Abstract
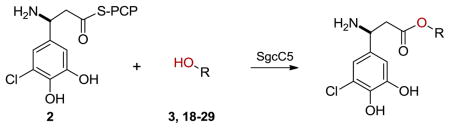
The SgcC5 condensation enzyme catalyzes the attachment of SgcC2-tethered (S)-3-chloro-5-hydroxy-β-tyrosine (2) to the enediyne core in C-1027 (1) biosynthesis. It is reported that SgcC5 (i) exhibits high stereospecificity towards the (S)-enantiomers of SgcC2-tethered β-tyrosine and analogues as donors, (ii) prefers the (R)-enantiomers of 1-phenyl-1,2-ethanediol (3) and analogues, mimicking the enediyne core, as acceptors, and (iii) can recognize a variety of donor and acceptor substrates to catalyze their regio- and stereospecific ester-bond formations.
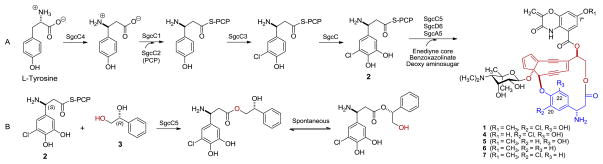
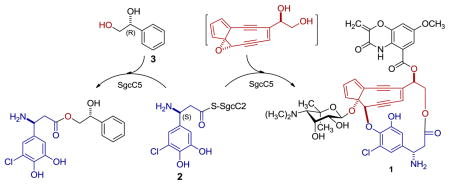
C-1027, a chromoprotein enediyne antitumor antibiotic produced by Streptomyces globisporus, consists of an apoprotein and the C-1027 chromophore (1).1 In vivo and in vitro characterization of the C-1027 biosynthetic machinery has unveiled a convergent strategy for production of 1, in which the central enediyne core is appended with three peripheral moieties of a deoxy aminosugar, a benzoxazolinate, and an (S)-3-chloro-5-hydroxy-β-tyrosine.2 We have previously shown that the (S)-3-chloro-5-hydroxy-β-tyrosine moiety is derived from L-tyrosine and activated in the form of (S)-3-chloro-5-hydroxy-β-tyrosyl-S-SgcC2 (2) before its incorporation into 1 and these steps are catalyzed by the SgcC4 aminomutase,3 the SgcC1 adenylation enzyme [as well as the SgcC2 peptidyl carrier protein (PCP)],4 the SgcC3 halogenase,5 and SgcC hydroxylase,6 respectively (Figure 1A). We further demonstrated that the incorporation of the (S)-3-chloro-5-hydroxy-β-tyrosine moiety of 2 into 1 was catalyzed by the SgcC5 condensation enzyme.7 Using (R)-1-phenyl-1,2-ethanediol (3) as an enediyne core substrate mimic, we have revealed that SgcC5 absolutely requires the tethering of (S)-3-chloro-5-hydroxy-β-tyrosine to SgcC2 in order to recognize it as a donor substrate, and catalyzes regiospecific ester bond formation to the C-2 hydroxyl group of 3 (Figure 1B).7
Figure 1.
(A) Structures of the enediyne chromophore of C-1027 (1) and engineered analogues 7″-desmethyl-C-1027 (4), 20-deschloro-C-1027 (5), 20-deschloro-22-deshydroxy-C-1027 (6), and 22-deshydroxy-C-1027 (7) and biosynthesis of the (S)-3-chloro-5-hydroxy-β-tyrosine moiety and its activation by tethering to SgcC2 (2) and incorporation into 1. (B) The SgcC5-catalyzed ester-bond formation between 2 and (R)-1-phenyl-1,2-ethanediol (3) as a substrate mimic of the enediyne core. The (S)-3-chloro-5-hydroxy-β-tyrosine moiety and the enediyne cores of 1 and 4–7 are highlighted in blue and red, respectively. The nascent ester product resulting from the coupling between 2 and 3 can undergo spontaneous rearrangement, affording a pair of regioisomer.7
As a hallmark structural feature for the enediyne family of antitumor antibiotics, the enediyne core is directly responsible for its potent cytotoxicity, whereas the peripheral moieties are known to be critical in fine-tuning bioactivity and enhancing the stability. These structural features have been exploited by manipulating the C-1027 biosynthetic machinery to generate the new C-1027 analogues 7″-desmethyl-C-1027 (4), 20-deschloro-C-1027 (5), 20-deschloro-22-deshydroxy-C-1027 (6), and 22-deshydroxy-C-1027 (7), all of which indeed exhibited varying activities or altered modes of action (Figure 1A).8 Intriguingly, the isolation of 5 and 6 from the ΔsgcC3 mutant strain SB10084a and 7 from the the ΔsgcC mutant strain SB10062a also suggested that SgcC5 must have exhibited some degree of substrate promiscuity as long as the β-tyrosyl moiety is tethered to SgcC2.
