Abstract
We have previously shown that a novel −74 C to T mutation in the promoter of the cyclin-dependent kinase inhibitor p18 gene was associated with a reduced p18 expression in B cells from mice carrying the Sle2c1 lupus susceptibility locus. To determine the function of the −74 C/T SNP, we have characterized the proximal promoter of the mouse p18 gene. Functional analysis of the 5' flanking region by sequential deletions revealed crucial elements between −300 and +1, confirming the in silico prediction that the −74 T allele created a novel YY-1 binding site adjacent to an existing one common to both alleles. Moreover, we found that YY-1, E2F1 and Sp-1 can synergistically enhance the activity of the p18 promoter. Mutational inactivation revealed that YY-1 binding regulates the p18 activity in an allele-dependent fashion. EMSAs with splenic B cell extracts directly demonstrated that YY-1 binds to the p18 promoter with differences between the C and the T alleles. We also determined in vivo by chromatin immunoprecipitation that the T allele resulted in increased YY-1 and decreased Nrf-2 binding to the p18 promoter as compared to the C allele in B cells. Thus, YY-1 is a direct regulator of p18 gene expression in an allele-dependent fashion that is consistent with the lupus-associated T allele inducing a lower p18 transcriptional activity by increasing YY-1 binding. These results establish the p18 −74 C/T mutation as the leading causal variant for the B1a cell expansion that characterizes the NZB and NZM2410 lupus-prone strains.
Keywords: p18INK4c, YY-1, Cell cycle, B-cells, lupus
Introduction
The mammalian mitotic cell cycle is divided into four distinct phases, G1, S, G2 and M (1). Progression through the cell cycle is regulated by the activation and subsequent inactivation of a family of serine/threonine kinases known as cyclin dependent kinases (CDKs) (2). In the G1 phase, positive and negative growth signals regulate CDK activity through numerous mechanisms, including the expression of CDK inhibitors (CDKIs). Association of a CDK with a positively regulating Cyclin D or E subunit leads to its activation, which is prevented by the binding of the CDKs by CDKIs (2). Based on binding properties, the CDKIs are divided into two families, the inhibitor of kinase 4 (Ink4: p16Ink4a, p15Ink4b, p18Ink4c and p19Ink4d) and CDK/kinase inhibitory proteins (p21Cip1, p27Kip1 and p57). p18Ink4c (p18) acts primarily on CDK4 and CDK6, and is involved in cell cycle arrest in a variety of cells, including B-lymphocytes (3). Increased p18 expression has been associated with specific cell stimulation and differentiation conditions, such as IL-6 stimulation and terminal differentiation of B cells into plasma cells (4). On the other hand, p18 expression is down-regulated by agents that stimulate cell proliferation such as genistein in breast cancer cells (5), and HTLV infection in T-cells (6). Relatively little is known about the regulation of p18 expression, most of it from studies in human cell lines. The p18 protein is very stable with a very low turnover (7), implying that p18 is mostly regulated at the transcriptional level. The human p18 promoter does not contain a TATA box, and is activated with the ubiquitous E2F1 and Sp-1 transcription factors, without any evidence of downstream regulatory elements (8).
