Abstract
Renal fibrosis and inflammation are associated with hypoxia, and tissue pO2 plays a central role in modulating the progression of chronic kidney disease. Key mediators of cellular adaptation to hypoxia are hypoxia-inducible factor (HIF)-1 and -2. In the kidney they are expressed in a cell type-specific manner; to what degree activation of each homolog modulates renal fibrogenesis and inflammation has not been established. To address this issue, we used Cre-loxP recombination to activate or to delete both Hif-1 and Hif-2 either globally or cell type-specifically in myeloid cells. Global activation of Hif suppressed inflammation and fibrogenesis in mice subjected to unilateral ureteral obstruction, while activation of Hif in myeloid cells suppressed inflammation only. Suppression of inflammatory cell infiltration was associated with down-regulation of CC chemokine receptors in renal macrophages. Conversely, global deletion or myeloid-specific inactivation of Hif promoted inflammation. Furthermore, prolonged hypoxia suppressed the expression of multiple inflammatory molecules in non-injured kidneys. Collectively, we provide experimental evidence that hypoxia and/or myeloid cell-specific HIF activation attenuates renal inflammation associated with chronic kidney injury.
Introduction
A discrepancy between oxygen availability and demand has been shown to associate with renal fibrosis and the progression of chronic kidney disease (CKD), and is thought to result from multiple causes, including capillary rarefaction, limited oxygen diffusion from extracellular matrix (ECM) expansion, atherosclerotic vascular disease, anemia, and alterations in oxygen consumption itself (1). Although this discrepancy is often considered a consequence rather than a cause of renal fibrogenesis, recent studies have indicated that hypoxia may act as an initiating factor in this process (2–5).
Hypoxia-inducible factor (HIF)-1 and -2 are the two predominant transcription factors that regulate cellular hypoxia responses. HIFs consist of an oxygen-sensitive α-subunit and a constitutively expressed β-subunit, which is also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) (6). Under normoxia pO2-dependent prolyl hydroxylase domain (PHD) dioxygenases hydroxylate specific proline residues in the HIF-α oxygen-dependent degradation domain, which leads to von Hippel-Lindau tumor suppressor (pVHL)-dependent poly-ubiquitylation and subsequent proteasomal degradation (7). Under hypoxia, hydroxylation is inhibited, resulting in HIF-α stabilisation and its translocation to the nucleus, where it hetero-dimerizes with ARNT to form transcriptionally active HIF. In the kidney, only HIF-1α has been detected in renal epithelial cells under acute hypoxic conditions in vivo, whereas HIF-2α can be found in endothelial, glomerular, as well as in peritubular interstitial cells, where it regulates erythropoietin (EPO) synthesis (8, 9).
The role of HIF in kidney diseases is context-dependent and differs between acute and chronic hypoxic injury with regard to disease outcome (8). We have previously demonstrated that renal proximal tubule-specific ablation of Hif-1α decreases fibrosis and inflammation in a model of unilateral ureteral obstruction (UUO), suggesting that epithelial Hif-1α acts as a pro-fibrogenic transcription factor (5). This notion is supported in a 5/6 renal ablation model, where activation of epithelial Hif-1 promoted fibrosis (10). In contrast to these findings, systemic Hif activation by pharmacologic means has been shown to improve renal injury in certain experimental CKD models {for reviews on these studies see (8, 11)} suggesting that Hif may have cell type-specific functions, which either promote or inhibit renal fibrogenesis and inflammation.
In order to address the role of HIF in experimental CKD, we have used a genetic approach to dissect cell type-specific Hif functions in the context of renal fibrosis and inflammation. Here we show that global activation of Hif ameliorates inflammation and reduces ECM accumulation following UUO, whereas global deletion of Hif enhances renal inflammation. These alterations in inflammatory responses are phenocopied when Hif is either activated or deleted cell type-specifically in myeloid cells. Hif signalling in myeloid cells may therefore contribute to the reno-protective effects of global Hif activation. Furthermore, we provide experimental evidence that hypoxia and/or activation of the Hif pathway in the kidney leads to a down-regulation of chemokine and chemokine receptor expression. Taken together our study establishes a critical role for myeloid cell-derived Hif in inflammatory cell recruitment associated with renal injury, and supports the notion that hypoxia and activation of the Hif system induces cell type-dependent molecular responses that result in different disease outcomes.
Methods
Generation of mice, genotyping and surgical procedures
The generation and genotyping of Hif1a, Hif2a (Epas1), or Vhl conditional alleles has been described elsewhere (12, 13). To achieve either inducible global, or myeloid-specific recombination, mice carrying the Hif1a, Hif2a, or Vhl conditional alleles were crossed to mice that either expressed a tamoxifen-inducible Cre-recombinase, which is under control of the ubiquitin c promoter, referred to as Ubc-cre/ERT2 (14), or LysM-cre, where Cre-recombinase is controlled by lysozyme M regulatory elements (15). Inducible conditional Vhl knockout mice, Vhl2lox/2lox;Ubc-cre/ERT2 are referred to as Ubc-Vhl2lox/2lox prior to tamoxifen treatment and Ubc-Vhl−/− following tamoxifen treatment. Inducible conditional Hif-1/Hif-2 double knockout mice, Hif1a2lox/2lox;Hif2a2lox/2lox;Ubc-cre/ERT2 are referred to as Ubc-Hif2lox/2lox prior to tamoxifen injection, and Ubc-Hif−/− following tamoxifen treatment. Vhl2lox/2lox;LysM-cre and Hif1a2lox/2lox;Hif2a2lox/2lox;LysM-cre are referred to as LysM-Vhl−/− or LysM-Hif−/− mice. For activation of the Cre-ERT2 transgenic system, tamoxifen (Sigma-Aldrich, St. Louis, MO) was injected intraperitoneally every other day for 10 days at a concentration of 10mg/ml (~1.5mg/mouse), dissolved in a mixture of 90% sunflower oil and 10% ethanol. To assess recombination in renal macrophages, LysM-cre mice were bred to a membrane bound tomato-red/GFP (mT/mGFP) double-fluorescent Cre reporter strain (16) generating LysM-mT/mGFP mice, which were then subjected to UUO. A mouse model of β-thalassemia (Hbbth3/th3) was generated by deletion of both β-globin genes, βmajor and βminor, and has been described previously (17, 18). UUO was performed in 8- to 10-week-old mice as previously described (5). Cre-negative (Cre−) littermates were always used as control mice. Mice were analyzed on day 8 following ureteral ligation. All procedures involving mice were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania, Cornell University and Vanderbilt University Institutional Animal Care and Use Committees.
DNA, RNA and protein analysis
DNA and RNA were isolated using DNeasy and RNeasy kits, respectively, according to manufacturer’s protocols (Qiagen Inc., Valencia, CA). For semi-quantitative real-time PCR, cDNA was prepared from 1 μg of total RNA and analyzed using SYBR green or TaqMan PCR master mix (Applied Biosystems, Carlsbad, CA). Primer sequences for Vegfa, Pgk-1, Epo, collagen-Iα, F4/80, iNos, Arg1, Mr, Tnfa, and Il10 have been described previously (5, 19–22). Primer sequences for other genes analyzed are listed in supplemental table S1. 18S rRNA was used to normalize mRNA. For the quantification of mRNA expression levels the relative standard curve method was used according to the manufacturer’s instructions (Applied Biosystems).