As a part of our continuing efforts to biochemically characterize the C-1027 biosynthetic machinery, we now report that SgcC5 (i) exhibits high stereospecificity towards the (S)-enantiomers of SgcC2-tethered β-tyrosine and analogues as donor substrates, (ii) prefers the (R)-enantiomers of 1-phenyl-1,2-ethanediol and analogues as acceptor substrates, and (iii) can recognize a variety of donor and acceptor substrates to catalyze their regio- and stereospecific ester-bond formations (Tables 1, 2, 3). These findings further motivate our on-going effort to engineer new C-1027 analogues by manipulating the C-1027 biosynthetic machinery via combinatorial biosynthesis strategies.
Table 1.
Enantiospecificity of SgcC5-catalyzed ester bond formation between selected donor and acceptor substrates
 | |||
|---|---|---|---|
| substrates | Km (μM) | kcat (min−1) | kcat/Km (μM−1 min−1) |
| 2 (at 5 mM 3)7 | 71 ± 9 | 22 ± 2 | 3.1 × 10−1 |
| 8 (at 5 mM 3) | - | no reaction | - |
| 9 (at 5 mM 3) | 36 ± 8 | 0.14 ± 0.01 | 3.9 × 10−3 |
| 10 (at 5 mM 3) | 79 ± 12 | (6.7 ± 1.7) × 10−3 | 8.4 × 10−5 |
| 3 (at 200 μM 2)7 | (1.2 ± 0.1) × 103 | 27 ± 2 | 2.3 × 10−2 |
| 11 (at 200 μM 2) | (3.5 ± 0.2) × 103 | 0.15 ± 0.02 | 4.3 × 10−5 |
Table 2.
Donor substrate promiscuity of SgcC5-catalyzed ester bond formation between selected β-tyrosines and 3
 | |
|---|---|
| donor substrates (X, Y) | relative activity |
| 2 (Cl, OH) | 100 |
| 9 (H, H) | 2 ± 1 |
| 12 (F, H) | 9 ± 2 |
| 13 (Cl, H) | 14 ± 2 |
| 14 (Br, H) | 27 ± 3 |
| 15 (I, H) | 2 ± 1 |
| 16 (CH3, H) | 4 ± 1 |
| 17 (OH, H) | 275 ± 22 |
Table 3.
Acceptor substrate promiscuity of SgcC5-catalyzed ester bond formation between selected aromatic and aliphatic alcohols and 2
 | ||
|---|---|---|
| no. | acceptor substrate | relative activity |
| 3 | 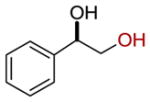 |
100 |
| 18 | 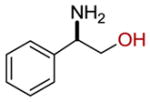 |
8 ± 2 |
| 19 | 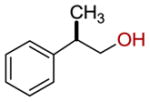 |
48 ± 2 |
| 20 | 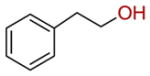 |
56 ± 3 |
| 21 | 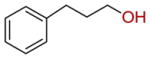 |
28 ± 2 |
| 22 | 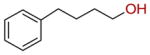 |
62 ± 4 |
| No activity was detected for the following compounds | ||
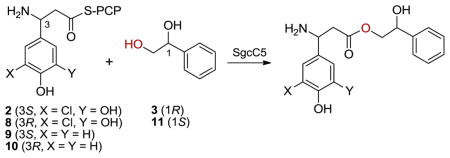
We first determined the enantiospecificity of SgcC5 towards both the β-tyrosine donor substrates (2, 8, 9, and 10) and the 1-phenyl-1,2-ethanediol acceptor substrates (3 and 11) (Table 1). We have previously developed the synthesis of 2 and determined its pseudo first-order kinetic parameters for the SgcC5-catalyzed ester bond-forming reaction with 3 as the acceptor substrate mimicking the enediyne core.7 The (R)-enantiomer of 2, (8) was similarly synthesized and evaluated as a potential donor substrate for SgcC5 with 2 as a positive control [see Supporting Information (SI)]. Strikingly, we did not detect any coupling between 8 and 3 even with 150-fold more SgcC5 (150 μM) and an 18-fold longer incubation time (3 hrs) than those used for the positive control of 2. This finding indicates that SgcC5 is highly stereospecific for the (S)-enantiomer of the 3-chloro-5-hydroxy-β-tyrosyl-S-SgcC2 donor substrate.
