain a series of Y-type fragment ions that are complimentary to the previously mentioned B-type ions. The series of Y-type ions results from the sequential losses of mannose residues (162 Da) from the glycan precursor ion. Depending on the number of mannoses, the Y-type fragment can produce ladder patterns of mannoses up to the total mannose content of the compound.
LC-MS/MS Analysis of Neutral Complex N-glycans
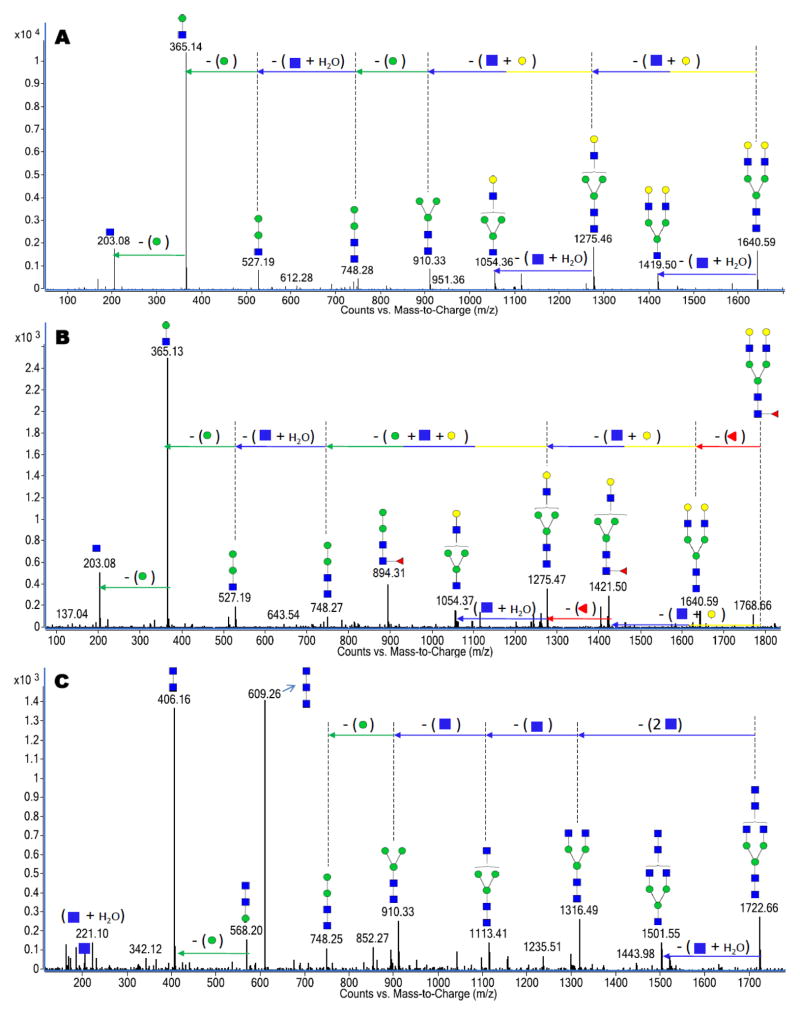
Neutral complex N-glycans refer to non-high mannose glycans lacking the sialic acid residues in their compositions. They are sometimes fucosylated with the residue bound to a GlcNAc reducing end and/or an antenna on a GlcNAc residue. Figure 3A, 3B and 3C are representative examples of the deconvoluted MS/MS spectra of three neutral complex N-glycans. The deconvoluted mass spectra represented in Figure 3A for the ion m/z 821.31 (z = +2) corresponds to a bi-antennary glycan assigned as [GlcNAc4+Hex5+2H]+2. Unlike the high mannose CID where mannose losses (162 Da) dominate the spectra, the spectrum reveals the loss of two disaccharide units (GlcNAc:Hex) corresponding to a 365 Da loss. Likewise, Figure 3B represents another bi-antennary glycan with m/z 894.34 assigned as [GlcNAc4+Hex5+Fuc1+2H]+2 and corresponds to the fucosylated oligomer of the compound in Figure 3A. As expected, both spectra (Figure 3A and 3B) share some similar fragment ions. However, a fucose residue loss (146 Da) from the precursor ion is observed in Figure 3B. Additionally, the 894 Da peak corresponding to (GlcNAc2:Man2:Fuc1) in the spectrum further confirms fucosylation while revealing the fucose residue on the trimannosyl core. IgG is a common protein found in both human and bovine milk sources 12,13 and has previously been shown to be associated with the glycans 30 represented in Figure 3A and 3B.
