Abstract
Haptoglobin (Hp) is a hemoglobin (Hb) binding protein whose major function is to prevent heme-iron mediated oxidation. The polymorphic nature of the Hp gene results in varying levels of antioxidant function associated with the protein products. Multiple clinical studies have now determined that the Hp 2-2 genotype is associated with an increased risk of developing vascular complications in patients suffering from diabetes. The mechanism for this phenomenon is a decrease in antioxidant capability associated with the Hp 2-2 protein. Specifically, heme iron associated with the Hp2-2/Hb complex is more redox active than other Hp type complexes and has been shown in a number of systems to lead to increased levels of oxidative stress in the form of oxidized lipids and decreased lipoprotein function. In addition, Hp 2-2/Hb complexes are cleared less efficiently from the circulation, leading to a buildup of iron in the plasma and in tissues. Recent analyses from clinical studies utilizing vitamin E treatment have shown beneficial results specifically in the diabetic Hp 2-2 genotype population. The use of vitamin E in the treatment of Hp 2-2 diabetics has the potential to greatly reduce medical costs and improve quality of life in the ever-growing diabetic population.
Keywords: Haptoglobin, Hemoglobin, Oxidative Stress, Vitamin E
1. Introduction
Haptoglobin (Hp) is an acute phase protein whose transcription is increased in response to inflammatory stimuli. Hp has several biological functions, but it is best known as a hemoglobin (Hb) binding protein. Hp/Hb complex formation serves to reduce the oxidative damage which would otherwise occur between heme derived iron and surrounding plasma or tissue proteins and lipids. Binding of Hb to Hp is especially important during hemolysis, when free Hb levels in the plasma increase, leading to Hb accumulation in the kidney and iron loss in the urine. By binding to Hp, the Hp/Hb complex is formed and cleared by receptor mediated endocytosis in the liver. In this manner, the iron is recycled and renal damage is prevented [1].
In humans the gene for Hp is polymorphic and this variability is reflected in the antioxidant capabilities of the different allelic protein products. These differences are thought to ultimately determine severity of disease. This review will summarize differences in Hp at the molecular level and will elaborate on the evidence showing that the Hp 2-2 genotype confers a higher risk of developing vascular complications in the setting of diabetes than other genotypes. Specific pathways by which heme derived iron in the Hp/Hb complex causes vascular damage will be presented as well as recent studies investigating antioxidant therapy in those individuals carrying high risk genotypes.
2. Molecular aspects of haptoglobin
2.1 Haptoglobin gene and protein structure
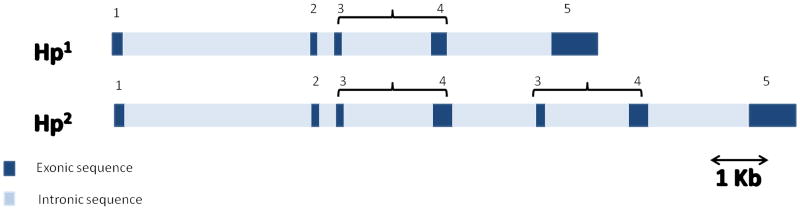
The Hp gene is located on chromosome 16q22 and has two major functional classes of alleles in humans. Those alleles, known as 1 and 2, can form three different genotypes: homozygous (1-1 or 2-2) and heterozygous (2-1). Allele 1 contains five exons and is similar in structure to Hp genes in all animals. Allele 2 is unique to humans, and was formed during evolution, probably from an unequal crossing over between two Hp1 alleles. As a result, allele 2 has a duplication of exons 3 and 4 of allele 1 (see figure 1) [1].
Figure 1. Genomic structure of Hp alleles 1 and 2.
Hp 1 contains 5 exons. The Hp2 allele evolved from the Hp 1 allele following duplication of exons 3 and 4. Brackets identify duplicated exons.
Hp is synthesized as a single polypeptide chain. The Hp monomer is formed after the nascent protein is cleaved into α and β chains which are linked to each other by disulfide bonds. The Hp 1 and Hp 2 proteins have the same β chain which is 45 kD. However, due the duplication, they differ in their α chain component, which is 9 kD for Hp 1 and 16 kD for Hp 2.
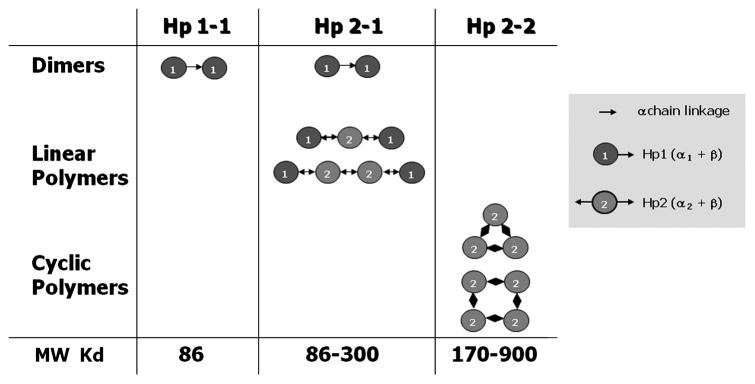
Since the duplication includes exon 3, which contains the Hp multimerization domain, the two alleles differ in their valences; Hp 1 is monovalent and Hp 2 is bivalent. This change affects the polymeric structure of the proteins, leading to dimers in Hp 1-1 individuals with the formula (α1 β)2, linear polymers in Hp 2-1 individuals with the formula (α1 β)2 + (α2 β)n (n = 0, 1, 2…) and cyclic polymers in Hp 2-2 individuals with the formula (α2 β)n (n = 3, 4, 5…) (see figure 2) [1]. Using Hb starch gel (or polyacrylamide gel) electrophoresis, Hp genotypes can be identified by their unique protein/complex patterns. Hp 1-1 shows one fast migrating band, Hp 2-2 shows series of slow migrating bands, and Hp 2-1 shows a weak fast migrating band and a series of slow migrating bands [2] (see figure 3).