We then selected β-tyrosine to examine the stereospecificity of SgcC5 towards alternative donor substrates since production of 6 by the ΔsgcC3 mutant strain SB1008 clearly suggested that it can be utilized. Both (S)- and (R)-β-tyrosine were then tethered to SgcC2, affording 9 and 10, respectively, and tested as donor substrates for SgcC5 (see SI). After incubation of 9 (100 μM) with 3 (5 mM) in the presence of SgcC5 (100 μM) at 25°C for 30 min, the reactions were quenched and analyzed by HPLC. Two new products, similar to those obtained from the assays with 2 as a positive control, were formed with either 9 or 10, which were collected and characterized, respectively. High resolution matrix-assisted laser desorption-ionization mass spectroscopy (HR-ESI-MS) analyses of the two compounds yielded the same [M + H]+ ion at m/z 302.1386, consistent with the molecular formula C17H19NO4 of the predicted ester products (calcd for C17H20NO4, 302.1391) (Table S1). This result agreed with our previous observations that coupling occurred regiospecifically at the C-2 hydroxyl group of 3, but subsequent non-enzymatic rearrangement yielded the benzylic ester derived from 3 (Figure 1B). We subsequently determined the pseudo first-order kinetic parameters of SgcC5 towards 9 and 10, using the same HPLC assay previously applied using substrate 2,7 with a fixed concentration of 3 (5 mM) and variable concentrations of 9 and 10, respectively (Table 1) (Figure S1). The catalytic efficiency of SgcC5 for 9 was ~45-fold greater than for 10, consistent with the (S)-stereochemistry of the β-amino acid moiety present in 1. These findings also support the early assignment of engineered analogues 5–7 as having the same (S)-configuration at the β-amino moieties as 1.2a,4a
The high stereospecificity of SgcC5 towards the β-tyrosine donor substrates was striking, since the SgcC2 PCP presumably provides the majority of the molecular recognition elements, without which none of the donor substrates can be recognized by SgcC5.7 Inspired by this exquisite molecular recognition feature, we further probed the stereospecificity of SgcC5 towards the acceptor substrates. The pseudo first-order kinetic parameters for the SgcC5-catalyzed ester bond-forming reaction between 2 and (S)-1-phenyl-1,2-ethanediol (11) was similarly determined, with the concentration of 2 kept at 200 μM (Table 1) (Figure S2); the identities of the resultant products were similarly confirmed by HPLC and high resolution MS analyses (Table S1). Remarkably, SgcC5 was highly stereoselective for 3 over 11 with the enantioselectivity (E = 530) for the (R)-enantiomer (Table 1). Indeed, when racemic 1-phenyl-1,2-ethanediol was used to explore the use of SgcC5 for kinetic resolution, coupling product could only be detected from 2 and 3 if the reaction was incubated with 10 μM SgcC5 (Figure S3).