The Whole Mouse Genome Oligo Microarray G4122A (Agilent Technology, Santa Clara, CA) was used for global gene expression profiling. Total RNA prepared from Hbbth3/th3 and control mice was quantified with, and purity and integrity verified, by Bioanalyzer (Agilent Technology). All procedures were performed according to instructions provided by Agilent Technologies. Arrays were scanned with Model G2565BA (Agilent Technology), and analyzed using the “SAM” statistical analysis package Version 3.1 (http://www-stat.stanford.edu/~tibs/SAM). Nuclear protein extracts were prepared and analyzed by Western blotting as previously described (13, 19).
Immunohistochemistry
To assess collagen deposition, paraffin-embedded kidney sections were stained with Sirius red (0.1% fast green FCF and 0.1% direct red 80 in saturated picric acid). A rat anti-F4/80 antibody was used for quantitative assessment of macrophage (MØ)/dendritic cell (DC) infiltration of renal tissue (Abcam Inc, Cambridge, MA, ab6640). Goat anti-rat secondary antibody (Vector Lab, Burlingame, CA) was used. 10 random high-power fields (HPF)/per kidney section were examined for both Sirius red and inflammatory cell analysis. Sirius red-positive area expressed as percent of total area analyzed was determined with NIH image J software (rsbweb.nih.gov/ij). For statistical analysis, Sirius red-positive area percentages, and F4/80+ cell numbers for individual mice were averaged across control and mutant cohorts. To visualize recombination event, UUO and contralateral kidneys from LysM-mT/mGFP mice were sacrificed at day 8 following ureteral ligation and stained for F4/80. Tissue sections were visualized with confocal fluorescent microscopy (Zeiss LSM510 META, Carl Zeiss Inc., Thornwood, NY).
Purification of renal macrophages
CD11b+ cells were isolated by immunomagnetic separation (MACS beads, Miltenyi Biotech, Auburn, CA) according to the manufacturer’s instructions at day 8 following UUO. Kidneys were harvested, minced, and homogenized, followed by incubation with 0.1% collagenase A (Roche, Madison, WI) and 100 μg/ml DNase I (BioRad, Hercules, CA) for 45 minutes at 37°C. In order to obtain sufficient quantities of cells, isolates from 3 to 5 littermates of the same genotype were pooled and then analyzed. Total RNAs isolated from the renal CD11b+ cells were subjected to real-time RT-PCR.
Primary cell culture and in vitro assays
Peritoneal exudates were collected by sterile lavage on day 3 following intraperitoneal injection of 3% thioglycollate (BD Biosciences, Sparks, MD). Isolated cells were washed and plated in RPMI1640 containing 10% FBS, which was changed 4 hours after plating to remove non-adherent cells. Cells were grown for 24 hours in normoxia (21% O2), hypoxia (1% O2), in the presence or absence of LPS (100ng/ml, Sigma-Aldrich), or IL-13 (10ng/ml, Invitrogen, Carlsbad, CA). Primary proximal renal tubule epithelial cells were isolated from tetracycline-inducible Hif1a2lox/2lox;R26-rtTA2;Lc-1-cre mice as previously described (5).
Statistical analysis
Statistical analysis was performed using 2-tailed Student’s t test. P values of less than 0.05 were considered significant.
Results
Global activation of Hif reduces renal fibrosis following UUO and is associated with a decrease in inflammation
We have previously demonstrated that proximal tubular Hif-1 promoted fibrogenesis in two experimental models of chronic renal injury (5, 10). In contrast, systemic Hif activation by pharmacologic prolyl-hydroxylase inhibition was found to ameliorate fibrosis and improved renal injury (23). These findings suggested that the effects of Hif activation with regard to renal fibrogenesis might be cell type-dependent. To examine the combined effects of Hif stabilization in different renal cell types, we used a genetic approach and globally activated Hif-1α and Hif-2α in a model of rapidly progressing renal fibrosis induced by unilateral ureteral obstruction (UUO). For this, we utilized a ubiquitously expressed tamoxifen-inducible Cre-recombinase (Ubc-Cre/ERT2) to generate mice with global inactivation of the Vhl tumor suppressor (Ubc-Vhl−/−), as Vhl germ line deletion results in embryonic lethality (24). Vhl−/− cells are unable to degrade Hif-α and are characterized by the activation of Hif transcriptional programs (24). Induction of Ubc-cre/ERT2 by tamoxifen resulted in efficient recombination in the kidney and other organs as determined by genomic PCR (Suppl. Fig. S1). As a consequence of Hif-1α and Hif-2α stabilization in the kidney, the expression of Hif-regulated genes, vascular endothelial growth factor A (Vegfa), phosphoglycerate kinase (Pgk)-1, lactate dehydrogenase (Ldh), and Epo, were increased (Fig. 1 and Suppl. Fig. S1).
Fig. 1. Global activation of Hif ameliorates renal fibrosis and inflammation.
(A) Shown are representative images of paraffin-embedded tissue sections from Cre− and Ubc-Vhl−/− kidneys stained for collagen with Sirius red (original magnification × 200) and for macrophages with F4/80 immunohistochemistry (original magnification × 200). For statistical analysis, 10 measurements per individual mice were averaged for Sirius red (n=7 for control and n=4 for mutants) and for F4/80 staining (n=6 for control and n=4 for mutants). (B) qRT-PCR analysis of F4/80, Ccr2, Ccl2, collagen Ia, Kim-1, and Vegfa mRNA levels in CTL and UUO kidneys 8 days after ligation. Relative expression values were normalized to 18S rRNA. Data points represent individual mice. Error bars represent mean values ± SEM; *p<0.05, **p<0.01. Abb.: CTL, contralateral kidney; UUO, obstructed kidney.
To investigate the effects of global Vhl inactivation on renal fibrosis, Ubc-Vhl−/− and Cre− littermate controls were subjected to UUO and analyzed on day 8 following ureteral ligation. Tamoxifen was administered prior to surgery to both Cre− littermate controls and Ubc-Cre/ERT2 expressing animals to control its potential effects on fibrosis development (25, 26). Tamoxifen administration by itself did not induce fibrosis or inflammation in wild type mice (Suppl. Fig. S1F). We found a ~30% reduction in collagen accumulation in Ubc-Vhl−/− UUO kidneys by Sirius red staining (Fig. 1A). Attenuation in collagen deposition was associated with a marked decrease in F4/80+ inflammatory cell infiltration as determined by F4/80 mRNA levels in whole kidney homogenates (~65% reduction) and by immunohistochemical (IHC) staining (63±8 vs. 13±4 cells/HPF) (Fig. 1A and B). The reduction in macrophage numbers was furthermore associated with decreased expression of chemokine (CC motif) receptor-2 (Ccr2) and its ligand chemokine (CC motif) ligand-2 (Ccl2), also known as monocyte chemotactic protein-1 (Mcp1) (Fig. 1B). Consistent with ameliorated injury was a ~40% reduction in Kim-1 mRNA levels (Fig. 1B). The expression of Kim-1, a transmembrane tubular protein, correlates positively with renal damage in both acute and chronic injuries (27). mRNA levels of neutrophil gelatinase-associated lipocalin (Ngal), another renal injury marker (28, 29), were also reduced but did not reach statistical significance (reduction by ~40%, Suppl. Fig. S1E). Ngal expression levels, however, are difficult to interpret in our model, as Hif-1 has been shown to be involved in the regulation of Ngal (30).