Figure 2. Polymeric structure of protein products resulting from the three Hp genotypes: Hp 1-1, Hp 2-1, and Hp 2-2.
All of the subunits in the polymers are disulfide bond linked through the alpha chain. Individuals homozygous for the Hp 1 allele form exclusively dimers containing two Hp 1 subunits. Heterozygotes form linear polymers containing a variable number of internal Hp 2 subunits flanked by two Hp 1 subunits at either end. Individuals homozygous for the Hp 2 allele form cyclic polymers of varying numbers of Hp 2 subunits [1].
Figure 3. Hp typing of Hb enriched serum by gel electrophoresis.
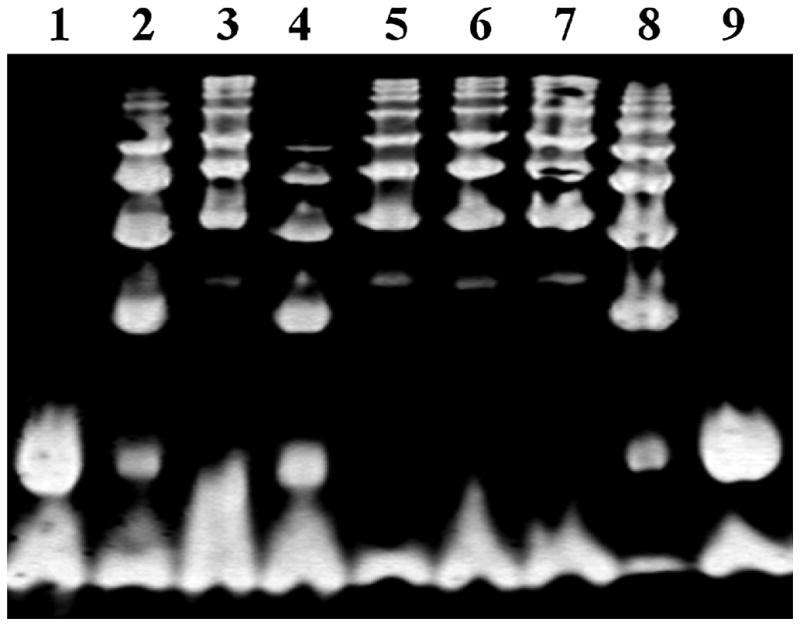
Each Hp type has a characteristic banding pattern. Free Hb present in all the samples appears as a single band at the bottom of the gel. Lanes 1 and 9 contain Hp 1-1 dimers which run as a single band immediately above Hb; lanes 2, 4, and 8 contain Hp 1-1 dimers as well as slower running Hp 2-1 linear polymers which appear as a series of bands in the upper part of the gel; lanes 3, 5, 6 and 7 contain Hp 2-2 cyclic polymers which appear as a series of bands running near the top of the gel [1].
2.2 Geographical distribution
During evolution, Hp 2 underwent positive selection and became more common in the northwestern European population. It is suspected that one reason is due to its role in protection against infectious disease. A study on streptococcus bacterium showed that the polymeric structure of Hp 2-2 can cause agglutination of bacteria and thereby interfere with its growth. Hp 1-1, a dimer, lacks this ability, and therefore doesn’t inhibit growth of the bacterium. Today the prevalence of the Hp 1-1, 2-2 and 2-1 genotypes in most western countries is 16%, 36% and 48% respectively. However, in Southeast Asia, about 90% of all individuals have the Hp 2-2 genotype [2].
2.3 Hp binding proteins
Each Hp monomer binds a Hb α-β dimer through a binding site situated in the β chain. The Kd of this reaction is very small (about 10−15), an indication of the high stability of Hp/Hb complex. Since the Hp β chain is the same for all Hp proteins, it is not surprising that the affinity of Hp 1-1 for Hb does not differ from that of Hp 2-2 [3]. However, as will be discussed below, the degree to which heme iron in the bound Hb can participate in oxidation reactions differs between Hp types.