The Cdkn2c gene, which encodes for p18, is located within Sle2c1, an NZM2410/NZB-derived systemic lupus erythematosus (SLE) susceptibility locus that we have associated with an expansion of peritoneal cavity B1a cells (9, 10). Previously, we have identified a novel −74 C/T single nucleotide polymorphism (SNP) in the NZB allele of the p18 promoter, which was associated with a significantly reduced p18 expression in the splenic B cells and peritoneal cavity B1a cells from Sle2c1-carrying mice. The presence of the T allele was also associated with a defective G1 cell cycle arrest in splenic B cells and an increased proliferation of peritoneal cavity B1a cells, leading to an expanded B1a cell population that has been associated with autoimmunity (10). Overall, these studies identified Cdkn2c as the lead candidate gene for Sle2c1, and suggested the −74 C/T as the causal polymorphism. Genetic linkage and association studies cannot distinguish a direct effect of this polymorphism on gene function from differences mediated by other neighboring polymorphisms that may be in linkage disequilibrium. To better understand the mechanism that modulates p18 expression, we performed studies to determine the functional significance of the −74 C/T exchange in the p18 promoter and the basis of its linkage with SLE. An in silico analysis has suggested that the −74 T mutation created a new Yin Yang 1 (YY-1) binding site adjacent to an existing one (10). YY-1 is a pleiotropic transcription factor that can both up- and down-regulate gene expression depending on the promoter context and the specific cellular environment (11, 12). It is a ubiquitously expressed 65-kD protein that binds to a consensus 5’-CCATNTT-3’ sequence (13). The mechanism by which YY-1 regulates gene expression is complex and most likely involves both co-activator and co-repressor complexes regulating histone acetylation (11, 12). YY-1-interacting proteins include basal transcription factors such as TBP (14) transcriptional coregulators such as p300/CREB-binding protein, poly(ADP-ribosyl) polymerase, and several transcription factors such as SP-1, c-Myc, and C/EBPβ (15). C to T promoter polymorphisms creating a novel YY-1 site have been identified in several human genes and have been associated with immune-related diseases. The −571 C/T in the IL-10 promoter and −509 C/T in the TGFβ promoter are associated with increased levels of both cytokines and with increased susceptibility to asthma (16, 17) and peridontitis (18). The −1993 C/T polymorphism in the TBX21 gene encoding for the TBET transcription factor has also been associated with an increased expression by the T allele (19), leading to an increased susceptibility to SLE (20), resistance to HBV infections (21) and asthma (22). The −1112 C/T mutation in the IL-13 promoter is associated with an increased transcription in individuals homozygous for the T allele, which also have a higher susceptibility to allergic inflammation (23). Finally, two other SNPs that increase YY-1 binding on the FCGRIIB promoter are associated with increased gene expression and susceptibility to SLE (24). Interestingly, mutations affecting YY-1 binding have not been reported in mice to our knowledge. In the present study, we show that the −74 region on the p18 promoter acts as an activator through the binding of YY-1 and transactivation by E2F1 and Sp-1. The −74 C to T base exchange is consistent with the creation of a second YY-1 site that relieves the activator and acts as a repressor of transcription. This is the first study to characterize the role of YY-1 in regulating the p18 promoter and to demonstrate a functional role for the −74 C to T SNP in decreasing p18 expression that is associated with the Sle2c1 lupus susceptibility locus.
Material and Methods
In silico p18 promoter analysis
A comprehensive library of hydroxyl radical cleavage profiles available at dna.bu.edu/orchid allows prediction of structural DNA profiles from sequence information in silico (25). Transcription factor binding sites on the p18 promoter were predicted using Chip MAPPER http://mapper.chip.org/ (26) and TFSEARCH (www.cbrc.jp/research/db/TFSEARCH.html).
Reagents
All chemicals, Schneider Drosophila medium, glutamine, trypsin, and antibiotics were purchased from Sigma. The luciferase reporter vector pGL-4 basic and the assay kit were purchased from Promega. RPMI, DMEM-high glucose and DMEM-F-12 culture media were purchased from Cellgro. FCS was obtained from Atlanta biotech; and cell culture plasticware was purchased from Corning.
Mice
The B6.Sle2c1 congenic strain is a C57BL/6 (B6) mouse that carries a ~ 6 Mb interval derived from the NZB genome on telomeric chromosome 4 that contains the Cdkn2c gene (10). B6 mice were obtained from the Jackson Laboratory. All experiments were conducted according to protocols approved by the University of Florida Institutional Animal Care and Use Committee.
Cell culture
The NIH3T3 (mouse fibroblasts), Raji (Human B-lymphocytes) and the Drosophila melanogaster Schneider-2 (SL2) cell lines were obtained from the American Type Culture Collection. NIH3T3 cells were grown in DMEM supplemented with 10% FCS, 2 mM glutamine, 100 units/ml penicillin and 50 µg/ml streptomycin. Raji cells were grown in RPMI supplemented with 10% v/v FCS, 100 units/ml penicillin and 50 µg/ml streptomycin. Cell density was kept between 0.20 and 1.25 × 106 cells/ml. SL2 cells were grown at 24°C without supplemental CO2 as a loosely attached monolayer in Schneider Drosophila medium supplemented with 10% FCS and antibiotics.
