Abstract
Leprosy is not eradicable with currently available diagnostics or interventions as evidenced by its stable incidence. Early diagnosis of Mycobacterium leprae infection should therefore be emphasized in leprosy-research. It remains challenging to develop tests based on immunological biomarkers that distinguish individuals controlling bacterial replication from those developing disease.
To identify biomarkers for field-applicable diagnostics, we determined cytokines/chemokines induced by M. leprae proteins in blood of leprosy patients and controls (EC) from high leprosy-prevalence areas (Bangladesh, Brazil, Ethiopia) and from South Korea where leprosy is not endemic anymore.
M. leprae- sonicate induced IFN-γ was similar for all groups, excluding M. leprae/IFN-γ as a diagnostic read-out. By contrast, ML2478 and ML0840 induced high IFN-γ concentrations in Bangladeshi EC, which were completely absent for South Korean controls. Importantly, ML2478/IFN-γ could indicate distinct degrees of M. leprae exposure, and thereby the risk of infection and transmission, in different parts of Brazilian and Ethiopian cities.
Notwithstanding these discriminatory responses, M. leprae proteins did not distinguish patients from EC in one leprosy endemic area based on IFN-γ. Analyses of additional cytokines/chemokines showed that M. leprae and ML2478 induced significantly higher concentrations of MCP-1, MIP-1β and IL-1β in patients compared to EC, whereas IP-10, like IFN-γ, differed between EC from areas with dissimilar leprosy prevalence.
This study identifies M. leprae-unique antigens, particularly ML2478, as biomarker tools to measure M. leprae exposure using IFN-γ or IP-10, and also shows that MCP-1, MIP-1β and IL-1β can potentially distinguish pathogenic immune responses from those induced during asymptomatic exposure to M. leprae.
Keywords: Mycobacterium leprae (M. leprae), biomarkers, leprosy, multiplex analysis
INTRODUCTION
Leprosy is a treatable immuno-pathogenic infection caused by Mycobacterium leprae (M. leprae). It mainly affects skin and peripheral nerves and ranks as the second most pathogenic mycobacterial infectious disease after tuberculosis (TB). Despite a spectacular decrease in global prevalence since 1982, leprosy is still considered a public health problem in 32 countries, mostly from the African, Asian and South American continents that cover 92% of all registered patients (1). Transmission of leprosy is sustained as evidenced by the hundreds of thousands of new cases of leprosy that keep being detected globally every year: 228,474 new cases were detected in 2010 amongst whom 20,472 were children (1). However, our understanding of the mode of M. leprae transmission has been complicated due to the long incubation time of leprosy and the lack of tests that detect asymptomatic M. leprae infection, a presumed major source of transmission, or predict possible progression of infection to clinical disease. Tests used in leprosy diagnostics include a serological test detecting IgM antibodies against phenolic glycolipid-1 (PGL-I), an M. leprae specific cell-surface antigen. Although it is useful for detection of most multibacillary (MB) leprosy patients, it has limited value in identifying paucibacillary (PB) leprosy patients, since the latter typically develop cellular rather than humoral immunity (32). The Mitsuda skin test, on the other hand, evaluates the in vivo immune response against M. leprae bacilli (lepromin) and is used for classification of leprosy. However, this test is not specific for M. leprae as it can also be mediated by lymphocytes responsive to M. tuberculosis and thus does not represent an adequate tool to measure M. leprae exposure or latent infection (29,36).
Since the methods and knowledge available to date have obviously not been sufficient to eliminate leprosy, the WHO 2011–2015 global strategy highlighted the need for early diagnosis and treatment (5) which will block development of nerve damage, disability and deformity, the hallmarks of leprosy. To design new diagnostic tests for early diagnosis, various studies have focused on identifying genes encoding M. leprae-unique antigens since the availability of the M. leprae genome sequence about one decade ago (9). Subsequently, these (hypothetical) antigens were used as recombinant proteins or synthetic peptides in in vitro T cell stimulation assays, mostly assessing IFN-γ production (3,12,15,16,41,43). Although it is not an immunological correlate of protection, the number of IFN-γ-releasing antigen-specific T cells and the amount of total IFN-γ released remain widely used as surrogate markers for the pro-inflammatory immune response against M. leprae and M. tuberculosis (45). A pitfall of the use of IFN-γ for leprosy diagnosis in a leprosy endemic area, however, is that not only infected individuals but also individuals with adequate immunity against M. leprae produce substantial concentrations of IFN-γ in response to M. leprae antigens.
In a previous study we tested recombinant proteins that had been selected based on their unique sequence in M. leprae (16). Notwithstanding this selection, IFN-γ production by EC-derived PBMC or whole blood was observed in response to most of these M. leprae proteins. Since these EC were living in areas with pockets of high leprosy prevalence (e.g. Dhaka and Karachi) and also responded to M. leprae whole cell sonicate (WCS) in vitro, the observed cellular responses towards the M. leprae-unique proteins may still have indicated M. leprae-specificity. The inclusion in the current study of groups of individuals with distinct degrees of exposure to M. leprae allowed us to investigate whether and to what extent the level of leprosy endemicity in a certain community influences the cellular immunity to M. leprae-unique antigens.
Since host immunity and immuno-pathogenicity in response to M. leprae involves complex interactions between a variety of cells expressing different effector and regulatory molecules, assessment of multiple rather than single biomarkers may be more representative of the immune status of the host and may identify patterns predisposing to leprosy. Therefore, here we have analyzed the concentrations of multiple cytokines, besides IFN-γ, after 24 hour whole blood stimulation with 17 M. leprae antigens in various cohorts from leprosy endemic areas in Bangladesh, Brazil and Ethiopia. This study describes the first identification of cellular host biomarkers, other than IFN-γ, that differ between leprosy patients and EC in one endemic area and thus could have value for early diagnosing leprosy and monitoring the response to MDT.
MATERIALS AND METHODS
General procedure of the study
Patients and controls were recruited at: International Center for Diarrhoeal Disease Research Bangladesh (ICDDR,B), Dhaka, Bangladesh, Yonsei University (YU), Seoul, South Korea, Fiocruz Fortaleza, Brazil and the Armauer Hansen Research Institute (AHRI) in Addis Ababa, Ethiopia. To ensure reproducibility of data throughout the study at each site, all experiments carried out by the laboratories involved were performed according to standard operating procedures (SOP) and each site was provided with identical reagents. Multiplex analyses were performed in one laboratory.
Recombinant proteins
M. leprae candidate genes were amplified by PCR from genomic DNA of M. leprae and cloned using the Gateway technology platform (Invitrogen, Carlsbad, CA) with pDEST17 expression vector containing an N-terminal histidine tag (Invitrogen) (14). Sequencing was performed on selected clones to confirm identity of all cloned DNA fragments. Recombinant proteins were overexpressed in E. coli BL21(DE3) and purified as described to remove any traces of endotoxin (14). Each purified recombinant protein was analyzed by 12% SDS-PAGE followed by Coomassie Brilliant Blue staining and Western-blotting with an anti-His antibody (Invitrogen) to confirm size and purity. Endotoxin contents were below 50 EU (endotoxin unit) per mg of recombinant protein as tested using a Limulus Amebocyte Lysate (LAL) QCL-1000 assay (Lonza Inc., Basel, Switzerland). Recombinant proteins tested in this study (n = 17) included: ML0009, ML0091, ML0755, ML0811, ML0840, ML0953, ML0957, ML1601, ML1976, ML2044, ML2055, ML2307, ML2313, ML2478, ML2531, ML2532 and ML2666. ML0091, ML0811, ML2044 and ML2055 were kindly provided by Dr. M.S. Duthie (Seattle, USA).
Recombinant proteins were tested to exclude protein non-specific T cell stimulation and cellular toxicity in IFN-γ release assays using PBMC of in vitro PPD-negative, healthy Dutch donors recruited at the Blood Bank Sanquin, Leiden, The Netherlands. None of these controls had experienced any known prior contact with leprosy or TB patients.