Figure 3.
Deconvoluted CID data for (A) a neutral bi-antennary complex N-glycan with m/z 821.31 corresponding in mass to [GlcNAc4+Hex5+2H]+2. (B) a neutral bi-antennary complex N-glycan with m/z 894.34 corresponding in mass to [GlcNAc4+Hex5+Fuc1+2H]+2. (C) a neutral bi-antennary complex N-glycan with m/z 862.34 corresponding in mass to [HexNAc6+Man3+2H]+2. Both glycans represented in Figures 3A and 3B were observed in human and bovine milk while the glycan represented in Figure 3C was unique to only the bovine milk source.
The third example presented in Figure 3C with m/z 862.34 corresponds to [HexNAc6+Man3+2H]+2. The deconvoluted CID data reveal a unique structural feature with three HexNAc residues connected owing to the unusual 609 Da trisaccharide peak corresponding to (HexNAc3) and an equally intense 406 Da disaccharide peak corresponding to (HexNAc2). The CID data further reveal four GlcNAc residues (203 Da for each residue) sequentially lost from the glycan precursor peak. The glycan is believed to be bi-antennary as there was no peak suggesting that the fourth antennae GlcNAc residue was also directly connected to the (HexNAc3) trisaccharide. The 609 Da and 406 Da peaks clearly disqualify the assumption of a tri- or tetra-antennary glycan in this example. The glycan represented in Figure 3C is unique to the bovine milk sample as it was not observed in the human milk glycan pool.
LC-MS/MS Analysis of Sialylated Complex N-glycans
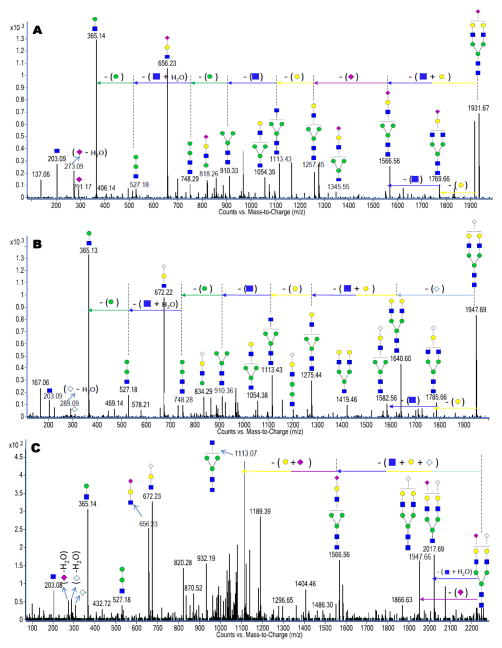
Sialylated N-glycans contain NeuAc and/or NeuGc. Particularly to NeuAc-sialylation, B-type ions corresponding to m/z 292.10 [NeuAc+H]+1, m/z 274.10 [NeuAc–H2O+H]+1 and m/z 657.24 [GlcNAc+Hex+NeuAc+H]+1 are observed as diagnostic peaks in the CID spectra. As an example, Figure 4A represents the deconvoluted CID data of a sialylated N-glycan with m/z 966.86 corresponding to [GlcNAc4+Hex5+NeuAc1+2H]+2. The 273 Da, 291 Da and 656 Da peaks are all observed in the deconvoluted tandem MS data as expected. The spectrum also reveals a NeuAc neutral loss (291 Da), which further verifies NeuAc in the composition of the sialylated N-glycan being investigated. The glycan represented in Figure 4A has been shown to be associated with IgG, 30 which was observed in both milk glycan pools 10,11.