While our data suggest that global Hif activation suppresses inflammation and reduces collagen accumulation in UUO kidneys, we could not completely rule out Hif-independent effects of Vhl inactivation. We therefore hypothesized that global deletion of both Hif-1α and Hif-2α together would increase inflammation and fibrosis in this model. To test this hypothesis, we used the Ubc-cre/ERT2 transgenic system to ablate both Hif-1a and Hif-2a globally (Ubc-Hif−/−). Tamoxifen treatment resulted in highly efficient deletion of both Hif-1a and Hif-2a (Suppl. Fig. S2B), and was associated with a 40% reduction in Vegfa levels (Fig. 2B) as well as a reduction of Hif-targeted genes Pgk-1 and Epo (Suppl. Fig. S2C). In contrast to Ubc-Vhl−/− kidneys, the number of infiltrating F4/80+ cells was significantly increased in Ubc-Hif−/− mice compared to controls (98±8 vs. 57±4 cells/HPF; Fig. 2A), while ECM accumulation was not different, dissociating renal inflammation from fibrosis (Fig. 2A). Taken together, our findings suggest that Hif functions as potent modulator of inflammatory responses in the kidney, and when activated, confers renoprotection by suppressing inflammation.
Fig. 2. Global deletion of Hif exacerbates renal inflammation.
(A) Shown are representative images of paraffin-embedded tissue sections from Cre− and Ubc-Hif−/− kidneys stained for collagen with Sirius red (original magnification × 200) and for macrophages with F4/80 immunohistochemistry (original magnification × 200). For statistical analysis, 10 measurements per individual mice were averaged for Sirius red (n=7 for control and n=5 for mutants) and for F4/80 staining (n=7 for control and n=5 for mutants). (B) qRT-PCR analysis of Vegfa mRNA level in CTL and UUO kidneys 8 days after ligation. Shown are the means of relative expression values normalized to 18S rRNA. Data points represent individual mice. Error bars represent mean ± SEM; *p<0.05, **p<0.01. Abb.: CTL, contralateral kidney; UUO, obstructed kidney.
Macrophage-restricted Hif activation attenuates, while Hif elimination enhances inflammation without altering the fibrotic response following UUO
Since we observed that global Hif activation suppressed inflammation and thereby attenuated renal injury, we investigated the role of MØ-specific Hif activation in UUO-induced renal injury. For this study, we generated myeloid cell-specific Vhl−/− mice, in which Hif-1α and Hif-2α were stabilized in MØ, from hereon referred to as LysM-Vhl−/−, and mice that lacked both Hif-1α and Hif-2α in MØ, which are from hereon referred to as LysM-Hif−/−. LysM-mediated inactivation of Vhl or Hif did not affect the number of circulating leukocytes and monocyte or neutrophil differential counts, which is consistent with previous reports (31). In agreement with Cramer and colleagues (31), recombination of >90% was observed in peritoneal M/M isolated from LysM-Vhl−/− or LysM-Hif−/− mice (data not shown). As Lysozyme M promoter activity is maturation stage-dependent (32), we assessed the degree of recombination in renal MØ with LysM-mT/mGFP reporter mice that were subjected to UUO. In this reporter strain membrane-targeted Tomato red (mT) is ubiquitously expressed at baseline and switches to membrane-bound green-flourescent protein (mGFP) in cells that have undergone Cre-mediated excision (16). By day 8 post UUO, most F4/80+ cells were GFP-positive, indicating significant recombination in renal MØ (Fig. 3A). Using real-time RT-PCR, a 40% reduction was found in the ratio of Hif exon 2/exon 8 (Hif exon 2 is flanked by loxP-sites and excised upon Cre-mediated recombination) in CD11b+ cells isolated from LysM-Hif−/− UUO kidneys, indicating efficient recombination (Suppl. Fig. S3A). Furthermore, an increase and a decrease of Hif-targeted gene, Vegfa, were observed by Vhl−/− and Hif−/− peritoneal MØ, respectively (Suppl. Fig. S3B).
Fig. 3. Activation of myeloid Hif attenuates inflammation.
(A) Shown are representative confocal laser microscopy images of CTL and UUO kidneys from LysM-mT/mGFP mice. Recombined cells express GFP (a), non-recombined cell express tomato red (b), F4/80+ cells are depicted by blue fluorescence (c), overlay of images a-c is shown in (d). Arrowheads point towards F4/80+/GFP+ myeloid cells. Asterisks depict F4/80−/GFP− tubular structures. (B) Representative images of paraffin-embedded sections from Cre− and LysM-Vhl−/− kidneys stained for collagen with Sirius red (original magnification × 200), and for macrophages with F4/80 immunohistochemistry (original magnification × 200). For statistical analysis, 10 measurements per individual mice were averaged for Sirius red (n=7 for control and n=6 for mutants) and for F4/80 staining (n=7 for control and n=6 for mutants). (C) qRT-PCR analysis of F4/80, collagen Ia, and Kim-1 mRNA levels in CTL and UUO kidneys 8 days after ligation. Relative expression values were normalized to 18S rRNA. Data points represent individual mice. Error bars represent mean ± SEM; *p<0.05. Abb.: CTL, contralateral kidney; UUO, obstructed kidney.