M. leprae whole cell sonicate (WCS)
Irradiated armadillo-derived M. leprae whole cells were probe sonicated with a Sanyo sonicator to >95% breakage. This material was provided through the NIH/NIAID “Leprosy Research Support” Contract N01 AI-25469 from Colorado State University (now available through the Biodefense and Emerging Infections Research Resources Repository listed at http://www.beiresources.org/TBVTRMResearchMaterials/tabid/1431/Default.aspx).
Study participants
The following HIV-negative individuals were recruited between August 2008 and February 2011: in Bangladesh (prevalence = 2.45/10,000): 10 TT/BT leprosy patients (Leprosy Control Institute & Hospital, Dhaka), 10 healthy household contacts of BL/LL patients (HHC), 10 healthy individuals from the same endemic area (EC); in South Korea (prevalence <1/10,000): 10 smear positive, pulmonary tuberculosis patients (TB) and 10 healthy controls (EC); in Brazil: 10 TT/BT leprosy patients, 10 HHC, 10 EC living in an area of Fortaleza with low prevalence (Mereiles; prevalence <0.2/10,000; EClow) and 10 healthy controls living in an area of Fortaleza with high prevalence (Bom Jardin; prevalence > 4/10,000; EChigh); in Ethiopia 35 healthy controls were tested: 18 EChigh who were derived from a sub city of Addis Ababa (Kolfe Keranio) with a prevalence rate of 1.5 per 10,000 (72 in 465,811), whereas17 EClow were derived from areas with a prevalence rate of 0.36 per 10,000 (10 in 273,310). Leprosy endemicity for each Ethiopian EC was based on the number of new cases and leprosy prevalence in nearby health centers per area.
Leprosy was diagnosed based on clinical, bacteriological and histological observations and classified by a skin biopsy evaluated according to the Ridley and Jopling classification (35) by qualified personnel. Patients were treated with chemotherapy for less than 3 months with no signs of leprosy reactions. HHC were defined as adults living in the same house as a BL/LL index case for at least the preceding six months. TB patients were diagnosed based on a positive culture of M. tuberculosis in sputum and were recruited at the outpatient clinic of the Pulmonary Division, Severans Hospital, Yonsei University Health System (YUHS) and had been on chemotherapy for at least 3 months to enable recovery of T cell function. EC were assessed for the absence of signs and symptoms of tuberculosis and leprosy. Staff members working in the leprosy centers or TB clinics were excluded as EC. Ethical approval of the study protocol was obtained through the appropriate local and national or institutional ethics committees, namely in Bangladesh: Ethical Review Committee of ICDDR,B; in South Korea: Institutional Review Board for the Protection of Human Subjects at YUHS; in Brazil: Brazilian National Council of Ethics in Research (CONEP); in Ethiopia: National Health Research Ethical Review committee (NERC). Informed consent was obtained from all individuals before venepuncture.
Whole blood assays (WBA)
Within 3 hours of collection, venous heparinized blood (450 μl per well) was incubated in 48-well plates at 37°C at 5% CO2, 90% relative humidity with 50 μl of antigen solution (100μg/ml). After 24 hour 150μl of supernatants were removed from each well and frozen in aliquots at −20°C until further analysis.
Lymphocyte stimulation tests (LST)
PBMC were isolated by Ficoll density centrifugation from venous, heparinized blood. and plated in triplicate cultures (2 × 105 cells/well) in 96-well round bottom plates (Costar Corporation, Cambridge, Mass.) in 200 μl/well of serum free Adoptive Immunotherapy medium (AIM-V, Invitrogen, Carlsbad, CA). Recombinant protein, M. leprae WCS or PPD (purified protein derivative of M. tuberculosis, Statens Serum Institut, Copenhagen, Denmark) were added at final concentrations of 10 μg/ml. As a positive control 1 μg/ml PHA (phytoheamagglutinin; Remel, Oxoid, Haarlem, The Netherlands) was used. After 6 days of culture at 37°C at 5% CO2, 90% relative humidity, 75 μl of supernatant were removed from each well, triplicates were pooled and frozen in aliquots at −20°C until further analysis.
IFN-γ ELISA
IFN-γ concentrations were determined by ELISA (U-CyTech, Utrecht, The Netherlands) as described (17). The cut-off value to define positive responses was set beforehand at 100 pg/ml. The assay sensitivity level was 40 pg/ml. Values for unstimulated cell cultures were typically <20 pg/ml. Lyophilized supernatant of PHA cultures of PBMC from an anonymous buffycoat (Sanquin, Leiden, The Netherlands) was provided to all laboratories as a reference positive control supernatant.
Serum Antibody ELISA
Recombinant protein ML2028 (M. leprae Ag85B), a synthetic analog of the M. leprae-specific phenolic glycolipid I (PGL-I; ND-O-BSA) and M. leprae lipoarabinomannan (LepLAM) were coated onto high-affinity polystyrene Immulon IV 96-well ELISA plates (Dynex Technologies, Chantilly, VA) using 50 ng per well in 100 μl of 0.1M sodium carbonate buffer, pH 9.0 at 4°C overnight. Unbound antigen was washed away using PBS, pH 7.4, containing 1% BSA and 0.05% Tween 80 (blocking buffer) six times. A 1:200 dilution of serum diluted in 100 μl blocking buffer was added to the wells and incubated for 2 h at room temperature. After incubating with the primary antibody, the wells were washed six times with PBS with 0.05% Tween 80 (wash buffer), followed by the addition of 100 μl of a 1:5,000 dilution of the secondary anti-human polyvalent antibody (Sigma) for 2 h. Following washing the wells with PBS six times, 100 μl of p-nitrophenylphosphate substrate (Kirkegaard and Perry Labs, Gaithersburg, MD) was added. The absorbance at 405 nm was read using a VersaMax Pro plate reader (Molecular Devices, Sunnyvale, CA) at 15 minutes. The cutoff for positivity was considered to be three times the background O.D. average for the non-endemic control sera (n = 23) determined by binding BSA with a 1:200 serum dilution (cutoff 0.411).
Multi-cytokine and -chemokine assay
The concentrations of 19 analytes [IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17A, IFN-γ, IP-10 (CXCL10), G-CSF, GM-CSF, MCP-1 (CCL2), MIG (CXCL9), MIP-1β (CCL4) and TNF] in supernatants from 24 hour WBA were measured using the Bio-Plex suspension array system powered by Luminex xMap multiplex technology (Bio-Rad Laboratories, Veenendaal, The Netherlands) and analyzed using the Bio-Plex Manager™ software 6.0 (Bio-Rad laboratories, Veenendaal, The Netherlands) (18). After pre-wetting the filter with assay-solution, the magnetic beads were washed twice with washing-solution using 96-well multiscreen filter plates (Millipore), an Aurum™ vacuum manifold and a vacuum pump (Bio-Rad Laboratories, Veenendaal, The Netherlands). Supernatant samples (50 μl) were added to the plates and the plates were incubated for 45 minutes at room temperature in the dark at 300 rpm on a plate shaker. After three washing steps, 12.5 μl detection antibody cocktail was added per well and plates were incubated at room temperature in the dark for 30 minutes on a plate shaker. After three washes, 25 μl strepavidin-PE solution was added per well and incubated for 10 minutes. After three washes, 80 μl of assay buffer was added to each well and the plates were placed in the Bio-Plex System. From each well, a minimum of 50 analyte-specific beads were analyzed for fluorescence. A curve fit was applied to each standard curve according to the manufacturer’s manual. Sample concentrations were interpolated from these standard curves. Analyte concentrations outside the upper- or lower limits of quantification were assigned the values of the limits of quantification of the cytokine or chemokine.