Figure 4.
Deconvoluted CID data for (A) a NeuAc-sialylated N-glycan with m/z 966.86 corresponding in mass to [GlcNAc4+Hex5+NeuAc1+2H]+2. (B) a NeuGc-sialylated N-glycan with m/z 974.85 corresponding in mass to [GlcNAc4+Hex5+NeuGc1+2H]+2. (C) a bi-sialylated N-glycan with m/z 1120.40 corresponding in mass to [GlcNAc4+Hex5+NeuAc1+NeuGc1+2H]+2. The numerous unassigned peaks in Figure 4C are probably due to isobaric interferences in isolating the precursor ion. The glycan represented in Figure 4A was observed in both the human and bovine milk sources while the glycans represented in Figures 4B and 4C were unique to only the bovine milk source.
With NeuGc sialylation, B-type ions corresponding to m/z 308.10 [NeuGc+H]+1, m/z 290.10 [NeuGc–H2O+H]+1 and m/z 673.23 [GlcNAc+Hex+NeuGc+H]+1 are observed as diagnostic peaks in their CID spectra. Figure 4B represents the deconvoluted MS/MS data of a sialylated N-glycan with m/z 974.85 corresponding to [GlcNAc4+Hex5+NeuGc1+2H]+2. The 289 Da, 307 Da and 672 Da peaks are observed, as described. The spectrum also reveals a NeuGc neutral loss (307 Da), which further verifies NeuGc in the sialylated N-glycan. Interestingly, some sialylated N-glycans were observed with both NeuAc and NeuGc residues on the same glycan. Figure 4C is representative of the deconvoluted CID spectrum of a bi-sialylated N-glycan with m/z 1120.40 corresponding to [GlcNAc4+Hex5 +NeuAc1+NeuGc1+2H]+2. As expected, the MS/MS data of this glycan contain a combination of peaks observed in Figure 4A and 4B. They include diagnostic peaks such as the 273 Da, 289 Da, 307 Da, 656 Da and 672 Da peaks. The glycan examples represented in Figure 4B and 4C were only observed in the bovine milk.
Glycan Isomer-specific Analysis
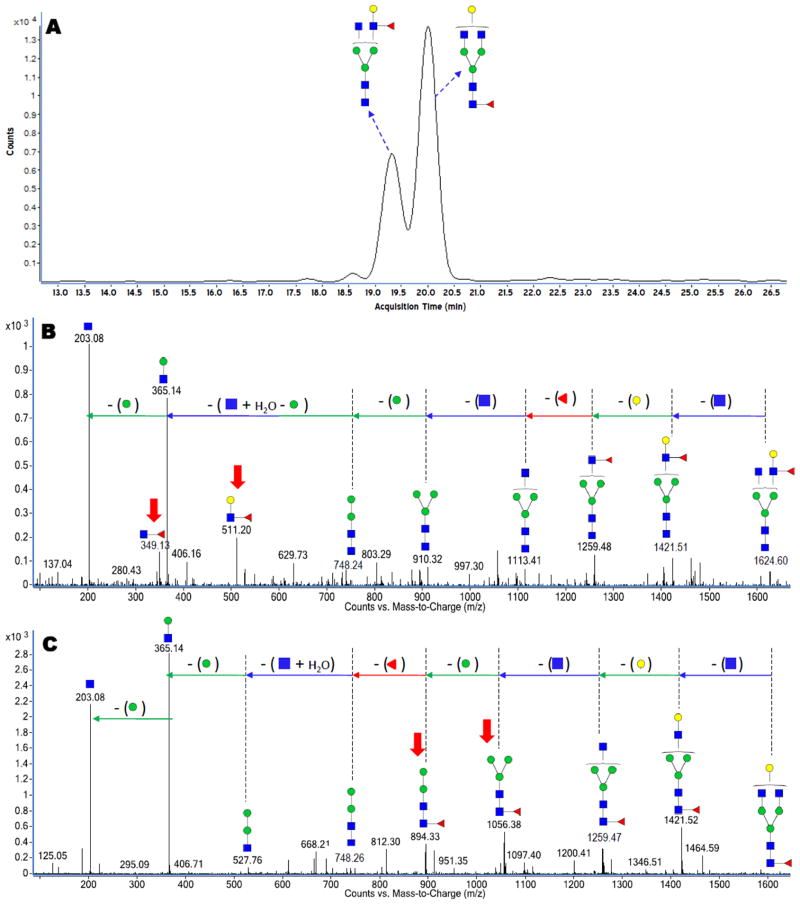
Separation of N-glycans 38 and N-glycopeptides 37 on the PGC chip occurs based on the unique structures of the glycans and their monosaccharide compositions. Owing to this separation, PGC particularly affords a suitable means for separation of glycan isomers 38, which in turn yields detailed MS/MS information providing compositions and connectivities of separated glycan isomers. Figure 5A is an extracted ion chromatogram (EIC, constructed with a precursor ion search within a 10 ppm error margin) for the N-glycan with m/z 813.31 corresponding in mass to [GlcNAc4+Hex4+Fuc1+2H]+2. The EIC of the fucosylated glycan shows two major peaks at 19.35 min and at 20.00 min, suggesting at least two isomers. Figure 5B and 5C show the deconvoluted CID spectra for the glycan eluting at 19.35 