The Hp-Hb complex is recognized by the CD163 receptor which is found on monocytes and to a greater extent on macrophages. The CD163 receptor recognizes a unique epitope on the Hp-Hb complex and does not bind either ligand separately with high affinity. The binding affinity of the Hp/Hb complex for CD163 is 1.9 nM for Hp 1-1 and 0.25nM for Hp 2-2. The apparent increase in affinity of the latter complex is likely due to the polymeric nature of the Hp 2-2 protein. Human macrophages in culture secrete interleukin-10 to a greater extent when incubated with Hp 1-1/Hb complexes than with Hp 2-2/Hb complexes. This response was shown to be CD163 dependent and suggests a molecular difference in receptor mediated signal transduction between the two Hp types.
Another protein to which Hp binds to is apolipoprotein A1 (apo A1), the major lipoprotein of HDL. Binding of Hp to ApoA1 also occurs when Hp is bound to Hb. The affinity of this binding interaction is low, and is estimated to be in the micromolar range (unpublished observations). The Hp binding site is on helix 6 of Apo-1, near the binding site of lecithin acetyl transferase (LCAT) [4]. This overlapping causes displacement of LCAT and has been shown to result in an inhibition of LCAT cholesterol esterification rate in vitro [5],[6].
3. Haptoglobin and diabetes
3.1 Human studies
In an early effort to understand the genetic basis for inter-individual differences in coronary collateral formation among patients suffering from cardiovascular disease, Hp was investigated as a possible genetic marker. Included in this analysis were patients suffering from retinopathy, another hypoxia related disease which is more common among diabetic patients. Although no relationship between collaterals and Hp type was found, the number of diabetic patients with retinopathy was significantly increased among individuals of the Hp 2-2 genotype. In addition, it was shown that individuals carrying the Hp 2-2 genotype were at increased risk of developing nephropathy as well as restenosis following angioplasty [7], [8], [9]. These results suggested that Hp genotype may also be related to heart disease in diabetic patients.
The first major study to investigate this association was done on samples collected in the Strong Heart Study [10]. This cohort included 206 case controlled samples from American Indians who were followed over a period of 6 years. The results showed that Hp 2-2 diabetic individuals were five times more likely to suffer from cardiovascular disease (CVD) than diabetic individuals carrying the Hp 1-1 genotype. Hp 2-1 diabetics were at an intermediate risk. Hp genotype was not predictive of heart disease in non-diabetic individuals. Since that time numerous studies have extended this finding and established that Hp 2-2 genotype is a risk factor for developing CVD complications in diabetic patients (see table 1). These studies included cohorts of varying ethnicity and geographic location as well as patients with type I and type II diabetes.
Table 1.
Clinical studies showing increased risk of vascular complications in Hp 2-2 diabetic individuals.
| Clinical Study | Increase risk in Hp 2-2 genotype | Ref. |
|---|---|---|
| Strong Heart Study | Three – to five-fold increased incidence CVD. | [10] |
| Munich Stent Study | Increase in the incidence of major adverse cardiac events (MACE), in the 1-year period after percutaneous transluminal coronary angioplasty | [11] |
| The Rambam Myocardial Infraction Outcomes in Diabetes Study | Eightfold increase incidence of death and heart failure, in the 30 day period after myocardial infarction | [12] |
| Israel Cardiovascular Vitamin E study (ICARE) | Two – to threefold increased risk of MACE | [13] |
| Epidemiology of Diabetes Complications Study (EDC) | Two fold increased risk of CVD in type 1 diabetic individuals | [14] |
| Coronary Artery Calcification Type I (CACTI) Study | Increase risk of development of coronary artery calcification in type 1 diabetic individuals | [15] |
| Epidemiology of Diabetes Complications Study (EDC) | Two fold increased risk for estimated glomerular filtration rate decline and end stage renal disease | [16] |
3.2 Haptoglobin transgenic mouse model
In order to investigate the mechanism of Hp genotype effect on vascular disease, a transgenic mouse model was developed. The mouse Hp 1 gene was used to engineer a mouse homolog of the human Hp 2 allele by inserting a duplication of exons 3 and 4 (see figure 1). The resulting mouse Hp 2 gene was inserted into the mouse Hp 1 locus by homologous recombination. The transgenic Hp 2 protein was shown to be of appropriate size and to form similar polymeric complexes with hemoglobin as the human Hp 2 protein. Mice were made diabetic by injection of streptozotocin and used in studies determining the relationship between Hp genotype and vascular disease as will be discussed below.
Using a number of different systems, it has now been established that studies using transgenic mice successfully recapitulate those results seen in the human studies, namely, that diabetic Hp 2-2 mice are at increased risk of developing vascular disease compared to their Hp 1-1 counterparts. Specifically, diabetic Hp 2-2 transgenic mice showed a significant increase in renal and glomerular hypertrophy compared to diabetic Hp 1-1 mice [17]. No difference was found between Hp 2-2 and Hp 1-1 mice in the absence of diabetes. Similarly, a highly significant increase in retinal capillary basement membrane thickness was observed in diabetic mice with the Hp 2-2 genotype. In a mouse model of myocardial infarction (MI) it was shown that diabetic Hp 2-2 mice suffered larger infarctions than diabetic Hp 1-1. There was no difference in infarction size between non diabetic Hp 2-2 and Hp 1-1 mice. In addition, in diabetic mice the Hp 2-2 genotype was associated with increased mortality and more severe cardiac remodeling 30 days after MI [18]. Hp 2-2 diabetic mice bred on an apo E knockout background showed increased iron, lipid peroxidation and macrophage accumulation in arterial plaques compared to apoE knockout Hp 1-1 diabetic mice [19]. In summary, these data establish the mouse transgenic model as an appropriate system to study Hp polymorphic effects on vascular disease.