Statistical analysis
Differences in cytokine concentrations between test groups were analysed with the two-tailed Mann-Whitney U test for non-parametric distribution using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego California USA; www.graphpad.com). P-values were corrected for multiple comparisons. The statistical significance level used was p<0.05.
RESULTS
IFN-γ responses to M. leprae antigens in WBA in Bangladesh and South Korea
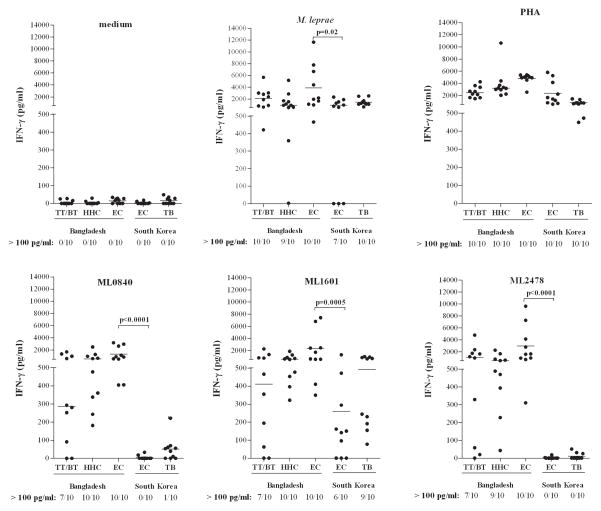
In a previous study IFN-γ production by T cells from EC was observed in response to M. leprae-unique proteins (16). However, since these EC were derived from areas with high leprosy prevalence and also responded to M. leprae WCS in vitro, the observed cellular responses towards the M. leprae-unique proteins could still indicate M. leprae-specificity. To investigate this, 17 M. leprae antigens were tested in an area highly endemic for leprosy (Dhaka, Bangladesh) and an area with low prevalence (South Korea) by analysis of IFN-γ production after 24 hour incubation of whole blood cultures stimulated with recombinant proteins in 10 TT/BT leprosy patients, 10 EC and 10 HHC from Bangladesh and the same numbers of EC and TB patients from South Korea. To ensure reproducibility, exactly the same batches of control antigens, recombinant M. leprae proteins and ELISA kits were provided to both sites. ML0755, ML0091, ML0811, ML0953, ML2044, ML2055, ML2307, ML2313 and ML2666 were only tested in the Bangladeshi groups, in which they showed low responses, in tuberculoid patients and/or in HHC (Supplementary Figure S1A), and were therefore not investigated in other cohorts. IFN-γ responses for the negative and positive controls (medium and PHA) were similar in individuals from both areas indicating that the blood samples used for all five groups were equally able to produce IFN-γ (Figure 1). M. leprae induced some variability in IFN-γ between the two EC groups. Nevertheless median values were comparable for all groups, thereby excluding the use of IFN-γ responses to M. leprae WCS as a discriminatory read-out. Importantly, significant differences in IFN-γ concentrations between exposed individuals versus individuals living in a population where they are less likely to be exposed were induced by ML0840 and ML2478 (both p<0.0001): all Bangladeshi EC and none of the EC from South Korea recognized these proteins (Figure 1). ML1601 was significantly better recognized in the EC group in Bangladesh (p=0.0005), whereas 9 out of 10 TB patients from South Korea also recognized this protein which has an orthologue in M. avium paratuberculosis (4). ML0009, ML0957, ML1976 and ML2531 did not show significant differences, although ML0009 (p=0.0686) and ML2531 (p=0.0342) showed a tendency towards higher responses in EC from Bangladesh (Supplementary Figure S1B). Thus, IFN-γ responses in 24 hour WBA using M. leprae-specific recombinant proteins ML2478 and ML0840, but not M. leprae WCS, correlate with differences in M. leprae exposure likelihood as estimated from EC living in high versus low leprosy prevalence areas.
Figure 1. IFN-γ responses in WBA from individuals in Bangladesh and South Korea.
IFN-γ production in response to control stimuli (medium, PHA and M. leprae WCS) or to recombinant proteins (ML0840, ML1601 and ML2478) in 24 hour WBA of leprosy patients (TT/BT; n = 10), healthy household contacts (HHC; n =10) and endemic controls (EC; n=10) from Bangladesh (prevalence = 2.45/10,000) or healthy controls (EC; n=10) and tuberculosis patients (TB; n=10) from South Korea (prevalence <1/10,000). For each group the number of IFN-γ responders (>100 pg/ml) versus the total number of individuals in the group is indicated below the x-axis. Background values were <50 pg/ml. Median values for each group are indicated by horizontal lines. Significant differences between test groups are indicated by p-values.
Next, sera from these individuals were analyzed for the presence of antibodies (Ab) to the M. leprae homolog of Ag85B (ML2028), a synthetic analog of the M. leprae-specific PGL-I (ND-O-BSA) and M. leprae lipoarabinomannan (LepLAM) (44). In contrast to the discriminatory IFN-γ patterns induced in 24 hour WBA of EC (South Korea) vs. EC (Bangladesh) with ML2478 and ML0840, the Ab concentrations to the three M. leprae antigens tested could not differentiate between these two EC groups (Supplemental Figure S2).
IFN-γ responses to M. leprae antigens in EChigh and EClow from the same city
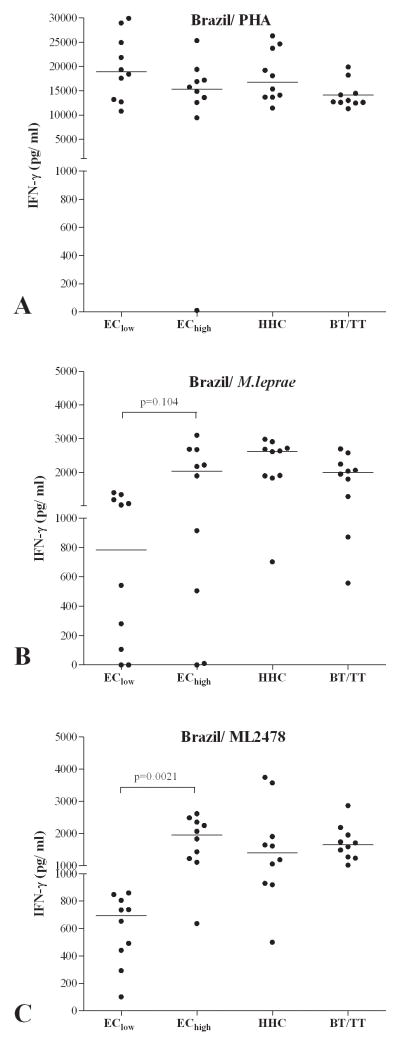
In order to expand these findings using healthy controls from an area with low numbers of new leprosy cases and a group from an area with much higher leprosy endemicity (EClow vs. EChigh), we investigated reactivity to the above M. leprae antigens in EC in Fortaleza (Brazil), where pockets in the city have a prevalence of less than 0.2 per 10,000 (EClow) and another area with a leprosy prevalence of more than 4 per 10,000 (EChigh). In addition, HHC and TT/BT patients from Fortaleza were included (Figure 2). Since comparison of WBA and lymphocyte stimulation tests (LST) showed similar IFN-γ responses (Supplementary Figure S3), 6 day LST with PBMC were used as a test format in this part of the study to allow testing of more antigens.
Figure 2. IFN-γ responses to M. leprae antigens in PBMC from EChigh and EClow in Brazil.
IFN-γ production (corrected for background values) induced using PHA (A), M. leprae (B) or ML2478 recombinant protein (C) in 6 day cultures of PBMC from healthy individuals from an area of Fortaleza, Brazil with low (EClow; prevalence <0.2/10,000; n=10) or high (EChigh; prevalence > 4/10,000; n=10) leprosy prevalence, healthy household contacts (HHC) of MB leprosy patients and TT/BT patients. Median values for each group are indicated by horizontal lines. Background values were <20 pg/ml.