min and 20.00 min, respectively. The spectrum in Figure 5B suggests the attachment of the fucose residue to the antenna GlcNAc residue based on the 349 Da and 511 Da peaks, which correspond in mass to the disaccharide (GlcNAc:Fuc) and the trisaccharide (GlcNAc:Hex:Fuc), respectively. However, the deconvoluted CID spectrum in Figure 5C shows two distinct fragment peaks that signify a fucose connected to a core GlcNAc residue. The 894 Da and 1056 Da peaks corresponding in mass to (Hex2:GlcNAc2:Fuc) and (Hex3:GlcNAc2:Fuc) distinguish this isomer as being core fucosylated in contrast to Figure 5B. The core-fucosylated glycan structure represented in Figure 5C is depicted with the fucose residue attached to the outer core GlcNAc based on biological precedence.
Figure 5.
(A) Extracted ion chromatogram (EIC) showing two isomers for the N-glycan with m/z 813.31 corresponding in mass to [GlcNAc4+Hex4+Fuc1+2H]+2. (B) Deconvoluted CID data for the isomer eluting at 19.35 min. (C) Deconvoluted CID data for the isomer eluting at 20.00 min. Both glycans represented in Figures 5B and 5C were observed in human and bovine milk. The red bold arrows correspond to diagnostic ions that signify core and antenna fucosylation.
Comparison of the N-Glycomes from Human and Bovine Milk
The use of nano-LC MS in this study affords the opportunity to investigate certain distinguishing features in the glycan profiles obtained from human (Figure 1A) and bovine (Figure 1B) milks. Table 2 represents a comparison of some of such features (high mannose, fucosylation and sialylation) observed in both chromatograms in terms of glycan compositions. While all five high mannose glycan compositions (GlcNAc2:Man5–9) were observed in both chromatograms, significant differences were observed in terms of fucosylated and sialylated glycan compositions in both chromatograms. With regards to fucosylation, 25 of the 38 human milk N-glycan compositions were observed as fucosylated while 21 of the 51 were observed for bovine. Of these numbers, about 60% of the fucosylated human milk glycan compositions were observed with higher degrees of fucosylation (di-, tri- and tetra-) compared to only about 30% of the bovine milk glycans. In terms of sialylation, 12 of the 38 human milk N-glycans were observed as sialylated compared to 22 of the 51 for bovine. Only about 10% of the sialylated human milk glycan compositions were multi-sialylated compared to about 30% for bovine.
Table 2.
Comparison of general features in glycosylation between the human and bovine milk as analyzed by nano-LC MS.