4. Molecular mechanisms of increased vascular disease in Hp 2-2 diabetic individuals
The basic mechanism by which Hp/Hb complexes mediate vascular damage involves the decreased ability of Hp 2-2 to prevent oxidation of lipids catalyzed by Hb derived iron. This effect is magnified when Hb is glycosylated. Molecular and cellular studies detailing this phenomenon will be presented. Exacerbating this effect is the fact that Hp 2-2/Hb complexes are cleared more slowly from the circulation than Hp 1-1/Hb complexes, resulting in increased accumulation of Hb in the plasma and the tissues, particularly in the proximal tubule of the kidney. Data describing this aspect of Hp/Hb mediated damage will be summarized.
4.1 Oxidation reactions catalyzed by heme iron
Iron derived from hemoglobin can catalyze a number of oxidative reactions which can be inhibited by Hp. First, ferrous heme iron (Fe2+) can react with hydrogen peroxide to yield ferric Hb (Fe3+) and the highly reactive hydroxyl radical species (see Figure 4 reaction 1). By abstracting a hydrogen atom from polyunsaturated fatty acids, hydroxyl radicals may initiate the process of lipid peroxidation. Second, ferrous Hb (Fe2+) can also react with hydrogen peroxide to produce ferrylhemoglobin (Fe4+) [21] (see Figure 4 reaction 2), a highly unstable molecule which readily reacts with a second molecule of hydrogen peroxide to yield ferric Hb (Fe3+) and superoxide anion (see Figure 4, reaction 3). The damaging effects of superoxide anion are two-fold: reduction of ferric iron (Fe3+) in Hb to ferrous iron (Fe2+) (see Figure 4 reaction 4), allowing for the production of addition hydroxyl radical as described in reaction 1, and dismutation of superoxide anion to produce hydrogen peroxide, again promoting the production of ROS by reaction 1.
Figure 4. Reactions of Hb with hydrogen peroxide (H2O2) and superoxide ion (O2−).
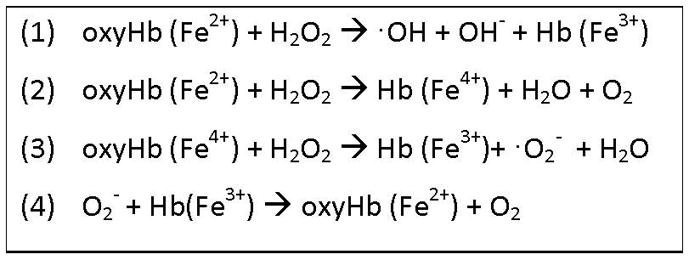
Iron present in Hb can exist in three oxidation states, +2, +3, and +4, indicating the net charge of the iron atom. Heme iron can undergo oxidation by H2O2 as shown in reactions 1 and 2, or reduction by H2O2 (reaction 3) or superoxide anion (reaction 4) as explained in further detail in section 4.1.
In all of the above reactions, the heme derived iron remains in the heme pocket of the globin molecule. However, ferric Hb (containing Fe3+), also known as methemoglobin, can spontaneously transfer its heme moiety resulting in heme entry into diverse lipophilic environments such as LDL or cell membranes [22]. Once intercalated into its new lipid environment, heme iron can undergo reactions with hydrogen peroxide as described above or with adjacent lipid peroxides generating a free radical cascade and leading to extensive lipid oxidation.
4.2 Differential inhibition of Hb redox activity by Hp types
As a part of the Hp/Hb complex, Hp stabilizes heme in the heme pocket of Hb, and prevents Hb from causing oxidative injury [23]. However, the degree to which Hp neutralizes the redox activity of heme iron differs among Hp types. This has been shown in a number of systems both in vitro and in vivo. For example, studies using linolenic acid showed that Hp 1-1 prevented oxidation (diene formation) as measured by an increase in absorbance at 232 nm to a greater extent than Hp 2-2 [24]. Another study examined LDL oxidation due to heme transfer from Hb to LDL. Heme transfer was measured by quenching of the fluorescence signal emitted by dansylated LDL. It was found that Hp 1-1 was superior in preventing heme transfer from Hb as compared to Hp 2-2 [25].
In a separate study redox active chelatable iron present in serum was measured using an in vitro kinetic fluorometric assay with dihydrorhodamine (DHR) in the presence of an iron chelator. The results showed a marked increase in the amount of chelatable redox active iron associated with Hp 2-2/Hb complexes as compared with Hp 1-1/Hb complexes. Differences in the amount of redox active chelatable iron between Hp 1-1/Hb and Hp 2-2/Hb complexes were enhanced when using in vitro glycated Hb. These results provide a plausible mechanistic explanation for differences in vascular disease seen between Hp genotypes specifically in the setting of diabetes.