Whereas PBMC of all groups were equally capable of producing IFN-γ after 6 days as indicated by the response to PHA (Figure 2A), ML2478 (p=0.0029) again showed significantly higher induction of IFN-γ responses in PBMC from TT/BT patients, HHC, and importantly, from EChigh as compared to PBMC from the EClow group from the same city. Thus, ML2478 (p=0.0021), but not M. leprae WCS (p=0.104), is useful to estimate differences in M. leprae exposure between EC defined by whether they reside in high versus low prevalence areas, even within the same city.
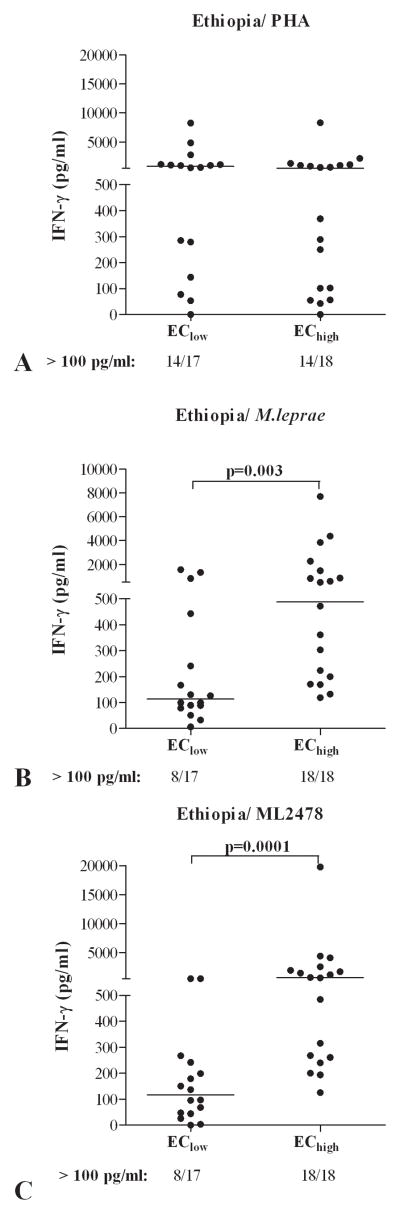
IFN-γ responses to M. leprae antigens in WBA in EChigh and EClow in Ethiopia
Based on the data obtained in Bangladesh, South Korea and Brazil, we next included an African setting by studying the response induced by selected M. leprae antigens in EC from Ethiopia. Eighteen EChigh were derived from a sub city of Addis Ababa (Kolfe Keranio) with a prevalence rate of 1.5 per 10,000, whereas 17 EClow were derived from areas in Addis Ababa with a prevalence rate of 0.36 per 10,000. All individuals responded equally well to the positive control stimulus PHA (Figure 3A) but responses to M. leprae WCS differed between the two EC groups (Figure 3B). Importantly, ML2478 again induced much higher concentrations (p=0.0001) of IFN-γ in the WBA of Ethiopian EChigh compared to Ethiopian EClow (Figure 3C; p=0.0001). In contrast to responses observed for EC from Bangladesh, ML0840 induced low responses in all Ethiopian EC (data not shown) and was not discriminatory with respect to M. leprae exposure. Thus, ML2478 combined with IFN-γ as a read-out, can also be used in 24h WBA to estimate differences in M. leprae exposure between EC in areas with different leprosy prevalence even when located in one city.
Figure 3. IFN-γ responses to M. leprae proteins in WBA from EChigh and EClow in Ethiopia.
IFN-γ production (corrected for medium values) in response to PHA (A), M. leprae WCS (B) or recombinant protein ML2478 (C) in 24 hour WBA of healthy individuals from areas in Addis Ababa, Ethiopia with low (EClow; prevalence = 0.36/10,000; n=17) and high (EChigh; prevalence =1.5/10,000; n=18) leprosy endemicity. Median values per test group are indicated by horizontal lines. For each group the number of IFN-γ responders versus the total number of individuals in the group is indicated below the x-axis.
Multiplex analysis of cytokines and chemokines in response to M. leprae antigens in WBA in Bangladesh, South Korea and Ethiopia
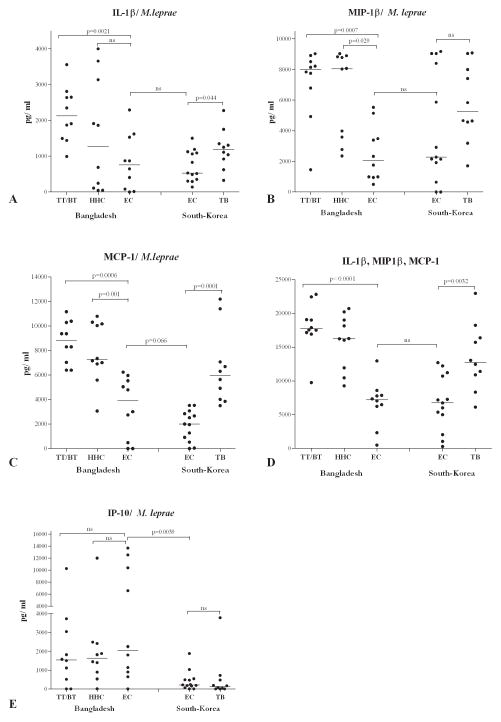
In our previous study (16) only IFN-γ was determined after stimulation of whole blood or PBMC. Recent studies on TB show that other (combinations of) cytokines are likely to be suitable for application in diagnostic assays (8,39,45). Since IFN-γ production induced by recombinant proteins was found in the current study not to be significantly different between the three different groups in Bangladesh (TT/BT, HHC and EC), IFN-γ cannot be used as a single biomarker to discriminate between leprosy patients (TT/BT) and those merely exposed to M. leprae (EC). Therefore, 18 additional cytokines and chemokines were tested using aliquots of WBA-supernatants (described in Figure 1). In striking contrast to IFN-γ, the concentrations of IL-1β, macrophage inflammatory protein-1β (MIP-1β or CCL4) and monocyte chemotactic protein-1 (MCP-1 or CCL2) were significantly enhanced in TT/BT patients after stimulation with M. leprae WCS compared to Bangladeshi EC (p=0.0006, p= 0.0007 and p= 0.0021 respectively; Figure 4A–C).
Figure 4. Multiplex cytokine analyses in WBA from individuals in Bangladesh and South Korea.
Concentrations (all corrected for background values) of IL-β (A), MIP-1β (B), MCP-1 (C) and IL-β, MIP-1β and MCP-1 combined (D) or IP-10 (E) induced by stimulation with M. leprae WCS in 24 hour WBA of leprosy patients (TT/BT; n = 10), healthy household contacts (HHC; n =10) and endemic controls (EC; n=10) from Bangladesh or healthy controls (EC; n=10) and tuberculosis patients (TB; n=10) from South Korea. Median values per test group are indicated by horizontal lines. Background values varied from <50 pg/ml for IFN-γ to <2000 pg/ml for MIP-1β. ns = not significant.
When cumulative values were considered (Figure 4D) even higher degrees of significance were observed between EC and TT/BT groups in Bangladesh (p<0.0001), as well as between EC and TB groups in South Korea (p=0.0032). Thus, in contrast to IFN-γ, the levels of MCP-1, MIP-1β and IL-1β induced in leprosy patients as well as TB patients are increased compared to EC from the same areas, potentially reflecting immune responses associated with mycobacterial infection.
To further analyze the potential of MCP-1, MIP-1β and IL-1β as biomarker tools for leprosy diagnostics, ROC (receiver operating characteristics) were analyzed (Table II), showing AUC (areas under the curve) ranging from 0.89 (IL-1β) to 0.94 (MIP-1β) thereby indicating good to excellent discrimination between the TT/BT and EC groups in Bangladesh. Combining the three biomarkers enhanced this diagnostic ability even more as evident from the AUC value (0.99).