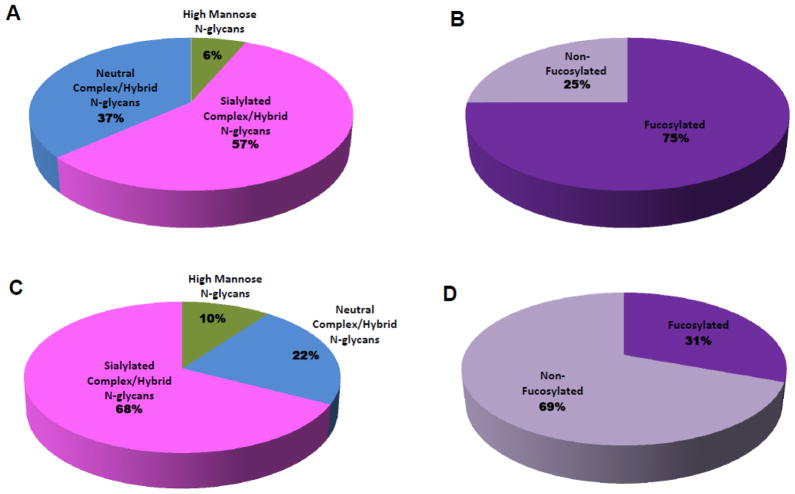
Relative quantitation of the different N-glycan types in both milks was performed based on the nano-LC MS analyses. Figure 6A and 6B are pie charts showing the relative abundances of all the different N-glycan types and the fucosylation distribution observed in the human milk analysis, respectively. Figure 6C and 6D are the corresponding pie charts for the bovine milk analysis representing the relative abundances of all the different N-glycan types and the fucosylation distribution observed, respectively. Fucosylation marks a significant difference between the N-glycans in both milk sources based on abundance. While 75% of the total N-glycan abundances corresponded to fucosylated glycans in human milk, only 31% of the total N-glycan abundance in bovine milk corresponded to fucosylated glycans. The results suggest that while high fucosylation and sialylation are general features of the human milk N-glycome, bovine milk is significantly less fucosylated and more highly sialylated. Although, mixtures of neutral and sialylated glycans will produce suppressed sialylated signals in positive ion mode MS analysis. However, when isolated or separated via LC-MS, the ionization efficiencies are not too different from each other.42 Hence, in this text, we have used glycan peak areas to compare the amounts of fucosylation and sialylation between the two milk samples. Another major difference between both glycan pools is the presence of NeuGc residues in bovine milk N-glycans, which was lacking in the human milk. Furthermore, 5% of the sialylation abundance in the bovine milk N-glycan pool corresponded to NeuGc residues with less than 1% containing both NeuAc and NeuGc on the same glycan. It is also important to state that of the 38 N-glycan compositions observed in human milk and the 51 N-glycan compositions observed in bovine milk, 20 glycan compositions were common to both milk sources.
Figure 6.
Pie charts showing the relative abundances of (A) all the N-glycan types in human milk. (B) fucosylated N-glycans in human milk. (C) all the N-glycan types in bovine milk. (D) fucosylated N-glycans in bovine milk.
Comparison of the Milk N-Glycome with Free Milk Oligosaccharides
Extensive analyses of free human milk oligosaccharides (HMOs) have previously been reported.7,10 The results from these studies suggest that HMOs and their corresponding protein-bound N-glycans observed in this study were significantly different in terms of sialylation. While 57% sialylation was observed for the human milk N-glycans in this study, less than 20% of HMOs was previously reported as being sialylated 7,10. This suggests that protein-bound N-glycans are potentially a major source of sialylation in breast-fed infants. With respect to fucosylation, results of both the human milk N-glycans and the HMOs revealed greater similarity. While 75% of human milk N-glycans was observed to be fucosylated in this study, 70% of HMOs were previously reported as fucosylated. No NeuGc residue was observed in the two human milk carbohydrates.