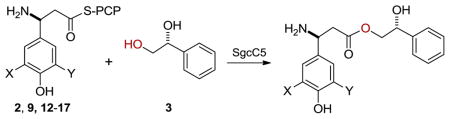
The isolation of 5–7 from the ΔsgcC34a and ΔsgcC2a mutant strains SB1008 and SB1006 also implied that SgcC5 should exhibit some degree of promiscuity towards the β-tyrosine donor substrates. By keeping the (S)-configuration, we next investigated the donor substrate promiscuity of SgcC5 by determining the relative rates of ester bond formation between a panel of 3-substituted β-tyrosines and 3 (Table 2). The selected (S)-tyrosines with varying 3-substitutions were synthesized and activated as SgcC2-tethered donor substrates (12–17) following the same procedure7 used for 2 and 9 (see SI). Under the identical conditions used to assay 2 and 9 with 3 as the acceptor substrate, SgcC5 remarkably recognized all of the SgcC2-tethered 3-substituted (S)-β-tyrosine analogues as donor substrates and catalyzed efficient formation of the predicted ester products with 3, the identities of which were confirmed by HPLC and high resolution MS analysis (Table S2). Although most of these β-tyrosine analogues were predictably poorer substrates than 2, they were as good as or better substrates than 9, which has been successfully incorporated into the engineered C-1027 analogue 64a (Table 2). Surprisingly, 17 was a significantly better (~3-fold) substrate, and this is consistent with the efficient production of the corresponding C-1027 analogue 5 from the ΔsgcC3 mutant strain SB1008.4a These findings unveil the intrinsic promiscuity of SgcC5 towards the donor substrate in the C-1027 biosynthetic machinery and will surely motivate future efforts to engineer new C-1027 analogues by incorporating these, as well as, other functional groups into the β-tyrosine moiety of C-1027 by manipulating its biosynthesis.8
Inspired by the donor substrate promiscuity, we finally explored the acceptor substrate specificity of SgcC5 by expanding the enediyne core mimics from 3 to a variety of aromatic (18–26) and aliphatic (27–29) alcohols and determined the relative rates of ester bond formation of those that formed ester products with 2 as the donor substrate (Table 3). Remarkably, under the identical assay conditions used for 3 and with 2 at 200 μM, SgcC5 utilized a variety of alcohols possessing a phenyl group as acceptor substrates (11 and 18–22), but not alcohols lacking a phenyl group (27–29), suggesting that the phenyl moiety is necessary for substrate recognition (Table 3). However, product was not detected from phenyl-containing acceptor substrates if the hydroxyl group was located at the C-1 position (24 and 25), further supporting the previously observed regiospecificity of SgcC5 for the C-2 hydroxyl of 3. Interestingly, 26 was not a substrate for SgcC5 despite its closer structural similarity to the putative enediyne core. Also surprising was the ability of SgcC5 to catalyze coupling of 2 with both 21 and 22 with activities comparable to that observed with 3, although 21 and 22 possess far less structural resemblance to the putative enediyne core substrate. Overall, while 3 was the best acceptor substrate examined, SgcC5 apparently exhibits significant substrate promiscuity towards the enediyne core mimics and can catalyze efficient coupling between 2 and a variety of aromatic alcohols as exemplified by 18–22 in this study. The enzymatic reaction between 19 and 2 was finally scaled up to isolate the product for complete 1H and 13C NMR analysis to definitively confirm ester formation between the donor and acceptor (SI). The identities for all other ester products were similarly confirmed by HPLC and high resolution MS analyses (Table S3).
In summary, we have previously unveiled a convergent strategy for 1 biosynthesis, in which coupling between the (S)-3-chloro-5-hydroxy-β-tyrosine and the enediyne core moieties is catalyzed by SgcC5, an unusual free standing condensation enzyme that absolutely requires the SgcC2 PCP to activate donor substrates and regiospecifically catalyzes both ester and amide bond formation between the donor and acceptor substrates.7 We now demonstrated that SgcC5 is highly enantioselective towards both donor and acceptor substrates, and its respective (S)- and (R)-specificities are consistent with the stereochemistry of C-1027 (Table 1, 9 and 10Figure 1). The relaxed enantioselectivity of SgcC5 towards indicates that the binding site is optimized for the physiological substrate (e.g. 2 but not the (R)-enantiomer 8) but smaller substrates (e.g. 9 and the (R)-enantiomer 10) may be accommodated with a relaxed stereospecificity as has been observed in the fourth condensation domain in tyrocidine biosynthesis.9 Together with the results obtained for SgcC4,3 SgcC1/SgcC2,4 SgcC3,5 and SgcC,6 the current study provided additional experimental evidence supporting the proposal that SgcC5 catalyzes the incorporation of the fully modified (S)-3-chloro-5-hydroxy-β-tyrosine moiety into C-1027 (Figure 1A).2,7
Finally, SgcC5 is capable of accepting a variety of 3-substituted β-tyrosine analogues as donor substrates (Table 2) and a variety of aromatic alcohols as acceptor substrates (Table 3). Although new C-1027 analogues with structural alterations at the enediyne core moiety have not been generated to date, the intrinsic substrate promiscuity of SgcC5 towards the enediyne core mimics revealed in the current study suggests that SgcC5 should not be a limiting factor preventing new enediyne cores from being incorporated into new C-1027 chromophores. In light of the substrate promiscuity of other enzymes in the C-1027 biosynthetic pathway, together, these findings will surely inspire future efforts in engineering new C-1027 analogues by applying combinatorial biosynthesis methods to its biosynthetic machinery, an endeavor that has already resulted in new analogues with altered activity, mode of action, or both.8
Supplementary Material
Acknowledgments
We thank Dr. Y. Li, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences, Beijing, China, for the wild-type S. globisporus strain and the Analytical Instrumentation Center of the School of Pharmacy, University of Wisconsin-Madison for support in obtaining MS and NMR data. This work is supported in part by NIH Grants CA78747 and CA113297. G.P.H. is the recipient of an NSERC (Canada) postdoctoral fellowship.