Table II.
Receiver operating characteristics (ROC) for cytokines induced in WBA
| Cytokine | median TT/BT | median EC | p-value | AUCa |
|---|---|---|---|---|
| MCP-1 | 8829 | 4794 | 0.0006 | 0.94 |
| MIP-1β | 7995 | 2045 | 0.0007 | 0.92 |
| IL-1β | 2129 | 760 | 0.0021 | 0.89 |
| MCP-1/MIP-1β/IL-1β | 17735 | 7109 | <0.0001 | 0.99 |
| IP-10 | 1578 | 2029 | 0.3258 | 0.63 |
| IFN-γ | 1268 | 1548 | 0.1417 | 0.70 |
AUC: area under the curve;
Data from 24h WBA including leprosy patient, HHC and EC from one area hyperendemic for leprosy (Bangladesh) were considered for the calculations of ROC-values.
It is of interest that IL-1β concentrations in HHC were very heterogeneous, resulting in two subgroups. This could indicate that some individuals in this group may induce similar immune responses as TT/BT patients. Longitudinal cytokine analysis of these HHC may reveal whether such immune responses could correlate with progression to disease. Interestingly, TB patients from South Korea produced significantly higher concentrations of MCP-1 than EC (p= 0.0001) arguing for a specific role of MCP-1 in mycobacterial diseases.
Despite some interindividual differences, the data revealed that the overall concentrations for most cytokines (IL-10, IL-17, IL-2, IL-6, IL-8, G-CSF, GM-CSF, IP-10, MIG and TNF) showed no significant differences between TT/BT, HHC and EC from Bangladesh (Figure 4 and data not shown). In all test groups the remaining cytokines IL-4, IL-5, IL-7, IL-12p70 and IL-13 were hardly detected (median <50 pg/ml; data not shown). Thus, these multiplex analyses demonstrate that cytokines/chemokines other than IFN-γ, namely IL-1β, MIP-1β and MCP-1, have the potential to distinguish pathogenic immune responses as present in patients of mycobacterial diseases from those induced during asymptomatic exposure to M. leprae.
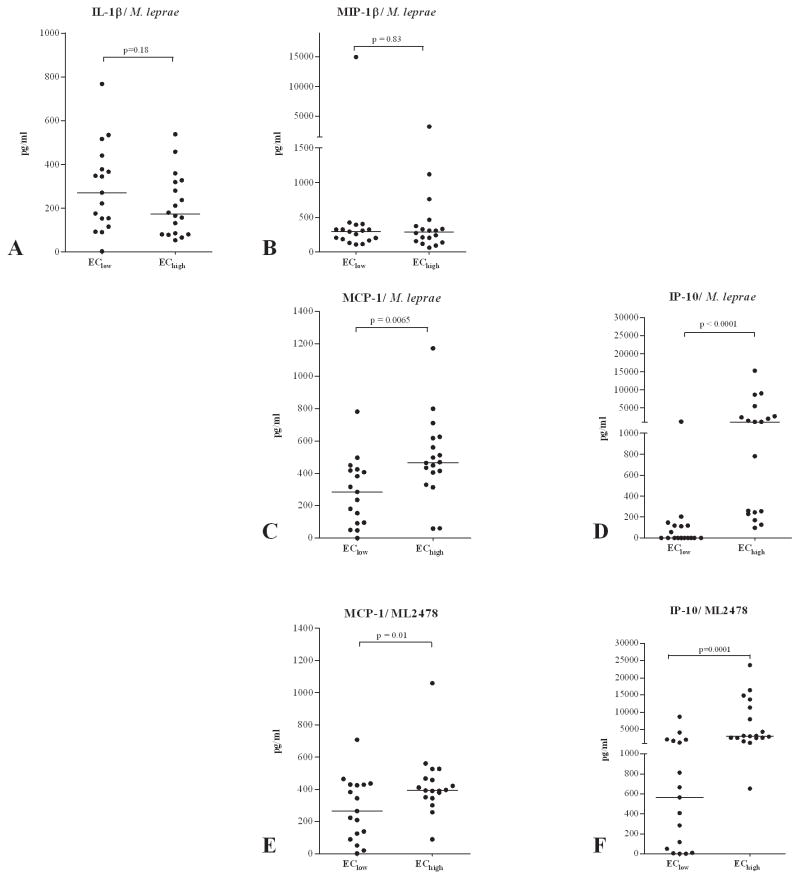
The multiplex cytokine analysis of WBA of Ethiopian EChigh and EClow (Figure 5) implied a comparison between two test groups of healthy individuals and thus does not necessarily reveal biomarkers related to pathogenic immune responses. IFN-γ induced protein 10 (IP-10 or CXCL10) has been shown to be a useful biomarker for diagnosis of M. tuberculosis infection (39). In Figure 5 it is shown that, in line with the differences in IP-10 observed between EC from Bangladesh and South Korea (Figure 4), IP-10 responses correlated with prevalence-estimated M. leprae exposure density, as EChigh produced substantially higher concentrations of IP-10 than EClow (p <0.0001).
Figure 5. Multiplex cytokine analyses in whole blood cultures from EC in Ethiopia.
Concentrations (all corrected for background values) of IL-β (A), MIP-1β (B), MCP-1 (C, E), IP-10 (D, F) induced by stimulation with M. leprae WCS (A–D) or ML2478 (E, F) in 24 hour WBA of leprosy patients (TT/BT; n = 10), healthy household contacts (HHC; n =10) and endemic controls (EC; n=10) from Bangladesh or healthy controls (EC; n=10) and tuberculosis patients (TB; n=10) from South Korea. Median values per test group are indicated by horizontal lines. Background values varied from <50 pg/ml for IFN-γ to <2000 pg/ml for MIP-1β.
Concentrations of MCP-1 were slightly increased in the EChigh group but not as significantly as IP-10. In contrast, IL-1β and MIP-1β that were increased in TT/BT patients in Bangladesh compared to EC from that area, did not show significant differences between the two Ethiopian EC groups. This is similar to the finding that these cytokines did not differ significantly between EC from Bangladesh and from South Korea either, whereas IP-10 concentrations could distinguish between these groups (Figure 4). None of the other cytokines tested displayed concentrations that differed sufficiently between patients and EC (data not shown).
Stimulation with the M. leprae-unique protein ML2478 instead induced a cytokine pattern similar to that of M. leprae WCS stimulated whole blood cultures for IP-10 and to a slightly lesser extent for MCP-1 (Figure 5E and 5F) indicating that, in addition to IFN-γ, IP-10 can also be used as a biomarker tool to measure M. leprae exposure. No MCP-1, MIP-1β and IL-1β was induced by ML2478 in NEC (Supplementary Figure S3B).
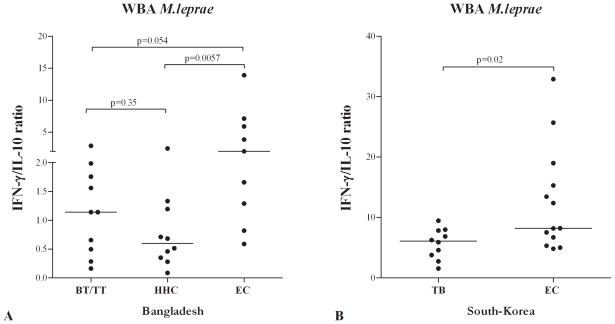
Determination of IFN-γ/IL-10 ratios in WBA
Since both pro- and anti-inflammatory cytokines play a role in protection from and pathogenesis of mycobacterial diseases, their balance may control or predict an eventual clinical outcome. In this respect the IFN-γ/IL-10 ratio has been described to significantly correlate with TB cure and severity (13,22,24,40). Determination of the IFN-γ/IL-10 ratio for individuals from Bangladesh showed a higher IFN-γ/IL-10 ratio for EC than for HHC and TT/BT, a difference that was not observed by separate analysis of these two cytokines (Figure 6). Similarly, TB patients in South Korea also had a decreased IFN-γ/IL-10 ratio compared to EC from that area. This corroborates the value of this ratio as an indicator for pathogenic responses to mycobacteria.