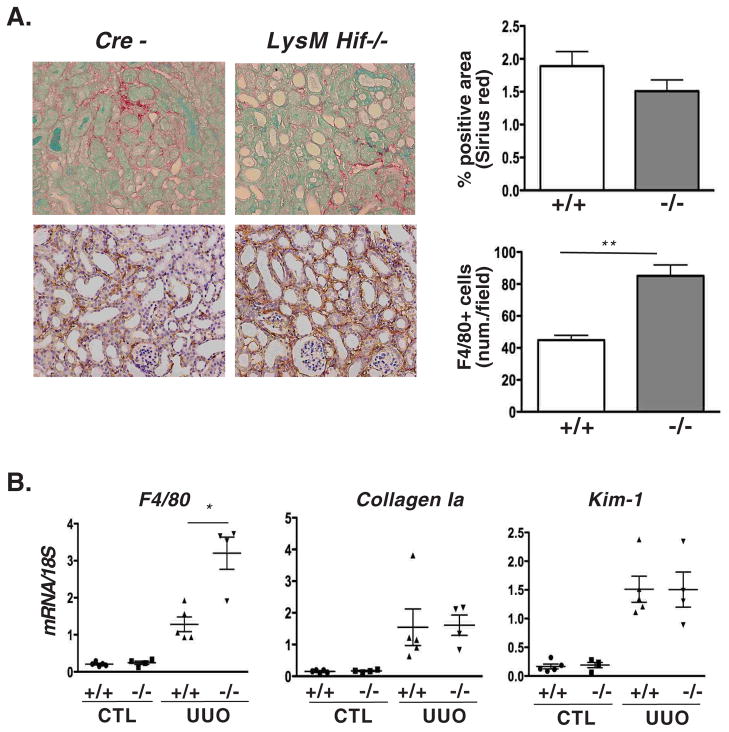
When LysM-Vhl−/− mice were subjected to UUO, we found a significant decrease in renal F4/80+ cell accumulation as determined by F4/80 mRNA levels (36% reduction) and by IHC (40% reduction; 119±5 vs. 71±6 cells/HPF) (Fig. 3B and C). However, despite the marked decrease in the F4/80+ cell numbers, no differences were observed in collagen-1a mRNA levels, or ECM accumulation as determined by Sirius red staining (Fig. 3B and C). Kim-1 expression levels between LysM-Vhl−/− and Cre− littermate controls were not different, consistent with comparable tubular injury (Fig. 3C). Taken together our data suggest that MØ-restricted Hif activation reduces the infiltration by F4/80+ cells, but does not influence the degree of ECM accumulation or tubular injury. In contrast to LysM-Vhl−/− mice, renal F4/80+ cell infiltration was significantly enhanced in LysM-Hif−/− mice subjected UUO as determined by F4/80 mRNA (2.5-fold increase) and F4/80 IHC (1.9-fold increase; 45±3 vs. 85±7 cells/HPF) (Fig. 4A and B). However, in spite of this increase in F4/80+ cell infiltration, collagen-1a expression, collagen accumulation, and Kim-1 levels were not different (Fig. 4A and B). Taken together our findings demonstrate that genetic manipulation of Hif in myeloid cells alone is sufficient to reproduce the changes in F4/80+ cell infiltration that were observed in Ubc-Vhl−/− or Ubc-Hif−/− UUO kidneys, suggesting that myeloid cell-derived Hif regulates renal inflammation. Surprisingly, however, Hif-dependent regulation of F4/80+ cell infiltration did not affect tubular injury as indicated by the absence of differences in Kim-1 levels or collagen accumulation between mutants and control.
Fig. 4. Deletion of myeloid Hif enhances inflammation.
(A) Shown are representative images of paraffin-embedded tissue sections from Cre− and LysM-Hif−/− kidneys stained with Sirius red to assess ECM expansion (upper panel, original magnification × 200) and analyzed by immunohistochemistry for macrophage marker F4/80 (original magnification ×200). For statistical analysis, 10 measurements per individual mice were averaged for Sirius red (n=7 for control; n=6 for mutants) and for F4/80 staining (n=3 for control and n=5 for mutants). (B) qRT-PCR analysis for F4/80, collagen Ia, and Kim-1 levels in CTL and UUO kidneys 8 days after ligation. Shown are the means of relative expression values normalized to 18S rRNA. Data points represent individual mice. Error bars represent mean ± SEM.; *p<0.05, **p<0.01. Abb.: CTL, contralateral kidney; UUO, obstructed kidney.
HIF regulates macrophage polarization
Since macrophage polarization (M1 versus M2 phenotype) has a major influence on the development and progression of renal disease (33), we next investigated the effects of Hif activation on macrophage polarization. For this study we isolated monocyte/macrophages (M/M) from LysM-Vhl−/− and LysM-Hif−/− mice. We then examined the expression of M1-associated inducible nitric oxide synthase (iNos) and M2-associated arginase I (Arg1) following stimulation with lipopolysaccaride (LPS) or interleukin 13 (IL-13); LPS treatment of M/M induces the M1 phenotype and increases iNos expression, whereas IL-13 induces the M2 phenotype and increases Arg1 expression. We found that the induction of iNos mRNA by LPS was significantly enhanced in Vhl−/− M/M compared to LPS-treated Cre− control cells (~45-fold), while Arg1 induction was further increased in IL-13-treated Vhl−/− M/M compared to Cre− controls (~3.5-fold) (Fig. 5A). In line with these findings and Hif dependence is the decreased expression of iNos and Arg1 in stimulated Hif−/− M/M (Fig. 5B). Furthermore, the hypoxic induction of iNos and ArgI in Cre− control M/M was almost completely abrogated in Hif−/− M/M (Fig. 5B), suggesting Hif-dependent regulation of these markers under hypoxia. Another hallmark of M2-MØ is the expression of high levels of mannose receptor (Mr), which did not differ between Vhl−/− M/M and Hif−/− M/M (data not shown).
Fig. 5. Hif regulates macrophage polarization.
qRT-PCR analysis of iNos and Arg1 levels in M/M isolated from LysM-Vhl−/− (A), LysM-Hif−/− (B), or LysM-Vhl/Hif-2a−/− or LysM-Vhl/Hif-1a−/− mice were treated with either normoxia (N), hypoxia (H), LPS, or IL-13 (C). M/M isolated from Cre− littermates served as control. Shown are the means of relative expression values normalized to 18S rRNA ± SEM; *p<0.05, **p<0.01.
To determine the contribution of individual Hifs to the regulation of iNos and Arg1, we compared M/M isolated from LysM-Vhl/Hif-1a and from LysM-Vhl/Hif-2a double-deletion mice (activation of Hif-2α or Hif-1α alone respectively) to Cre− controls. We found that the enhanced induction of iNos following LPS treatment noted in Vhl−/− M/M was blocked in Vhl/Hif-1a−/− M/M, but persisted in Vhl/Hif-2a−/− M/M, indicating Hif-1α regulation, whereas increased Arg1 induction by IL-13 was blocked in Vhl/Hif-2a−/− M/M, but preserved in Vhl/Hif-1a−/−, indicating Hif-2α regulation (Fig. 5C). Taken together, our observations suggest that in Vhl-deficient M/M, Hif-1α promotes gene expression changes that are consistent with the M1 phenotype, while Hif-2α regulates genes that are associated with M2-MØ. However, simultaneous activation of both Hif-1α and Hif-2α does not introduce a polarization bias, as the induction of genes associated with both phenotypes is not inhibited.
HIF modulates immune responses in renal macrophages
To elucidate the mechanisms by which myeloid-derived Hif suppressed renal inflammation, we isolated MØ from UUO kidneys to gain further insight into underlying mechanisms. CD11b+ cells from kidneys of LysM-Vhl−/− or LysM-Hif−/− mice were isolated using the MACS beads immuno-magnetic purification system, and inflammatory gene expression was analyzed with real-time RT-PCR. With regard to M1/M2-associated gene expression, we found that iNos was up-regulated in Hif−/− MØ and down-regulated in Vhl−/− MØ compared to control MØ from Cre− littermates. While we observed a down-regulation of Arg I, Mr, tumor necrosis factor a (Tnfa), and interleukin-10 (Il10) in Vhl−/− MØ compared to control, differential regulation was not found between and Vhl−/− MØ and Hif−/− MØ (Table 1). Because of their critical role in renal inflammation (34), we next examined the expression of chemokine receptors. We found that Ccr2 and Ccr5 mRNA levels were down-regulated in CD11b+ cells from UUO kidneys of LysM-Vhl−/− mice compared to Cre− littermate controls (Table 1). In contrast to renal Vhl−/− MØ, Ccr2 and Ccr5 were increased in renal Hif−/− MØ compared to controls (Table 1), which is consistent with increased F4/80+ cell infiltration in UUO kidneys from LysM-Hif−/− mice. Taken together, gene expression analysis of renal MØ is consistent with the LysM-cre knockout phenotype, and raise the possibility that Hif-mediated inhibition of inflammatory cell recruitment in UUO kidneys is mechanistically linked to a suppression of pro-inflammatory molecules in renal MØ.