As with HMOs, bovine milk oligosaccharides (BMOs) have been previously studied.11 Contrary to the sialylation and fucosylation trends between human milk N-glycans and HMOs, the bovine milk reveals a different result. While the degree of sialylation was similar for bovine milk N-glycans and BMOs, the levels of fucosylation differed. Specifically, 68% sialylation was observed for the bovine milk N-glycans in this study, while 70% of the BMOs was previously reported as being sialylated.11 On the other hand, 31% of bovine milk N-glycans was observed to be fucosylated in this study as compared to less than 1% for BMOs.11 This comparison suggests that protein-bound N-glycans are a major source of fucosylation in bovine milk. It is important to also note that NeuGc residues were observed in both bovine milk N-glycans and BMOs.
Conclusion
The approach described used LC-MS and LC-MS/MS analyses of N-glycans from human and bovine whey proteins on a well validated nano-LC/Q-TOF MS platform. This analysis ensured the separation, detection and characterization of a complicated mixture of N-glycans investigated in this study. The N-glycans were separated on a PGC chip based on their unique types and monosaccharide compositions. Isomeric glycans differing in their monosaccharide connectivities and/or compositions were separated on the PGC chip into unique components during the analysis. This separation enabled structural elucidation for the individual species during the LC/MS/MS analysis. In all, 38 N-glycan compositions were observed in the human milk N-glycome while 51 N-glycan compositions were observed in the bovine milk N-glycome. Again, these numbers do not include isomers as over a hundred total glycan compounds were observed in each milk source. The study was comprehensive as high mannose, neutral complex/hybrid and sialylated complex/hybrid N-glycans were all observed. The sialylated N-glycans in the human milk source contained only NeuAc while both NeuAc and NeuGc residues were observed in the bovine milk source. To the best of our knowledge, this study is the first reported MS and tandem MS analyses confirming NeuGc in milk glycoproteins. The NeuGc residue was not found in human samples. The development of this strategy affords a platform for direct comparison between the N-glycome of both milks.
SYNOPSIS.
The isolation of whey proteins from human and bovine milks followed by profiling of their entire N-glycan repertoire using nano-flow liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (nano-LC/Q-TOF MS) is described.
Acknowledgments
Funds provided by the National Institute of Health (GM049077, HD061923, P42 ES02710 and HD059127) and California Dairy Research Foundation Grant 06 LEC-01-NH are gratefully acknowledged. The Agilent QTOF MS instrument was obtained through a grant (S10RR027639). Special thanks to Henry Young for preparing and providing the bovine milk samples.
References
- 1.Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. 1999;88(430):89–94. doi: 10.1111/j.1651-2227.1999.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 2.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Ann Rev Nutr. 2000;20(1):699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 3.Kunz C, Rudloff S. Health promoting aspects of milk oligosaccharides. Int Dairy J. 2006;16(11):1341–1346. [Google Scholar]
- 4.Boehm G, Stahl B. Oligosaccharides from milk. J Nutr. 2007;137(3):847S–849S. doi: 10.1093/jn/137.3.847S. [DOI] [PubMed] [Google Scholar]
- 5.Veh RW, Michalski JC, Corfield AP, Sander-Wewer M, Gies D, Schauer R. New chromatographic system for the rapid analysis and preparation of colostrum sialyloligosaccharides. J Chromatogr A. 1981;212(3):313–322. doi: 10.1016/s0021-9673(01)84044-9. [DOI] [PubMed] [Google Scholar]
- 6.Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nurt. 2006;136(8):2127–2130. doi: 10.1093/jn/136.8.2127. [DOI] [PubMed] [Google Scholar]