Figure 6. IFN-γ/IL-10 ratio in M. leprae stimulated WBA.
Ratios of IFN-γ concentrations (corrected for background values) with respect to IL-10 concentrations (corrected for background values) induced by stimulation with M. leprae WCS in 24 hour WBA in individuals from Bangladesh (A) and South Korea (B).
DISCUSSION
The stagnant decline in new leprosy cases demonstrates that transmission of M. leprae is persistent and not affected sufficiently by current control measures (1,37,42). In part this is the consequence of the present practice of leprosy diagnosis which is mainly based on recognition of clinical symptoms, requiring special, frequently not available, expertise. Major obstacles in leprosy diagnostics are the lack of good surrogate markers for subclinical or latent M. leprae infection, as well as the long incubation time that hinder early detection of leprosy and its modes of transmission. Thus, to overcome inadequate leprosy diagnostics, the development of rapid tests that can be applied in non-expert settings and allow identification of leprosy at early (subclinical) stages is high on the research agenda.
In the present study we show that IFN-γ production induced by M. leprae-unique proteins can identify individuals highly exposed to M. leprae and therefore more at risk of developing disease and/or transmitting the bacterium.
Since an M. leprae resistant phenotype is generally believed to be associated with the emergence of a protective Th1-based response characterized by consistent secretion of IFN-γ in association with moderate amounts of pro-inflammatory cytokines, we and others have previously used IFN-γ release assays (IGRAs) as a readout of cell-mediated immune responses (CMI) to investigate which M. leprae antigens can be useful for the diagnosis of leprosy (3,15,43). This was partly based on the initial promising reports on QuantiFERON®-TB, an IGRAs for diagnosis of TB (33). However, a recent meta-analysis showed that neither IGRA nor the tuberculin skin tests have high accuracy for the prediction of incident active TB in endemic areas (34). Our study shows that this is also the case for leprosy since the positive IFN-γ responses measured in WBA after stimulation with M. leprae-unique antigens depended on the level of endemicity in the investigated area and was not specific for disease. Importantly, however, here we have identified M. leprae-unique proteins, in particular ML2478, which can be used with IFN-γ as a read-out in the context of various genetic backgrounds (African, Asian, and South American) to point out distinct degrees of M. leprae exposure even if these occur in individuals residing in distinct areas of the same city. Therefore, such M. leprae proteins, combined with IGRAs, can be relevant as new tools for predicting the magnitude of M. leprae transmission in a given population and for identification of individuals who are at risk of acquiring M. leprae infection and possibly developing leprosy. Besides these data for ML2478, which is a hypothetical unknown protein lacking transmembrane regions and weakly similar to a probable metallopeptidase from Streptomyces avermitilis (33% identity), similar data, were recently found by us using M. leprae-specific peptides instead of proteins, further support our findings (Martins et al. submitted; (4). The M. leprae-specific IFN-γ response detected in this study in EC in areas hyperendemic for leprosy are consistent with earlier findings on the presence of M. leprae in nasal swaps of EC in Indonesia (21). Thus, this indicates that a vast proportion of leprosy patients probably acquire M. leprae infection from unidentified infected individuals or subclinical leprosy cases in the community and not necessarily from diagnosed leprosy patients.
The IP-10 production measured in WBA in this study displayed a pattern similar to that of IFN-γ, although the overall IP-10 concentrations were higher. Thus, our finding that IP-10 can differentiate between M. leprae exposure levels in two Ethiopian EC groups, corroborates the potential of this cytokine as a biomarker for M. tuberculosis exposure/infection (38). In this respect it is noteworthy that IP-10 has also been shown to be a promising biomarker for TB in HIV+ individuals, as the use of IP-10 as a read-out, with or without IFN-γ, was reported to be much less influenced by CD4 cell count than the QuantiFERON®-TB Gold In-Tube (2). Although IFN-γ is directly involved in inducing IP-10 production, IP-10 is produced primarily by monocytes and might be induced by CD4 T-cell- and IFN-γ-independent pathways. Alternatively, the higher concentrations of IP-10 produced may render this biomarker less sensitive to the effect of immune suppression.
The outcome of the immune response to M. leprae is determined by chemokines and cytokines that act as molecular signals for communication between cells of the immune system which renders them useful biomarkers predicting either protection or progression to disease. In this study, we identified secreted chemokines/cytokines (IL-1β, MIP-1β and MCP-1) that, in contrast to IFN-γ, could discriminate in 24h WBA between patients (leprosy and TB) and healthy EC in the same endemic areas, thereby possibly reflecting differences between M. leprae exposure and pathogenic immunity against M. leprae.
The chemokine that was very significantly increased in TT/BT leprosy patients compared to healthy EC from Bangladesh was MCP-1 (or CCL2). This molecule recruits monocytes, memory T cells and dendritic cells to sites of tissue injury and infection (7) and it has been suggested to play a role in maintaining the integrity of the granuloma in asymptomatic individuals with latent infection in high TB burden settings has been suggested (23). For TB patients MCP-1 production by M. tuberculosis-stimulated PBMC was associated with TB disease severity (19). On the other hand, for lepromatous leprosy (LL) patients MCP-1 was found to be lower than for TB patients (20). Similar data for tuberculoid leprosy patients have not been reported, yet the data in this study indicate that TT/BT patients are more inclined towards a phenotype resembling that of TB patients with elevated MCP-1 production.
The second potential immunological biomarker we identified, MIP-1β (or CCL4), is a chemo-attractant for monocytes and can inhibit T cell activation by interfering with TCR signaling (25). The exact role of MIP-1β in leprosy pathogenesis is still not clear.
Thirdly, our data showed increased IL-1β concentrations in WBA of TT/BT compared to EC in Bangladesh. IL-1β is produced by activated macrophages, plays a major role in host resistance to M. tuberculosis (30) and is involved in the TLR2/1-induced vitamin D antimicrobial pathway leading to induction of the antimicrobial peptide defensin β4A. Recently, reduced expression of the IL1B gene was reported for lesions of LL patients who typically lack good cellular responses (28). In view of our finding that TT/BT patients produce more IL-1β in response to M. leprae, this cytokine could be useful to indicate leprosy subtypes as well. Thus, although we can not absolutely explain the observed difference in IL-1β, MIP-1β and MCP-1 secretion in the WBA in the various test groups we can not rule out any effect of M. leprae-specific recall responses that may affect these innate responses (26).
In leprosy the quality and quantity of the innate and adaptive immune response, determine the outcome of infection: whereas the pro-inflammatory cytokine IFN-γ provides protection against mycobacteria, the anti-inflammatory cytokine IL-10 has been shown to be associated with dampening Th1 cells’ responses towards mycobacteria (27,31). Besides measuring single cytokines, the ratios of such cytokines can provide important information since both pro- and anti-inflammatory cytokines play a role in protection from and pathogenesis of mycobacterial diseases and their balance may control or predict the eventual clinical outcome. The IFN-γ/IL-10 ratio has been described to significantly correlate with TB cure (13,22,24,40). Also, the IFN-γ/IL-10 ratio positively correlated with TST induration suggesting that the ratio between PPD induced IFN-γ and IL-10 in peripheral blood may be important in controlling TST reactivity (6). In this study IFN-γ/IL-10 ratios were higher for EC compared to either leprosy or TB patients, despite the lack of significant differences if only IFN-γ was measured. Thus, changes in the IFN-γ/IL-10 ratio, especially when measured longitudinally in one individual, may provide information about potential disease development or response to treatment.