Table 1. Hif modulates immune responses in renal macrophages.
CD11b+ cells were isolated from UUO kidneys 8 days after ureteral ligation using the MACS beads system. For each experimental set, CD11b+ cells were pooled from 3 to 5 littermates (mutant and control mice each). qRT-PCR analysis was performed with pooled CD11b+ cells and gene expression was normalized to 18S rRNA. Alterations in gene expression are presented as fold-changes over pooled littermate controls.
| Gene | LysM-Hif−/− | LysM-Vhl−/− | ||
|---|---|---|---|---|
| Set 1 | Set 2 | Set 1 | Set2 | |
| iNos | 2.13 | 3.22 | 0.75 | 0.72 |
| Arginase I | 0.65 | 0.81 | 0.65 | 0.81 |
| Mr | 0.53 | 1.66 | 0.80 | 0.51 |
| Tnfa | 0.62 | 0.89 | 0.77 | 0.55 |
| Il-10 | 0.63 | 1.00 | 0.89 | 0.29 |
| Ccr 2 | 2.03 | 1.83 | 0.76 | 0.81 |
| Ccr 5 | 2.99 | 2.53 | 0.72 | 0.71 |
Pro-inflammatory gene expression is suppressed in chronically hypoxic kidneys
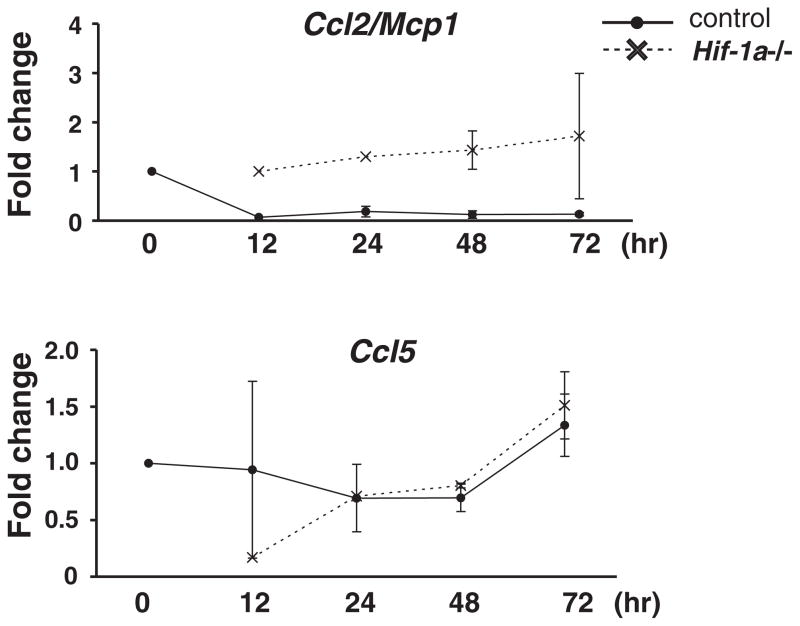
Since we used conditional Vhl inactivation to stabilize Hif either globally or in myeloid cells specifically, we asked the question whether activation of Hif in the kidney under hypoxic conditions alone, i.e. in the presence of wild type Vhl, would result in a suppression of pro-inflammatory genes. For this study, we used genome-wide RNA expression analysis and examined kidneys from mice with mutated β-hemoglobin (Hbbth3/th3), which develop severe anemia resulting in substantial Hif activation in the kidney (Fig 6A). We found that a large number of genes which were differentially expressed and encoded inflammatory molecules were down-regulated in Hbbth3/th3 kidneys compared to control (data accessible at NCBI GEO database, accession# GSE36312,://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE36312), supporting our hypothesis that hypoxia and/or activation of Hif signalling suppresses inflammation in the kidney (Table 2). Consistent with our findings in renal Vhl−/− MØ is the decreased expression of Ccr2 and Ccr5 in Hbbth3/th3 kidneys (Fig. 6B). Furthermore, the main ligand for Ccr2, Ccl2/Mcp1 was down regulated in Hbbth3/th3 kidneys (Fig. 6B). Since Ccl2/Mcp1 increases in renal tubular cells upon injury (35) and initiates monocyte recruitment, we determined Ccl2/Mcp1 expression levels in primary proximal tubular epithelial cells (PTEC) grown under hypoxia (1% O2). While Ccl5 was not differentially expressed in either anemic kidneys or hypoxic PTEC (Fig. 6B and Fig. 7), we found Hif-1-dependent down-regulation of Ccl2/Mcp1 in PTEC (Fig. 7), raising the possibility that Hif activation in proximal renal epithelial cells impinges on M/M recruitment. These data are consistent with in vivo findings from global and myeloid-specific Vhl knockout mice and support the notion that activation of Hif signalling in both myeloid and epithelial cells contributes to the renal inflammatory response.
Fig. 6. Ccr2 and Ccl2/Mcp1 expression is suppressed in chronically hypoxic kidneys.
(A) Immunoblot analysis for Hif-1α was performed with nuclear protein extracts isolated from kidneys of Hbbth3/th3 and control mice. Ponceau S staining is shown to demonstrate equal protein loading. (B) qRT-PCR analysis of Ccr2, Ccr5, Ccl2, and Ccl5 in Hbbth3/th3 and control kidneys. Shown are the means of relative expression levels normalized to 18S rRNA ± SEM; *p<0.05, **p<0.01.
Table 2. Differentially regulated inflammatory pathway genes in kidneys from chronically hypoxic mice.
Relative expression levels of genes assigned to inflammatory pathways that were at least ≥ 1.5 fold up- or down-regulated and had less than 10% FDR (total of 1170 probes and 912 genes) in kidneys from mice with mutated β–hemoglobin (Hbbth3/th3) compared to wild type littermates. Inflammatory pathway genes were considered those genes with ontologies of: Inflammatory response, activation of plasma proteins involved in acute inflammatory response, cytokine activity, and chemokine activity (http://david.abcc.ncifcrf.gov/).