- 7.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Ann Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 8.Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145(3):297–303. doi: 10.1016/j.jpeds.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Huang PW, Zhong WM, Tan M, Farkas T, Morrow AL, Newburg DS, Ruiz-Palacios GM, Pickering LK. Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J Infect Dis. 2004;190(10):1850–1859. doi: 10.1086/425159. [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9(8):4138–4151. doi: 10.1021/pr100362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao N, DePeters EJ, Freeman S, German JB, Grimm R, Lebrilla CB. Bovine milk glycome. J Dairy Sci. 2008;91(10):3768–3778. doi: 10.3168/jds.2008-1305. [DOI] [PubMed] [Google Scholar]
- 12.Liao Y, Alvarado R, Phinney B, Lo nnerdal B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J Proteome Res. 2011;10(4):1746–1754. doi: 10.1021/pr101028k. [DOI] [PubMed] [Google Scholar]
- 13.Le A, Barton LD, Sanders JT, Zhang Q. Exploration of bovine milk proteome in colostral and mature whey using an ion-exchange approach. J Proteome Res. 2011;10(2):692–704. doi: 10.1021/pr100884z. [DOI] [PubMed] [Google Scholar]
- 14.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 15.An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr Opin Chem Biol. 2009;13(4):421–426. doi: 10.1016/j.cbpa.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newburg DS. Human milk glycoconjugates that inhibit pathogens. Curr Med Chem. 1999;6(2):117–128. [PubMed] [Google Scholar]
- 17.Hamosh M. Protective function of proteins and lipids in human milk. Biol Neonate. 1998;74(2):163–176. doi: 10.1159/000014021. [DOI] [PubMed] [Google Scholar]
- 18.Schroten H, Hanisch F, Plogmann R, Hacker J, Uhlenbruck G, Nobis-Bosch R, Wahn V. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human milk fat globule membrane components: a novel aspect of the protective function of mucins in the nonimmunoglobulin fraction. Infect Immun. 1992;60(7):2893–2899. doi: 10.1128/iai.60.7.2893-2899.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yolken R, Peterson J, Vonderfecht S, Fouts E, Midthun K, Newburg D. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992;90(95):1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamauchi K, Wakabayashi H, Shin K, Takase M. Bovine lactoferrin: Benefits and mechanism of action against infections. Biochem Cell Biol. 2006;84(3):291–296. doi: 10.1139/o06-054. [DOI] [PubMed] [Google Scholar]
- 21.Harmsen MC, Swart PJ, de Bethune MP, Pauwels R, De Clercq E, The TH, Meijer DK. Antiviral effects of plasma and milk proteins: Lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis. 1995;172(2):380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- 22.Hanson LA, Ahlstedt S, Andersson B, Carlsson B, Fallstrom SP, Mellander L, Porras O, Soderstrom T, Eden CS. Protective factors in milk and the development of the immune system. Pediatrics. 1985;75(1):172–176. [PubMed] [Google Scholar]
- 23.Hamosh M, Peterson JA, Henderson TR, Scallan CD, Kiwan R, Ceriani RL, Armand M, Mehta NR, Hamosh P. Protective function of human milk: The milk fat globule. Semin Perinatol. 1999;23(3):242–249. doi: 10.1016/s0146-0005(99)80069-x. [DOI] [PubMed] [Google Scholar]
- 24.Paterson JA, Patton S, Hamosh M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol Neonate. 1998;74(2):143–162. doi: 10.1159/000014020. [DOI] [PubMed] [Google Scholar]
- 25.Ofek I, Sharon N. Adhesins as lectins: Specificity and role in infection. Curr Top Microbiol Immunuol. 1990;151:91–113. doi: 10.1007/978-3-642-74703-8_5. [DOI] [PubMed] [Google Scholar]
- 26.Ashorn P, Vilja P, Shorn R, Rohn K. Lectin binding affinities of human milk fat globule (HMFG) membrane antigens. Mol Immunol. 1986;23(3):221–230. doi: 10.1016/0161-5890(86)90046-5. [DOI] [PubMed] [Google Scholar]