Further support of this hypothesis was obtained from human macrophage cells (THP-1) exposed to Hb/Hp complexes when grown in a hyperglycemic environment. Cells were loaded with 2, 7-dichlorofluorescein, a compound which becomes fluorescent following oxidation. Specifically, there was a greater increase in flourescence in cells exposed to Hb-Hp 2-2 complexes compared to Hb/Hp 1-1 complexes. This effect was not seen when the cells were grown in normal levels of glucose. Furthermore, this increase was inhibited by desferroxamine, a high affinity iron chelator, confirming that the increased oxidative stress induced by hyperglycemia was likely due to an increased amount of redox active iron. Similar results were obtained by looking at oxidized lipids from cells treated in an identical manner. The presence of increased redox active iron was also demonstrated in the plasma of transgenic Hp 2-2 diabetic mice compared with Hp 1-1 diabetic mice [3].
4.3 Hp and high density lipoprotein (HDL)
One of the targets of oxidation by Hp/Hb complexes in Hp 2-2 diabetics is HDL. An important function of HDL is reverse cholesterol transport (RCT), a multi-step process resulting in the net movement of cholesterol from peripheral tissues back to the liver via the plasma compartment. Cellular cholesterol efflux occurs by binding of HDL to the ATP-binding cassette transporter present in the cell membrane. Cholesterol is then transferred to HDL, converted by LCAT to cholesterol ester, and subsequently taken up by the liver via the scavenger receptor B1.
As mentioned earlier, Hp binds to apo A1 present in HDL. In order to determine whether Hp binding affects RCT human macrophages were cultured in vitro and loaded with tritiated cholesterol. HDL (in the form of serum) from diabetic patients was added to the cultures and the amount of label released into the medium was measured. It was found that serum from Hp 2-2 diabetic patients was less active than serum from Hp 1-1 patients in promoting cholesterol efflux from the cells. In a second study mice were injected with macrophages loaded with tritiated cholesterol. After 48 hours there was significantly less radioactivity observed in the liver, feces, and plasma of diabetic Hp 2-2 mice compared to diabetic Hp 1-1 mice. The labeled cholesterol in the plasma, liver, and feces, was shown to be cholesterol ester, and not simply leakage of cholesterol from the injected macrophages, thereby confirming that the Hp 2-2 genotype is associated with a decrease in RCT [5].
Further experiments established that glycated Hb alone can oxidize HDL as well as cause a decrease in RCT. This reduction was shown to be rescued by Hp 1-1 to a greater extent than Hp 2-2. The mechanism of decreased RCT in Hp 2-2 diabetics appears to be increased oxidation of lipid components of HDL. However, oxidation of apo A1 at Tyr166 has also been shown to inhibit binding of LCAT to apo A1 [26]. It is likely that oxidation of both protein and lipid components of HDL lead to RCT dysfunction. Oxidation of HDL may also be detrimental due to inactivation of other anti-oxidant enzymes associated with HDL, such as paraoxonase and glutathione peroxidase [27].
4.4 Differential ability of Hp types to clear extracellular iron and its accumulation in the circulation and tissues
As mentioned above, CD163 plays an important role in clearance of Hb/Hp complexes from the circulation, especially after hemorrhage. In general diabetes results in a decrease in the percentage of peripheral blood monocytes which express CD163 and a decrease in the percentage of macrophages expressing CD163 present in atherosclerotic plaque. Specifically however, it was found that Hp 2-2 diabetic individuals showed a greater decrease in CD163 expression than Hp 1-1 diabetic individuals, both in percentage CD163 positive monocytes in the peripheral blood as well as CD163 positive macrophages in atherosclerotic plaque. In addition, Hp 2-2 diabetics contained higher amounts of plasma soluble CD163 when compared to Hp 1-1 diabetics [28].
A study conducted on Chinese hamster ovary cells stably transfected with CD163 receptor examined the rate of uptake of the Hb- Hp complex by the receptor. The results showed that while more Hp 2-2/Hb was bound to the cell surface, Hp 2-2/Hb complexes were endocytosed more slowly compared to Hp 1-1/Hb complexes [28]. An alternative approach to looking at Hp/Hb clearance was performed by injecting transgenic mice with 125I labeled Hp/Hb complexes intravenously and measuring the loss of radioactivity in the blood over time. It was found that the half-life of Hp 1-1/Hb complexes was 20 minutes with or without diabetes. The half-life of Hp2-2/Hb complexes was 50 min without diabetes and greater than 100 min with diabetes [29]. In summary, the above studies indicate that Hp 2-2 diabetic individuals have impaired Hb/Hp clearance due to altered regulation of CD163 expression.