Since the HIV burden in most leprosy endemic areas is quite severe, it should be analyzed whether IL-1β, MIP-1β, MCP-1, IFN-γ and IP-10 as well as the ratios of Th1/Th2 cytokines can be applied as biomarkers in immuno-compromised individuals. Therefore, we are currently investigating such potential biomarkers, in combination with M. leprae specific antigens, in HIV+ individuals as well as HIV+ leprosy patients.
WBA using M. leprae antigens thus induce a ‘fingerprint’ of (the ratio of) Th1 or Th2 cytokines that may, combined with detection of anti-PGL-I antibodies, be used to specify disease type in the leprosy spectrum. Recently, we reported the development of a robust, user-friendly lateral flow assay based on up-converting phosphor technology (UCP-LF) that allows simultaneous detection of cellular and humoral immune responses in one sample (10,11). Using ML2478-stimulated WBA, this UCP-LF assay can now be used in poorly equipped laboratories to estimate levels of M. leprae exposure, by measuring both Th1 (IFN-γ/IP-10) and Th2 (IL-10) as well as anti-PGL-I IgM antibodies. Currently, the development of this rapid lateral flow assay for detection of IL-1β, MIP-1β and MCP-1 is in progress.
Since the majority of those exposed to M. leprae develop a protective immune response against the bacterium, large-scale, longitudinal follow-up studies, allowing intra-individual comparison of immune profiles in healthy controls from leprosy-endemic areas worldwide, will be essential to analyze whether the biomarkers identified here can be applied as tools for prediction of pathogenic immune responses to M. leprae.
Supplementary Material
Table I.
Participating study sites and study groups
| Site | Prevalencea | Categoryb | BIc | Sex ratio (M/F) | Mean age (yr) | Age range (yr) |
|---|---|---|---|---|---|---|
| Bangladesh (Dhaka) | 0.28 | TT/BT | 0 | 7/3 | 38.5 | 22–65 |
| HHC | -d | 6/4 | 35.7 | 20–70 | ||
| EC | -d | 7/3 | 28.1 | 24 35 | ||
| South Korea (Seoul) | <0.1 | EC | -d | 9/1 | 23 | 21–25 |
| TB | -d | 4/6 | 51.2 | 24 77 | ||
| Ethiopia (Addis Ababa) | 0.36 | EClow | -d | 5/13 | 27.6 | 18–40 |
| 1.5 | EChigh | -d | 8/9 | 23.1 | 18–38 | |
| Brazil (Fortaleza) | <0.2 | EClow | -d | 5/5 | 34.7 | 22–60 |
| > 4 | EChigh | -d | 5/5 | 36.6 | 18–58 |
Prevalence per 10,000 individuals at the end of 2010.
TT/BT: tuberculoid leprosy/borderline tuberculoid leprosy; HHC: healthy household contact; EC: endemic control.
BI: bacterial index (mean).
not applicable.
Acknowledgments
AHRI, CSU, Fiocruz, ICDDR, B, LSHTM and LUMC are part of the IDEAL (Initiative for Diagnostic and Epidemiological Assays for Leprosy) Consortium. We thank Yonas Fantahun (AHRI) for help with recruitment of blood donors, Dr. Young Ae Kang (Severance Hospital, YUHS) for recruitment of tuberculosis patients and Dr. William Wheat for critically reading the manuscript.
Footnotes
This study was supported by the Netherlands Leprosy Relief Foundation (NLR; ILEP#: 7.01.02.48) together with the Turing Foundation as part of the IDEAL Consortium, and the Order of Malta-Grants-for-Leprosy-Research. AG received additional support for this study from the Q.M. Gastmann-Wichers Foundation. JSS and HJK received some support from the NIH/NIAID grant R01 AI-082575. The authors declare no conflict of interest.
References
- 1.Leprosy update, 2011. Wkly Epidemiol Rec. 2011;86:389–399. [PubMed] [Google Scholar]
- 2.Aabye MG, Ruhwald M, Praygod G, Jeremiah K, Faurholt-Jepsen M, Faurholt-Jepsen D, Range N, Friis H, Changalucha J, Andersen AB, Ravn P. Potential of interferon-gamma-inducible protein 10 in improving tuberculosis diagnosis in HIV-infected patients. Eur Respir J. 2010;36:1488–1490. doi: 10.1183/09031936.00039010. [DOI] [PubMed] [Google Scholar]
- 3.Araoz R, Honore N, Banu S, Demangel C, Cissoko Y, Arama C, Uddin MK, Hadi SK, Monot M, Cho SN, Ji B, Brennan PJ, Sow S, Cole ST. Towards an immunodiagnostic test for leprosy. Microbes Infect. 2006;8:2270–2276. doi: 10.1016/j.micinf.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Bobosha K, van der Ploeg-van Schip JJ, Esquenazi DA, Guimaraes MM, Martins MV, Bekele Y, Fantahun Y, Aseffa A, Franken KL, Gismondi RC, Pessolani MC, Ottenhoff TH, Pereira GM, Geluk A. Peptides Derived from Mycobacterium leprae ML1601c Discriminate between Leprosy Patients and Healthy Endemic Controls. J Trop Med. 2012;2012:132049. doi: 10.1155/2012/132049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burki T. Fight against leprosy no longer about the numbers. Lancet Infect Dis. 2010;10:74. doi: 10.1016/s1473-3099(10)70015-3. [DOI] [PubMed] [Google Scholar]
- 6.Burl S, Adetifa UJ, Cox M, Touray E, Whittle H, McShane H, Rowland-Jones SL, Flanagan KL. The tuberculin skin test (TST) is affected by recent BCG vaccination but not by exposure to non-tuberculosis mycobacteria (NTM) during early life. PLoS ONE. 2010;5:e12287. doi: 10.1371/journal.pone.0012287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chegou NN, Black GF, Kidd M, van Helden PD, Walzl G. Host markers in QuantiFERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm Med. 2009;9:21. doi: 10.1186/1471-2466-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 10.Corstjens PL, de Dood CJ, van der Ploeg-van Schip JJ, Wiesmeijer KC, Riuttamaki T, van Meijgaarden KE, Spencer JS, Tanke HJ, Ottenhoff TH, Geluk A. Lateral flow assay for simultaneous detection of cellular- and humoral immune responses. Clin Biochem. 2011;44:1241–1246. doi: 10.1016/j.clinbiochem.2011.06.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corstjens PL, Zuiderwijk M, Tanke HJ, van der Ploeg-van Schip JJ, Ottenhoff TH, Geluk A. A user-friendly, highly sensitive assay to detect the IFN-gamma secretion by T cells. Clin Biochem. 2008;41:440–444. doi: 10.1016/j.clinbiochem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dockrell HM, Brahmbhatt S, Robertson BD, Britton S, Fruth U, Gebre N, Hunegnaw M, Hussain R, Manandhar R, Murillo L, Pessolani MC, Roche P, Salgado JL, Sampaio E, Shahid F, Thole JE, Young DB. A postgenomic approach to identification of Mycobacterium leprae-specific peptides as T-cell reagents. Infect Immun. 2000;68:5846–5855. doi: 10.1128/iai.68.10.5846-5855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellner JJ. Immunoregulation in TB: observations and implications. Clin Transl Sci. 2010;3:23–28. doi: 10.1111/j.1752-8062.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franken KL, Hiemstra HS, van Meijgaarden KE, Subronto Y, Hartigh Jd, Ottenhoff TH, Drijfhout JW. Purification of his-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr Purif. 2000;18:95–99. doi: 10.1006/prep.1999.1162. [DOI] [PubMed] [Google Scholar]