Footnotes
Supporting Information Available: Experimental procedures, Tables S1–S3, and Figures S1–S5. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Hu J, Xue YC, Xie MY, Zhang R, Otani T, Minami Y, Yamada Y, Marunaka T. J Antibiot. 1988;41:1575–1579. doi: 10.7164/antibiotics.41.1575. [DOI] [PubMed] [Google Scholar]; (b) Otani T, Minami Y, Marunaka T, Zhang R, Xie MY. J Antibiot. 1988;41:1580–1585. doi: 10.7164/antibiotics.41.1580. [DOI] [PubMed] [Google Scholar]
- 2.(a) Liu W, Christenson SD, Standage S, Shen B. Science. 2002;297:1170–1173. doi: 10.1126/science.1072110. [DOI] [PubMed] [Google Scholar]; (b) Van Lanen SG, Shen B. Curr Top Med Chem. 2008;8:448–459. doi: 10.2174/156802608783955656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Christenson SD, Liu W, Toney MD, Shen B. J Am Chem Soc. 2003;125:6062–6063. doi: 10.1021/ja034609m. [DOI] [PubMed] [Google Scholar]; (b) Christenson SD, Wu W, Spies MA, Shen B, Toney MD. Biochemistry. 2003;42:12708–12718. doi: 10.1021/bi035223r. [DOI] [PubMed] [Google Scholar]; (c) Christianson CV, Montavon TJ, Van Lanen SG, Shen B, Bruner SD. Biochemistry. 2007;42:7205–7214. doi: 10.1021/bi7003685. [DOI] [PubMed] [Google Scholar]
- 4.(a) Van Lanen SG, Dorrestein PC, Christenson SD, Liu W, Ju JH, Kelleher NL, Shen B. J Am Chem Soc. 2005;127:11594–11595. doi: 10.1021/ja052871k. [DOI] [PubMed] [Google Scholar]; (b) Van Lanen SG, Lin S, Dorrestein PC, Kelleher NL, Shen B. J Biol Chem. 2006;281:29633–29640. doi: 10.1074/jbc.M605887200. [DOI] [PubMed] [Google Scholar]
- 5.Lin S, Van Lanen SG, Shen B. J Am Chem Soc. 2007;129:12432–12438. doi: 10.1021/ja072311g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin S, Van Lanen SG, Shen B. J Am Chem Soc. 2008;130:6616–6623. doi: 10.1021/ja710601d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin S, Van Lanen SG, Shen B. Proc Natl Acad Sci U S A. 2009;106:4183–4188. doi: 10.1073/pnas.0808880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Kennedy DR, Gawron LS, Ju J, Liu W, Shen B, Beerman TA. Cancer Res. 2007;67:773–781. doi: 10.1158/0008-5472.CAN-06-2893. [DOI] [PubMed] [Google Scholar]; (b) Kennedy DR, Ju J, Shen B, Beerman TA. Proc Natl Acad Sci U S A. 2007;104:17632–17637. doi: 10.1073/pnas.0708274104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Beerman TA, Gawron LS, Shin S, Shen B, McHugh MM. Cancer Res. 2009;69:593–598. doi: 10.1158/0008-5472.CAN-08-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Linne U, Marahiel MA. Biochemistry. 2000;39:10439–10447. doi: 10.1021/bi000768w. [DOI] [PubMed] [Google Scholar]; (b) Clugston SL, Sieber SA, Marahiel MA, Walsh CT. Biochemistry. 2003;42:12095–12104. doi: 10.1021/bi035090+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.