The increased presence of Hp 2-2/Hb complexes in the circulation of diabetics allows not only for more oxidation but also increased iron deposits in the tissues. A number of studies confirm this hypothesis. For example, it was found that Hp 2-2 diabetic mice have increased levels of iron in the proximal tubule as measured by Perl’s staining, compared to other diabetic Hp genotypes. As mentioned in section 3.2, it was found that in diabetic, knockout apo E mice carrying the Hp 2-2 genotype, there was increased intraplaque iron as measured by Perl’s stain compared to their Hp 1-1 counterparts [19]. Similar results were seen in human atherosclerotic plaques from Hp 2-2 diabetics compared to Hp 1-1 diabetics [30]. Another recent study has also reported increased iron deposits in carotid artery plaque in Hp 2-2 diabetic patients [31]. This increase in plaque iron may be the result of decreased clearance of Hb in Hp 2-2 mice after intraplaque hemorrhage. The higher prevalence of plaque iron in Hp 2-2 diabetic individuals may contribute to the increased incidence of atherothrombotic events in these patients.
5. Antioxidant treatment of high risk individuals
5.1 Human studies with vitamin E
Based on preclinical studies suggesting the importance of oxidized LDL in atherosclerosis and observation studies showing increased vitamin E intake among individuals with less coronary artery disease [32], vitamin E supplements were routinely prescribed by cardiologists in the prevention of CVD. However, randomized placebo controlled trials have failed to show benefit from vitamin E. Furthermore, in a large meta-analysis published in 2005 it was reported that mortality was slightly increased in individuals who received high doses of vitamin E [33]. Therefore, the prevailing opinion is that high dose vitamin E supplements should be reconsidered [34]. One explanation for the lack of effective results following treatment with vitamin E is related to the proper selection of patients for treatment. In several studies that will be discussed below it was found that vitamin E supplementation had a beneficial effect specifically on individuals with diabetes and the Hp 2-2 genotype.
The Heart Outcomes Prevention Evaluation (HOPE) trial evaluated the 4.5-year effects of 400 IU vitamin E daily on the primary composite of CV death, myocardial infarction, and stroke. No significant benefit of vitamin E supplementation was detected in either the entire group or in the diabetic subset, consistent with what was previously reported for the entire HOPE cohort [35]. In Hp 2-2 diabetic participants, there was a trend toward a reduced primary composite outcome and a statistically significant reduction in the risk of CV death and nonfatal myocardial infarction [36].
In order to validate the results of the HOPE analysis, a prospective placebo controlled double blinded clinical trial, known as the Israel Cardiovascular Vitamin E study (ICARE), was carried out. In this study, diabetic individuals with the Hp 2-2 genotype were treated with vitamin E or with placebo, and the incidence of cardiovascular complications such as non-fatal myocardial infarction, cardiovascular death and stroke were measured. Also, Hp 1-1 and Hp 2-1 individuals were followed, even though they didn’t receive vitamin E. The results showed a 50% decrease in the event rate of cardiovascular complications in diabetic Hp 2-2 individuals randomized to vitamin E down to a rate similar to that of Hp 1-1 and Hp 2-1 individuals [13].
Vitamin E supplementation has also been used as a treatment for correcting HDL dysfunction. Twenty Hp 2-2 diabetic individuals were treated with vitamin E in a double-blinded crossover study. Lipid peroxidation and HDL function were measured in the serum at the beginning of the study, after 2 month of treatment with vitamin E or placebo, and again after another 2 month in which each group received the opposite treatment. It was found that vitamin E dramatically reduced HDL lipid peroxidation and improved HDL cholesterol efflux [29]. A second study, which included 36 Hp 2-1 and Hp 2-2 diabetic individuals showed a similar benefit in Hp 2-2 individuals, but with an opposite result in Hp 2-1 individuals, namely, that vitamin E supplementation caused a decreasing in RCT in diabetic Hp 2-1 individuals [37]. At present the mechanism of this difference in vitamin E effect between Hp 2-2 and Hp 2-1 individuals is not understood.
5.2 Animals studies with vitamin E
As mention above, the Hp 2-2 genotype is associated with early signs of kidney disease in diabetic mice. Specifically, glomerular and proximal tubular hypertrophy was significantly increased in Hp 2-2 diabetic mice. Histological analysis demonstrated that Hp 2-2 diabetic mice had significantly more collagen type IV, smooth muscle actin, and increased renal iron deposition. Studies of renal function demonstrated creatinine clearance time and albuminuria were increased in Hp 2-2 diabaetic mice. Treatment with vitamin E provided significant protection against the development of functional and histological features characteristic of diabetic nephropathy to Hp 2-2 mice but not to Hp 1-1 mice [38].
In a separate study vitamin E was given to Hp 1-1 or Hp 2-2 diabetic mice for 4 weeks. After this period, lipid peroxidation and cholesterol efflux were measured. It was found that vitamin E significantly improved cholesterol efflux and reduced lipid peroxidation in Hp 2-2 diabetic mice, but had no effect on HDL function or lipid peroxidation in Hp 1-1 diabetic mice. This data supports a role for vitamin E in preventing HDL dysfunction in Hp 2-2 diabetic individuals only [29].