| Gene | Description | Entrez Gene ID | Fold Induction |
|---|---|---|---|
| Ighg | Immunoglobulin heavy chain (gamma polypeptide) | BC092269 | −5.5 |
| Cxcl9 | chemokine (C-X-C motif) ligand 9 | NM_008599 | −3.5 |
| Cxcl10 | chemokine (C-X-C motif) ligand 10 | NM_021274 | −2.9 |
| Ccl2 | chemokine (C-C motif) ligand 2 | NM_011333 | −2.5 |
| Ccr2 | chemokine (C-C motif) receptor 2 | NM_020008 | −2.4 |
| Cxcr3 | Chemokine (C-X-C motif) receptor 3 | NM_009910 | −2.1 |
| Clec7a | C-type lectin domain family 7, member a | NM_020008 | −2.0 |
| C2ta | class II transactivator | NM_007575 | −1.9 |
| C8a | complement component 8, alpha polypeptide | NM_146148 | −1.9 |
| Ltb | lymphotoxin B | NM_008518 | −1.9 |
| Serpinf2 | serine (or cysteine) peptidase inhibitor, clade F, member 2 | NM_008878 | −1.9 |
| Cd74 | CD74 antigen | NM_001042605 | −1.9 |
| Ly86 | lymphocyte antigen 86 | NM_010745 | −1.8 |
| Mbl1 | mannose binding lectin (A) | NM_010775 | −1.8 |
| Prlr | Prolactin receptor | NM_011169 | −1.8 |
| Ccl7 | chemokine (C-C motif) ligand 7 | NM_013654 | −1.7 |
| Fn1 | fibronectin 1 | NM_010233 | −1.7 |
| Il16 | interleukin 16 | NM_010551 | −1.7 |
| Cxcr6 | Chemokine (C-X-C motif) receptor 6 | NM_030712 | −1.7 |
| Il10ra | Interleukin 10 receptor, alpha | NM_008348 | −1.7 |
| Il1b | Interleukin 1 beta | NM_008361 | −1.6 |
| Tlr2 | Toll-like receptor 2 | NM_011905 | −1.6 |
| Calca | Calcitonin/calcitonin-related polypeptide, alpha | NM_001033954 | −1.6 |
| Cd44 | CD44 antigen | NM_009851 | −1.6 |
| Blr1 | Burkitt/lymphoma receptor 1 | NM_007551 | −1.6 |
| Il2rb | Interleukin 2 receptor, beta chain | NM_008368 | −1.6 |
| Ccr5 | Chemokine (C-C motif) receptor 5 | NM_009917 | −1.5 |
| Masp2 | Mannan-binding lectin serine peptidase 2 | NM_010767 | −1.5 |
| C1qa | Complement component 1, q subcomponent, alpha polypeptide | NM_007572 | −1.5 |
| Il2rg | Inteleukin 2 receptor, gamma chain | NM_013563 | −1.5 |
| Cmtm3 | CKLF-like MARVEL transmembrane domain containing 3 | NM_024217 | −1.5 |
| Osmr | Oncostatin M receptor | NM_011019 | 1.5 |
| Csf2rb2 | Colony stimulating factor 2 receptor, beta 2 | NM_007781 | 1.6 |
| Cxcl4 | Chemokine (C-X-C motif) ligand 4 | NM_019932 | 1.6 |
| Chi3l4 | Chitinase 3-like 4 | NM_145126 | 1.7 |
| Il17rb | Interleukin 17 receptor B | AF208109 | 1.8 |
| Cfd | Complement factor D (adipsin) | NM_013459 | 1.9 |
| Cd163 | CD163 antigen | NM_053094 | 1.9 |
| Cxcl7 | Chemokine (C-X-C motif) ligand 7 | NM_023785 | 2.1 |
| Serpina1b | Serine (or cysteine) preptidase inhibitor, clade A, member 1b | BC037008 | 2.2 |
| Hc | Haemolytic complement | NM_010406 | 2.7 |
| Ccbp2 | Chemokine binding protein 2 | NM_021609 | 2.8 |
| Grem2 | Gremlin 2 homolog, cystein knot superfamily | NM_011825 | 3.5 |
| Ahsg | Alpha-2-HS-glycoprotein | NM_013465 | 4.2 |
| Chi3l3 | Chitinase 3-like 3 | NM_009892 | 6.4 |
Fig. 7. Hif-1-dependent down-regulation of Ccl2/Mcp1 in hypoxic primary proximal tubular epithelial cells (PTEC).
PTEC were isolated from tetracycline-inducible Hif1a2lox/2lox;R26-rtTA2;Lc-1-cre or Cre− control mice and cultured under hypoxia (1% O2) for the indicated time period. qRT-PCR analysis for Ccl2Mcp1 and Ccl5 mRNA levels was performed. Shown are the means of relative expression levels compared to normoxic Cre− littermate controls following normalization to 18S rRNA ± SEM.
Discussion
The role of cell type-specific Hif signalling in the pathogenesis of progression of chronic kidney disease is not well understood and is controversial (1). In this report we have used genetic means to investigate the effects of global and myeloid-cell specific Hif activation or inactivation in a model of rapidly progressing fibrosis and inflammation induced by UUO. Our studies demonstrate that global Hif activation ameliorates fibrosis and F4/80+ cell infiltration, which is associated with decreased expression of Ccl2 chemokine and its receptor Ccr2 in the injured kidney. Specifically, we have identified myeloid-derived Hif as a key factor in the suppression of renal inflammation.
The general role of Hif in the regulation of inflammatory responses is complex and appears to be context-dependent. While our studies indicate that Hif suppresses F4/80+ cell infiltration in a model of chronic renal disease, myeloid Hif-1 has been shown to promote inflammation in experimental models of dermatitis and rheumatoid arthritis (31). In these settings, Hif is thought to support inflammation by a) facilitating anaerobic ATP production, which enhances cellular survival and enables defensive immune functions at inflammatory sites that are often characterized by a very low pO2 (<1%); b) by promoting motility and migration to sites of inflammation; and c) by positively regulating cytokine production (31, 36, 37). Genetic studies have furthermore indicated that myeloid Hif-1 is required for efficient bacterial killing under hypoxic conditions and that it promotes a septic phenotype (38). In contrast to these reports, hypoxia and Hif have also been shown to suppress immune responses by producing anti-inflammatory and anti-apoptotic effects in inflamed tissues, and by suppressing cytokine production in T-cells (39–44). In support of the latter findings are recent studies, which demonstrated that, pharmacologic or genetic manipulation of HIF prolyl-hydroxylation is protective in experimental models of inflammatory bowel disease, where it inhibits inflammation (45, 46). This anti-inflammatory effect of Hif activation is likely to involve HIF-dependent and - independent, as well as NF-κB-mediated, signals, as there is complex and bidirectional interplay between both pathways (47, 48). While PHD1 and 2 inhibit IκB kinase-β (IKK-β), which keeps NF-κB inactive, transcription of Hif-1α is stimulated by NF-κB (48, 49). PHD3 on the other hand has been shown to promote neutrophil survival in a Hif-independent manner (50). In conjunction with these studies, our findings support further investigations into the role of pharmacologic PHD inhibition as a therapeutic strategy for the treatment of certain inflammatory conditions. However, careful characterization of the injured tissue’s microenvironment is required to better understand the molecular basis of Hif-induced pro- or anti-inflammatory effects. It is plausible that differences in tissue-specific chemokine/cytokine production and/or in local pO2 may explain Hif’s opposing roles in the regulation of inflammatory responses.