- 27.Jones J, Krag SS, Betenbaugh MJ. Controlling N-linked glycan site occupancy. Biochim Biophys Acta. 2005;1726(2):121–137. doi: 10.1016/j.bbagen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Picariello G, Ferranti P, Mamone G, Roepstorff P, Addeo F. Identification of N-linked glycoproteins in human milk by hydrophilic interaction liquid chromatography and mass spectrometry. Proteomics. 2008;8(18):3833–3847. doi: 10.1002/pmic.200701057. [DOI] [PubMed] [Google Scholar]
- 29.Hurley WL, Grieve RCJ, Magura CE, Hegarty HM, Zou S. Electrophoretic Comparisons of Lactoferrin from Bovine Mammary Secretions, Milk Neutrophils, and Human Milk. J Dairy Sci. 1993;76(2):377–387. doi: 10.3168/jds.S0022-0302(93)77356-7. [DOI] [PubMed] [Google Scholar]
- 30.Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics. 2008;8(14):2858–2871. doi: 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- 31.Royle L, Roos A, Harvey D, Wormald M, Gijlswijk-Janssen DV, Redwan EM, Wilson IA, Daha MR, Dwek RA, Rudd PM. Secretory IgA N- and O-Glycans Provide a Link between the Innate and Adaptive Immune. Systems J Biol Chem. 2003;278(22):20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 32.Landberg E, Huang Y, Stromqvist M, Mechref Y, Hansson L, Lundblad A, Novotny MV, Pahlsson P. Changes in glycosylation of human bile-salt-stimulated lipase during lactation. Arch Biochem Biophys. 2000;377(2):246–254. doi: 10.1006/abbi.2000.1778. [DOI] [PubMed] [Google Scholar]
- 33.Charlwood J, Hanrahan S, Tyldesley R, Langridge J, Dwek M, Camilleri P. Use of proteomic methodology for the characterization of human milk fat globular membrane proteins. Anal Biochem. 2002;301(2):314–324. doi: 10.1006/abio.2001.5498. [DOI] [PubMed] [Google Scholar]
- 34.Stanley P, Schachter H, Taniguchi N. N-Glycans. In: Varki A, editor. Essentials of Glycobiology. 2. Vol. 1 Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. [Google Scholar]
- 35.An HJ, Peavy TR, Hedrick JL, Lebrilla CB. Determination of N-glycosylation sites and site heterogeneity in glycoproteins. Anal Chem. 2003;75(20):5628–5637. doi: 10.1021/ac034414x. [DOI] [PubMed] [Google Scholar]
- 36.Stefansson M, Novotny M. Resolution of the branched forms of oligosaccharides by high-performance capillary electrophoresis. Carbohydr Res. 1994;258:1–9. doi: 10.1016/0008-6215(94)84070-9. [DOI] [PubMed] [Google Scholar]
- 37.Nwosu CC, Seipert RR, Strum JS, Hua SS, An HJ, Zivkovic AM, German BJ, Lebrilla CB. J Proteome Res. 2011;10(5):2612–2624. doi: 10.1021/pr2001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu CS, Ninonuevo MR, Clowers BH, Perkins PD, An HJ, Yin H, Killeen K, Miyamoto S, Grimm R, Lebrilla CB. Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics. 2009;9(7):1939–1951. doi: 10.1002/pmic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies M, Smith R. Use of a porous graphitized carbon column for the high-performance liquid chromatography of oligosaccharides, alditols and glycopeptides with subsequent mass spectrometry analysis. J Chromatogr. 1993;646(2):317–326. doi: 10.1016/0021-9673(93)83344-r. [DOI] [PubMed] [Google Scholar]
- 40.Davies M, Smith KD, Harbin AM, Hounsell EF. High performance liquid chromatography of oligosaccharide alditols and glycopeptides on a graphitized carbon column. J Chromatogr. 1992;609(1–2):125–131. doi: 10.1016/0021-9673(92)80155-n. [DOI] [PubMed] [Google Scholar]
- 41.Arnold JN, Wormald, Suter DM, Radcliffe CM, Harvey, Dwek RA, Rudd, Sim RB. Human Serum IgM Glycosylation: Identification of Glycoforms that can bind to Mannan-Binding Lectin. J Biol Chem. 2005;280(32):29080–29087. doi: 10.1074/jbc.M504528200. [DOI] [PubMed] [Google Scholar]
- 42.An HJ, Lebrilla CB. Suppression of Silaylated by Sulfated Oligosaccharides in Negative MALDI-FTMS. Isr J Chem. 2001;41(2):117–127. [Google Scholar]