In conclusion
Diabetes Mellitus is a worldwide disease, whose prevalence is increasing dramatically primarily due to poor nutrition and lack of physical activity. This disease can cause severe cardiovascular complications, especially in the later stages of the disease. Although the common treatment today for preventing diabetes, which includes controlling diet and maintaining exercise, is readily available to all, most diabetes patients are unable to achieve normal levels of blood glucose without eventual drug intervention. The mechanism responsible for the increase in complications in Hp 2-2 diabetics is iron induced oxidative stress. Not only is the Hp 2-2/Hb complex more redox active, it remains in the circulation for longer periods of time and heme derived iron accumulates in the tissues. Vitamin E has been shown in multiple human and animal studies to prevent diabetic complications. The findings of the studies described in section 5 concerning the benefits of vitamin E supplementation for diabetic Hp 2-2 individuals have the potential to greatly reduce health costs and improve quality of life for those individuals at high risk.
Acknowledgments
This work was supported by grants from the Israeli Science Foundation and the National Institutes of Health (NIH RO1DK085226).
Footnotes
Disclosure Statement
Dr. Levy is the author of a series of patents which claim that the Hp genotype is predictive of CVD in individuals with DM and that vitamin E may be used in combination with the Hp genotype to reduce CVD risk. He is also the chief scientific officer of Haptocure which has licensed these patents from his university.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hagit Goldenstein, Email: hagitity@gmail.com.
Nina S. Levy, Email: ninal@tx.technion.ac.il.
Andrew P. Levy, Email: alevy@tx.technion.ac.il.
References
- 1.Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, et al. Haptoglobin: Basic and clinical aspects. Antioxid Redox Signal. 2010 Feb;12(2):293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 2.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996 Oct;42(10):1589–600. [PubMed] [Google Scholar]
- 3.Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res. 2005 Mar 4;96(4):435–41. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 4.Spagnuolo MS, Cigliano L, D’Andrea LD, Pedone C, Abrescia P. Assignment of the binding site for haptoglobin on apolipoprotein A-I. J Biol Chem. 2005 Jan 14;280(2):1193–8. doi: 10.1074/jbc.M411390200. [DOI] [PubMed] [Google Scholar]
- 5.Asleh R, Miller-Lotan R, Aviram M, Hayek T, Yulish M, Levy JE, et al. Haptoglobin genotype is a regulator of reverse cholesterol transport in diabetes in vitro and in vivo. Circ Res. 2006 Dec 8;99(12):1419–25. doi: 10.1161/01.RES.0000251741.65179.56. [DOI] [PubMed] [Google Scholar]
- 6.Balestrieri M, Cigliano L, Simone ML, Dale B, Abrescia P. Haptoglobin inhibits lecithin-cholesterol acyltransferase in human ovarian follicular fluid. Mol Reprod Dev. 2001 Jun;59(2):186–91. doi: 10.1002/mrd.1021. [DOI] [PubMed] [Google Scholar]
- 7.Nakhoul FM, Zoabi R, Kanter Y, Zoabi M, Skorecki K, Hochberg I, et al. Haptoglobin phenotype and diabetic nephropathy. Diabetologia. 2001 May;44(5):602–4. doi: 10.1007/s001250051666. [DOI] [PubMed] [Google Scholar]
- 8.Levy AP, Roguin A, Hochberg I, Herer P, Marsh S, Nakhoul FM, et al. Haptoglobin phenotype and vascular complications in patients with diabetes. N Engl J Med. 2000 Sep 28;343(13):969–70. doi: 10.1056/NEJM200009283431313. [DOI] [PubMed] [Google Scholar]
- 9.Roguin A, Hochberg I, Nikolsky E, Markiewicz W, Meisel SR, Hir J, et al. Haptoglobin phenotype as a predictor of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 2001 Feb 1;87(3):330, 2, A9. doi: 10.1016/s0002-9149(00)01368-0. [DOI] [PubMed] [Google Scholar]
- 10.Levy AP, Hochberg I, Jablonski K, Resnick HE, Lee ET, Best L, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The strong heart study. J Am Coll Cardiol. 2002 Dec 4;40(11):1984–90. doi: 10.1016/s0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- 11.Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP. Haptoglobin genotype is predictive of major adverse cardiac events in the 1-year period after percutaneous transluminal coronary angioplasty in individuals with diabetes. Diabetes Care. 2003 Sep;26(9):2628–31. doi: 10.2337/diacare.26.9.2628. [DOI] [PubMed] [Google Scholar]
- 12.Suleiman M, Aronson D, Asleh R, Kapeliovich MR, Roguin A, Meisel SR, et al. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes. 2005 Sep;54(9):2802–6. doi: 10.2337/diabetes.54.9.2802. [DOI] [PubMed] [Google Scholar]
- 13.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: A prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008 Feb;28(2):341–7. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 14.Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: A determinant of cardiovascular complication risk in type 1 diabetes. Diabetes. 2008 Jun;57(6):1702–6. doi: 10.2337/db08-0095. [DOI] [PubMed] [Google Scholar]