- 15.Geluk A, Klein MR, Franken KL, van Meijgaarden KE, Wieles B, Pereira KC, Buhrer-Sekula S, Klatser PR, Brennan PJ, Spencer JS, Williams DL, Pessolani MC, Sampaio EP, Ottenhoff TH. Postgenomic approach to identify novel Mycobacterium leprae antigens with potential to improve immunodiagnosis of infection. Infect Immun. 2005;73:5636–5644. doi: 10.1128/IAI.73.9.5636-5644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geluk A, Spencer JS, Bobosha K, Pessolani MC, Pereira GM, Banu S, Honore N, Reece ST, Macdonald M, Sapkota BR, Ranjit C, Franken KL, Zewdie M, Aseffa A, Hussain R, Stefani MM, Cho SN, Oskam L, Brennan PJ, Dockrell HM. From genome-based in silico predictions to ex vivo verification of leprosy diagnosis. Clin Vaccine Immunol. 2009;16:352–359. doi: 10.1128/CVI.00414-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geluk A, van der Ploeg-van Schip JJ, Teles RO, Franken KL, Prins C, Drijfhout JW, Sarno EN, Sampaio EP, Ottenhoff TH. Rational combination of peptides derived from different Mycobacterium leprae proteins improves sensitivity for immunodiagnosis of M. leprae infection. Clin Vaccine Immunol. 2008;15:522–533. doi: 10.1128/CVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geluk A, van der Ploeg-van Schip JJ, van Meijgaarden KE, Commandeur S, Drijfhout JW, Benckhuijsen WE, Franken KL, Naafs B, Ottenhoff TH. Enhancing sensitivity of detection of immune responses to Mycobacterium leprae peptides in whole-blood assays. Clin Vaccine Immunol. 2010;17:993–1004. doi: 10.1128/CVI.00046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasan Z, Cliff JM, Dockrell HM, Jamil B, Irfan M, Ashraf M, Hussain R. CCL2 responses to Mycobacterium tuberculosis are associated with disease severity in tuberculosis. PLoS ONE. 2009;4:e8459. doi: 10.1371/journal.pone.0008459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan Z, Jamil B, Zaidi I, Zafar S, Khan AA, Hussain R. Elevated serum CCL2 concomitant with a reduced mycobacterium-induced response leads to disease dissemination in leprosy. Scand J Immunol. 2006;63:241–247. doi: 10.1111/j.1365-3083.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- 21.Hatta M, van Beers SM, Madjid B, Djumadi A, de Wit MY, Klatser PR. Distribution and persistence of Mycobacterium leprae nasal carriage among a population in which leprosy is endemic in Indonesia. Trans R Soc Trop Med Hyg. 1995;89:381–385. doi: 10.1016/0035-9203(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, Peters P, Ellner JJ. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–2073. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 23.Hussain R, Ansari A, Talat N, Hasan Z, Dawood G. CCL2/MCP-I genotype-phenotype relationship in latent tuberculosis infection. PLoS ONE. 2011;6:e25803. doi: 10.1371/journal.pone.0025803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain R, Kaleem A, Shahid F, Dojki M, Jamil B, Mehmood H, Dawood G, Dockrell HM. Cytokine profiles using whole-blood assays can discriminate between tuberculosis patients and healthy endemic controls in a BCG-vaccinated population. J Immunol Methods. 2002;264:95–108. doi: 10.1016/s0022-1759(02)00092-3. [DOI] [PubMed] [Google Scholar]
- 25.Joosten SA, van Meijgaarden KE, Savage ND, de BT, Triebel F, van der WA, de HE, Klein MR, Geluk A, Ottenhoff TH. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A. 2007;104:8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26:186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Lima MC, Pereira GM, Rumjanek FD, Gomes HM, Duppre N, Sampaio EP, Alvim IM, Nery JA, Sarno EN, Pessolani MC. Immunological cytokine correlates of protective immunity and pathogenesis in leprosy. Scand J Immunol. 2000;51:419–428. doi: 10.1046/j.1365-3083.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu PT, Wheelwright M, Teles R, Komisopoulou E, Edfeldt K, Ferguson B, Mehta MD, Vazirnia A, Rea TH, Sarno EN, Graeber TG, Modlin RL. MicroRNA-21 targets the vitamin D-dependent antimicrobial pathway in leprosy. Nat Med. 2012;18:267–273. doi: 10.1038/nm.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda SM, Rotta O, Michalany NS, Camargo ZP, Sunderkotter C, Tomimori-Yamashita J. Comparison between anti-PGL-I serology and Mitsuda reaction: clinical reading, microscopic findings and immunohistochemical analysis. Lepr Rev. 2003;74:263–274. [PubMed] [Google Scholar]
- 30.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misra N, Selvakumar M, Singh S, Bharadwaj M, Ramesh V, Misra RS, Nath I. Monocyte derived IL 10 and PGE2 are associated with the absence of Th 1 cells and in vitro T cell suppression in lepromatous leprosy. Immunol Lett. 1995;48:123–128. doi: 10.1016/0165-2478(95)02455-7. [DOI] [PubMed] [Google Scholar]
- 32.Oskam L, Slim E, Buhrer-Sekula S. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr Rev. 2003;74:196–205. [PubMed] [Google Scholar]
- 33.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 34.Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, Fielding K, Wilkinson RJ, Pai M. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–273. [PubMed] [Google Scholar]
- 36.Roberts PP, Dockrell HM, McAdam KP. Evidence that the Mitsuda reaction to Mycobacterium leprae can be mediated by lymphocytes responsive to Mycobacterium tuberculosis. Clin Exp Immunol. 1988;72:390–393. [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues LC, Lockwood DN. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis. 2011;11:464–470. doi: 10.1016/S1473-3099(11)70006-8. [DOI] [PubMed] [Google Scholar]
- 38.Ruhwald M, Bjerregaard-Andersen M, Rabna P, Eugen-Olsen J, Ravn P. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res Notes. 2009;2:19. doi: 10.1186/1756-0500-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruhwald M, Dominguez J, Latorre I, Losi M, Richeldi L, Pasticci MB, Mazzolla R, Goletti D, Butera O, Bruchfeld J, Gaines H, Gerogianni I, Tuuminen T, Ferrara G, Eugen-Olsen J, Ravn P. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis (Edinb ) 2011;91:260–267. doi: 10.1016/j.tube.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Sahiratmadja E, Alisjahbana B, de BT, Adnan I, Maya A, Danusantoso H, Nelwan RH, Marzuki S, van der Meer JW, van CR, van dV, Ottenhoff TH. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun. 2007;75:820–829. doi: 10.1128/IAI.00602-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampaio LH, Stefani MM, Oliveira RM, Sousa AL, Ireton GC, Reed SG, Duthie MS. Immunologically reactive M. leprae antigens with relevance to diagnosis and vaccine development. BMC Infect Dis. 2011;11:26. doi: 10.1186/1471-2334-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer JS, Dockrell HM, Kim HJ, Marques MA, Williams DL, Martins MV, Martins ML, Lima MC, Sarno EN, Pereira GM, Matos H, Fonseca LS, Sampaio EP, Ottenhoff TH, Geluk A, Cho SN, Stoker NG, Cole ST, Brennan PJ, Pessolani MC. Identification of specific proteins and peptides in mycobacterium leprae suitable for the selective diagnosis of leprosy. J Immunol. 2005;175:7930–7938. doi: 10.4049/jimmunol.175.12.7930. [DOI] [PubMed] [Google Scholar]
- 44.Spencer JS, Kim HJ, Wheat WH, Chatterjee D, Balagon MV, Cellona RV, Tan EV, Gelber R, Saunderson P, Duthie MS, Reece ST, Burman W, Belknap R, Mac Kenzie WR, Geluk A, Oskam L, Dockrell HM, Brennan PJ. Analysis of antibody responses to Mycobacterium leprae phenolic glycolipid I, lipoarabinomannan, and recombinant proteins to define disease subtype-specific antigenic profiles in leprosy. Clin Vaccine Immunol. 2011;18:260–267. doi: 10.1128/CVI.00472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, Zumla A. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375:1920–1937. doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.