In support of Hif’s suppressive role in renal inflammation, the expression of CC chemokine receptors Ccr2 and Ccr5 was decreased in renal CD11b+ cells. Suppression most likely occurred indirectly via Hif-dependent regulation of transcriptional repressors (51–53) that are furthermore modulated by disease microenvironment and cell-type specific signals. For example, Ccr5 and to a lesser degree Ccr2 levels were both suppressed in Vhl−/− peritoneal MØ treated with LPS in vitro, but were not increased in LPS-treated Hif−/− peritoneal macrophages (data not shown). Chemokine receptors and their ligands function as major regulators of inflammatory cell recruitment in renal injury (54). Inhibition of Ccr1 or Ccr2 reduces renal MØ infiltration and the development of interstitial fibrosis (55–57). In the setting of acute ischemia-reperfusion injury, deletion of Ccr2 was protective and decreased inflammation (58). In line with these observations are studies where blockade of the Ccr2 ligand Ccl2/Mcp1 decreased MØ recruitment in Col4a3-deficient Alport mice (59). In contrast to Ccr2, deletion of Ccr5 led to disease exacerbation in autoimmune nephritis (60), but improved the long-term outcome of renal allografts (61, 62). Although our data raise the possibility that Hif-mediated inhibition of inflammatory cell recruitment is mechanistically linked to a suppression of pro-inflammatory molecules in MØ, it is likely that Hif activation in other renal cell types, such as epithelial cells, has contributed to the attenuation of renal inflammation under certain conditions.
The notion that Hif could act as a more general suppressor of inflammation in the kidney is furthermore supported by our analysis of chronically hypoxic mice. Here, we examined kidneys with high levels of Hif activity and found suppression of both Ccr2 and its ligand Ccl2/Mcp1. We also found that Ccl2/Mcp-1 is expressed in renal epithelial cells and is negatively regulated by hypoxia in a Hif-1α dependent fashion, as we have shown in primary cell culture with conditional Hif-1α deletion. These results are consistent with findings from Ubc-Vhl−/− UUO kidneys, and illustrate that the anti-inflammatory effects of renal hypoxia/Hif activation involve more than one cell type, in this case both infiltrating MØ as well as renal epithelial cells, which initiate MØ recruitment during injury. The hypoxic regulation of Ccl2/Mcp1 appears to be cell type-dependent, as HIF has been shown to increase CCL2/MCP1 in astrocytes but not in renal epithelial cells (63, 64). A more recent example of Hif-dependent anti-inflammatory signals in the kidney is axonal guidance molecule netrin-1, which is expressed in endothelial and renal epithelial cells and attenuates inflammation by inhibiting myeloid cell migration (65).
We previously reported that renal epithelial Hif promoted fibrosis in a model of UUO and 5/6 nephrectomy (5, 10). Although these data appear to be in contrast to our present study, where global Hif activation was beneficial, we believe that these are not contradictory findings, and rather support the notion that Hif has cell type-specific functions, which impact inflammation and fibrosis differentially. It is likely that the DNA binding sites, to which Hif is recruited, differ between macrophages and epithelial cells, thus permitting activation of specialized transcriptional programs that could impact biological outcomes (66, 67).
We found that activation of both renal Hif-1α and Hif-2α in Ubc-Vhl−/− kidneys led to a suppression of UUO-associated fibrosis and inflammation. Further dissection of the Hif response in LysM-Cre mutant mice revealed that reduced MØ infiltration did not impact on indicators of fibrosis. This implies that the inhibition of inflammatory cell recruitment in this model is not simply a consequence of the degree of fibrotic injury, but rather represents a mechanistically independent change in the ability to recruit and/or retain MØ. While it is well established that inflammatory cells contribute to and modulate renal fibrosis (34), differences in functional outcomes following experimental manipulations of MØ numbers have been reported. Depletion of MØ following UUO on day 4 reduced interstitial fibrosis in a diphtheria-toxin-based transgenic model (68), while no correlation between the degree of MØ infiltration and interstitial fibrosis was observed following MØ blockade with a c-fms kinase inhibitor (69). These opposing effects can be explained by the high degree of cellular and functional heterogeneity in MØ populations (70), as ex vivo induction of different MØ phenotypes had substantial effects on disease outcome in adriamycin-induced nephrosis (20, 71). Consistent with Takeda et al. (72) was our in vitro finding that Hif-1α promoted M1 marker expression, while Hif-2α enhanced M2 marker expression in normoxic peritoneal M/M. Although no polarization bias was found when MØ from UUO kidneys were examined, it should be noted that the use of CD11b magnetic beads will yield a mixed population of inflammatory cells; thus, a shift from an M1 to M2 phenotype in a subpopulation of CD11b+ cannot be completely excluded.
In summary, our study established a critical role for myeloid cell Hif in bone marrow-derived monocytic inflammatory cell recruitment and activation associated with renal injury, and suggests that Hif activation in the kidney suppresses renal inflammation. Whether pharmacologic activation of Hif can be used therapeutically to target inflammation in chronic kidney disease warrants further investigation.
Supplementary Material
Acknowledgments
VHH holds the Krick-Brooks chair in Nephrology. The authors would like to thank Drs. Peter White and Klaus Kaestner (University of Pennsylvania) for help with the microarray analysis.
Grant support:
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), DK081646 and R01 DK081646-02S1 (VHH), and R01 DK081420 and R01 DK081420-02S1 (DS), and by the Penn Institute for Diabetes, Obesity, and Metabolism (P30-DK19525).
Footnotes
Conflict of interest: The authors declare that no conflict of interest exists
References
- 1.Haase VH. Pathophysiological Consequences of HIF Activation: HIF as a modulator of fibrosis. Ann N Y Acad Sci. 2009;1177:57–65. doi: 10.1111/j.1749-6632.2009.05030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orphanides C, Fine LG, Norman JT. Hypoxia stimulates proximal tubular cell matrix production via a TGF-beta1-independent mechanism. Kidney Int. 1997;52:637–647. doi: 10.1038/ki.1997.377. [DOI] [PubMed] [Google Scholar]
- 3.Norman JT, I, Clark M, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 4.Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. doi: 10.1097/01.asn.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- 5.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 7.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, Haase VH. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol. 2008;110:e1–7. doi: 10.1159/000148256. [DOI] [PubMed] [Google Scholar]
- 12.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 13.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 16.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 17.Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N, Smithies O. A mouse model for beta 0-thalassemia. Proc Natl Acad Sci U S A. 1995;92:11608–11612. doi: 10.1073/pnas.92.25.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciavatta DJ, Ryan TM, Farmer SC, Townes TM. Mouse model of human beta zero thalassemia: targeted deletion of the mouse beta maj- and beta min-globin genes in embryonic stem cells. Proc Natl Acad Sci U S A. 1995;92:9259–9263. doi: 10.1073/pnas.92.20.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Mol Cell Biol. 2005;25:3163–3172. doi: 10.1128/MCB.25.8.3163-3172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 21.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe F, Dafferner AJ, Donkor M, Westphal SN, Scholar EM, Solheim JC, Singh RK, Hoke TA, Talmadge JE. Myeloid-derived suppressor cells in mammary tumor progression in FVB Neu transgenic mice. Cancer Immunol Immunother. 2010;59:47–62. doi: 10.1007/s00262-009-0719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song YR, You SJ, Lee YM, Chin HJ, Chae DW, Oh YK, Joo KW, Han JS, Na KY. Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol Dial Transplant. 2010;25:77–85. doi: 10.1093/ndt/gfp454. [DOI] [PubMed] [Google Scholar]