- 15.Simpson M, Snell-Bergeon JK, Kinney GL, Lache O, Miller-Lotan R, Anbinder Y, et al. Haptoglobin genotype predicts development of coronary artery calcification in a prospective cohort of patients with type 1 diabetes. Cardiovasc Diabetol. 2011 Nov 20;10:99. doi: 10.1186/1475-2840-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costacou T, Ferrell RE, Ellis D, Orchard TJ. Haptoglobin genotype and renal function decline in type 1 diabetes. Diabetes. 2009 Dec;58(12):2904–9. doi: 10.2337/db09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller-Lotan R, Herskowitz Y, Kalet-Litman S, Nakhoul F, Aronson D, Zoabi R, et al. Increased renal hypertrophy in diabetic mice genetically modified at the haptoglobin locus. Diabetes Metab Res Rev. 2005 Jul-Aug;21(4):332–7. doi: 10.1002/dmrr.556. [DOI] [PubMed] [Google Scholar]
- 18.Asaf R, Blum S, Roguin A, Kalet-Litman S, Kheir J, Frisch A, et al. Haptoglobin genotype is a determinant of survival and cardiac remodeling after myocardial infarction in diabetic mice. Cardiovasc Diabetol. 2009 Jun 2;8:29. doi: 10.1186/1475-2840-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy AP, Levy JE, Kalet-Litman S, Miller-Lotan R, Levy NS, Asaf R, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2007 Jan;27(1):134–40. doi: 10.1161/01.ATV.0000251020.24399.a2. [DOI] [PubMed] [Google Scholar]
- 20.Sadrzadeh SM, Graf E, Panter SS, Hallaway PE, Eaton JW. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984 Dec 10;259(23):14354–6. [PubMed] [Google Scholar]
- 21.Rifkind JM, Ramasamy S, Manoharan PT, Nagababu E, Mohanty JG. Redox reactions of hemoglobin. Antioxid Redox Signal. 2004 Jun;6(3):657–66. doi: 10.1089/152308604773934422. [DOI] [PubMed] [Google Scholar]
- 22.Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal. 2010 Feb;12(2):233–48. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunn HF, Jandl JH. Exchange of heme among hemoglobin molecules. Proc Natl Acad Sci U S A. 1966 Sep;56(3):974–8. doi: 10.1073/pnas.56.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001 Dec 15;98(13):3693–8. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- 25.Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry. 2004 Apr 6;43(13):3899–906. doi: 10.1021/bi0362626. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, 3rd, Smith JD, et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007 Sep;14(9):861–8. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 27.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: Proatherogenic HDL--an evolving field. Nat Clin Pract Endocrinol Metab. 2006 Sep;2(9):504–11. doi: 10.1038/ncpendmet0245. [DOI] [PubMed] [Google Scholar]
- 28.Levy AP, Purushothaman KR, Levy NS, Purushothaman M, Strauss M, Asleh R, et al. Downregulation of the hemoglobin scavenger receptor in individuals with diabetes and the hp 2-2 genotype: Implications for the response to intraplaque hemorrhage and plaque vulnerability. Circ Res. 2007 Jul 6;101(1):106–10. doi: 10.1161/CIRCRESAHA.107.149435. [DOI] [PubMed] [Google Scholar]
- 29.Asleh R, Blum S, Kalet-Litman S, Alshiek J, Miller-Lotan R, Asaf R, et al. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes. 2008 Oct;57(10):2794–800. doi: 10.2337/db08-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno PR, Purushothaman KR, Purushothaman M, Muntner P, Levy NS, Fuster V, et al. Haptoglobin genotype is a major determinant of the amount of iron in the human atherosclerotic plaque. J Am Coll Cardiol. 2008 Sep 23;52(13):1049–51. doi: 10.1016/j.jacc.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Lioupis C, Barbatis C, Drougou A, Koliaraki V, Mamalaki A, Klonaris C, et al. Association of haptoglobin genotype and common cardiovascular risk factors with the amount of iron in atherosclerotic carotid plaques. Atherosclerosis. 2011 May;216(1):131–8. doi: 10.1016/j.atherosclerosis.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993 May 20;328(20):1450–6. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 33.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005 Jan 4;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 34.Blum S, Vardi M, Levy NS, Miller-Lotan R, Levy AP. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Atherosclerosis. 2010 Jul;211(1):25–7. doi: 10.1016/j.atherosclerosis.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. the heart outcomes prevention evaluation study investigators. N Engl J Med. 2000 Jan 20;342(3):154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 36.Levy AP, Gerstein HC, Miller-Lotan R, Ratner R, McQueen M, Lonn E, et al. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Diabetes Care. 2004 Nov;27(11):2767. doi: 10.2337/diacare.27.11.2767. [DOI] [PubMed] [Google Scholar]
- 37.Farbstein D, Blum S, Pollak M, Asaf R, Viener HL, Lache O, et al. Vitamin E therapy results in a reduction in HDL function in individuals with diabetes and the haptoglobin 2-1 genotype. Atherosclerosis. 2011 Nov;219(1):240–4. doi: 10.1016/j.atherosclerosis.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakhoul FM, Miller-Lotan R, Awad H, Asleh R, Jad K, Nakhoul N, et al. Pharmacogenomic effect of vitamin E on kidney structure and function in transgenic mice with the haptoglobin 2-2 genotype and diabetes mellitus. Am J Physiol Renal Physiol. 2009 Apr;296(4):F830–8. doi: 10.1152/ajprenal.90655.2008. [DOI] [PubMed] [Google Scholar]