- 24.Kapitsinou PP, V, Haase H. The VHL tumor suppressor and HIF: insights from genetic studies in mice. Cell Death Differ. 2008;15:650–659. doi: 10.1038/sj.cdd.4402313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delle H, Rocha JR, Cavaglieri RC, Vieira JM, Jr, Malheiros DM, Noronha IL. Antifibrotic effect of tamoxifen in a model of progressive renal disease. J Am Soc Nephrol. 2012;23:37–48. doi: 10.1681/ASN.2011010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asada N, Takase M, Nakamura J, Oguchi A, Asada M, Suzuki N, Yamamura K, Nagoshi N, Shibata S, Rao TN, Fehling HJ, Fukatsu A, Minegishi N, Kita T, Kimura T, Okano H, Yamamoto M, Yanagita M. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest. 2011;121:3981–3990. doi: 10.1172/JCI57301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 28.Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, Racusen L, Rabb H. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol. 2010;298:F1472–1483. doi: 10.1152/ajprenal.00619.2009. [DOI] [PubMed] [Google Scholar]
- 29.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cross M, Mangelsdorf I, Wedel A, Renkawitz R. Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci U S A. 1988;85:6232–6236. doi: 10.1073/pnas.85.17.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30:234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prodjosudjadi W, Gerritsma JS, van Es LA, Daha MR, Bruijn JA. Monocyte chemoattractant protein-1 in normal and diseased human kidneys: an immunohistochemical analysis. Clin Nephrol. 1995;44:148–155. [PubMed] [Google Scholar]
- 36.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. J Immunol. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 39.Lukashev D, Klebanov B, Kojima H, Grinberg A, Ohta A, Berenfeld L, Wenger RH, Sitkovsky M. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol. 2006;177:4962–4965. doi: 10.4049/jimmunol.177.8.4962. [DOI] [PubMed] [Google Scholar]
- 40.Kojima H, Gu H, Nomura S, Caldwell CC, Kobata T, Carmeliet P, Semenza GL, Sitkovsky MV. Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1alpha -deficient chimeric mice. Proc Natl Acad Sci U S A. 2002;99:2170–2174. doi: 10.1073/pnas.052706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiel M, Caldwell CC, Kreth S, Kuboki S, Chen P, Smith P, Ohta A, Lentsch AB, Lukashev D, Sitkovsky MV. Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS One. 2007;2:e853. doi: 10.1371/journal.pone.0000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 43.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer Metastasis Rev. 2007;26:273–279. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- 45.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 46.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliver KM, Taylor CT, Cummins EP. Hypoxia. Regulation of NFkappaB signalling during inflammation: the role of hydroxylases. Arthritis Res Ther. 2009;11:215. doi: 10.1186/ar2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walmsley SR, Chilvers ER, Thompson AA, Vaughan K, Marriott HM, Parker LC, Shaw G, Parmar S, Schneider M, Sabroe I, Dockrell DH, Milo M, Taylor CT, Johnson RS, Pugh CW, Ratcliffe PJ, Maxwell PH, Carmeliet P, Whyte MK. Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J Clin Invest. 2011;121:1053–1063. doi: 10.1172/JCI43273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207–217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 54.Panzer U, Steinmetz OM, Stahl RA, Wolf G. Kidney diseases and chemokines. Curr Drug Targets. 2006;7:65–80. doi: 10.2174/138945006775270213. [DOI] [PubMed] [Google Scholar]
- 55.Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Grone HJ, Onuffer J, Horuk R, Nelson PJ, Schlondorff D. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest. 2002;109:251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eis V, Luckow B, Vielhauer V, Siveke JT, Linde Y, Segerer S, Perez De Lema G, Cohen CD, Kretzler M, Mack M, Horuk R, Murphy PM, Gao JL, Hudkins KL, Alpers CE, Grone HJ, Schlondorff D, Anders HJ. Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J Am Soc Nephrol. 2004;15:337–347. doi: 10.1097/01.asn.0000111246.87175.32. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–1537. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clauss S, Gross O, Kulkarni O, Avila-Ferrufino A, Radomska E, Segerer S, Eulberg D, Klussmann S, Anders HJ. Ccl2/Mcp-1 blockade reduces glomerular and interstitial macrophages but does not ameliorate renal pathology in collagen4A3-deficient mice with autosomal recessive Alport nephropathy. J Pathol. 2009;218:40–47. doi: 10.1002/path.2505. [DOI] [PubMed] [Google Scholar]
- 60.Turner JE, Paust HJ, Steinmetz OM, Peters A, Meyer-Schwesinger C, Heymann F, Helmchen U, Fehr S, Horuk R, Wenzel U, Kurts C, Mittrucker HW, Stahl RA, Panzer U. CCR5 deficiency aggravates crescentic glomerulonephritis in mice. J Immunol. 2008;181:6546–6556. doi: 10.4049/jimmunol.181.9.6546. [DOI] [PubMed] [Google Scholar]
- 61.Fischereder M, Luckow B, Hocher B, Wuthrich RP, Rothenpieler U, Schneeberger H, Panzer U, Stahl RA, Hauser IA, Budde K, Neumayer H, Kramer BK, Land W, Schlondorff D. CC chemokine receptor 5 and renal-transplant survival. Lancet. 2001;357:1758–1761. doi: 10.1016/s0140-6736(00)04898-4. [DOI] [PubMed] [Google Scholar]
- 62.Dehmel S, Wang S, Schmidt C, Kiss E, Loewe RP, Chilla S, Schlondorff D, Grone HJ, Luckow B. Chemokine receptor Ccr5 deficiency induces alternative macrophage activation and improves long-term renal allograft outcome. Eur J Immunol. 2010;40:267–278. doi: 10.1002/eji.200939652. [DOI] [PubMed] [Google Scholar]
- 63.Li X, Kimura H, Hirota K, Sugimoto H, Yoshida H. Hypoxia reduces constitutive and TNF-alpha-induced expression of monocyte chemoattractant protein-1 in human proximal renal tubular cells. Biochem Biophys Res Commun. 2005;335:1026–1034. doi: 10.1016/j.bbrc.2005.07.175. [DOI] [PubMed] [Google Scholar]
- 64.Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grenz A, Dalton JH, Bauerle JD, Badulak A, Ridyard D, Gandjeva A, Aherne CM, Brodsky KS, Kim JH, Tuder RM, Eltzschig HK. Partial netrin-1 deficiency aggravates acute kidney injury. PLoS One. 2011;6:e14812. doi: 10.1371/journal.pone.0014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lefterova MI, Steger DJ, Zhuo D, Qatanani M, Mullican SE, Tuteja G, Manduchi E, Grant GR, Lazar MA. Cell-specific determinants of peroxisome proliferator-activated receptor gamma function in adipocytes and macrophages. Mol Cell Biol. 2010;30:2078–2089. doi: 10.1128/MCB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma FY, Liu J, Kitching AR, Manthey CL, Nikolic-Paterson DJ. Targeting renal macrophage accumulation via c-fms kinase reduces tubular apoptosis but fails to modify progressive fibrosis in the obstructed rat kidney. Am J Physiol Renal Physiol. 2009;296:F177–185. doi: 10.1152/ajprenal.90498.2008. [DOI] [PubMed] [Google Scholar]
- 70.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 71.Cao Q, Wang Y, Zheng D, Sun Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.