Plasmids
pCaags-YY1 and pCaags empty vector were provided by Prof. Yuki Yamaguchi (PRESTO, Japan), pPac-SP-1 and pPac empty vector were provided by Prof. Yasumasa Iwasaki (Kochi University, Japan), and pSG5L-E2F1 and pSG5L empty vector were provided by Dr. Shiwu Li (University of Florida). All plasmid sequences were confirmed by sequence analysis at the University of Florida Sequencing Core. Side by side transfections showed a clear p18 induction associated with the expression of each transcription factor above that of the empty vector control (data not shown).
Reporter constructs
The −279 p18 reporter construct was generated by cloning the promoter segment 279 nucleotide upstream from the putative translation initiation site of the mouse p18 gene into the luciferase reporter plasmid pGL-4-basic (10). −74 C- and T allele containing plasmids were cloned from the B6.Sle2c1 and B6 genomic DNA, respectively. Another −52 construct which does not include the −74 SNP was also constructed as a negative control. Promoter DNA was amplified using touchdown PCR with the primers shown in Table 1. Fragments were digested with XhoI and Bglll restriction enzymes and ligated into the pGL-4.10 basic vector digested with the same restriction enzymes. All constructs were confirmed by sequencing. Mutations in the p18 promoter were generated by site-directed mutagenesis (SDM) on both promoters carrying the C and T alleles with the primers shown in Table 1. The location and sequence of the mutations are depicted in Fig. 1A and 1B. PCR products were digested with DpnI to cleave the parental strand, and transformed into Super Competent cells (Invitrogen). The accuracy of the sequence of each mutated promoter was verified by sequencing before usage.
Table1.
List of oligonucleotides used in this study
| Oligonucleotides | Sequences |
|---|---|
| C allele F1,2 | 5’ – TCC TTA AAA CTC TGC CGT TAA AAT GGG GGC – 3’ |
| T allele F1,2 | 5’ – TCC TTA AAA CTC TGC TGT TAA AAT GGG GGC – 3’ |
| C mutant allele F1,2,3 | 5’ – TCC TTA AAA CTC TGC CGT TAA CGC AGG GGC –3’ |
| T mutant allele F1,2,3 | 5’ – TCC TTA AAA CTC TGC TGT TAA CGC AGG GGC – 3’ |
| YY-1 F1 | 5’ – CGC TCC CCG GCC ATC TTG GCG GCT GGT – 3’ |
| ChIP F | 5' - CTA ACT CGG CGG AGC CTC CTT AAA-3' |
| ChIP R | 5’ - TCT GAA GGT ACT GTG CCG GTT TCT-3' |
| −279 construct F4 | 5’- AGG GCTC GAG GAG ACT AGC GAA GCG AGA - 3’ |
| −279 construct R5 | 5’ – ACT GAG ATC TTG CCG GTT TCT TAT CCC T - 3’ |
| −52 construct F4 | 5' – TTC TCG AGT CAA CTC AAA AAG CGCT CAAT - 3’ |
| −52 construct R5 | 5' – GAC AAG ATC TCT TGC GCG TCT TTC CTT TA - 3’ |
| YY-1 SDM F | 5' – GAG CCT CCT TAA AAC TCT GCC GTT AAC GCA GGG GCG GGT TTT TCA - 3’ |
| YY-1 SDM R | 3' – CTC GGA GGA ATT TTG AGA CGG CAA TTG CGT CCC CGC CCA AAA AGT - 5’ |
| Sox3 F | 5' – ACGGCGAGCCTGTCAATCACGGGACCCGCA - 3’ |
| Sox3 R | 5' – TGCGGGTCCCGTGATTGACAGGCTCGCCGT -3’ |
Oligonucleotides used for double-stranded DNA EMSA probes. Only the sequences of the biotinylated forward oligotinucleotides are shown.
The underlined bolded nucleotides indicate the −74 C/T SNP.
The nucleotides mutated by SDM are indicated by a double-underline.
The Xho1 site is underlined
The BglII site is underlined
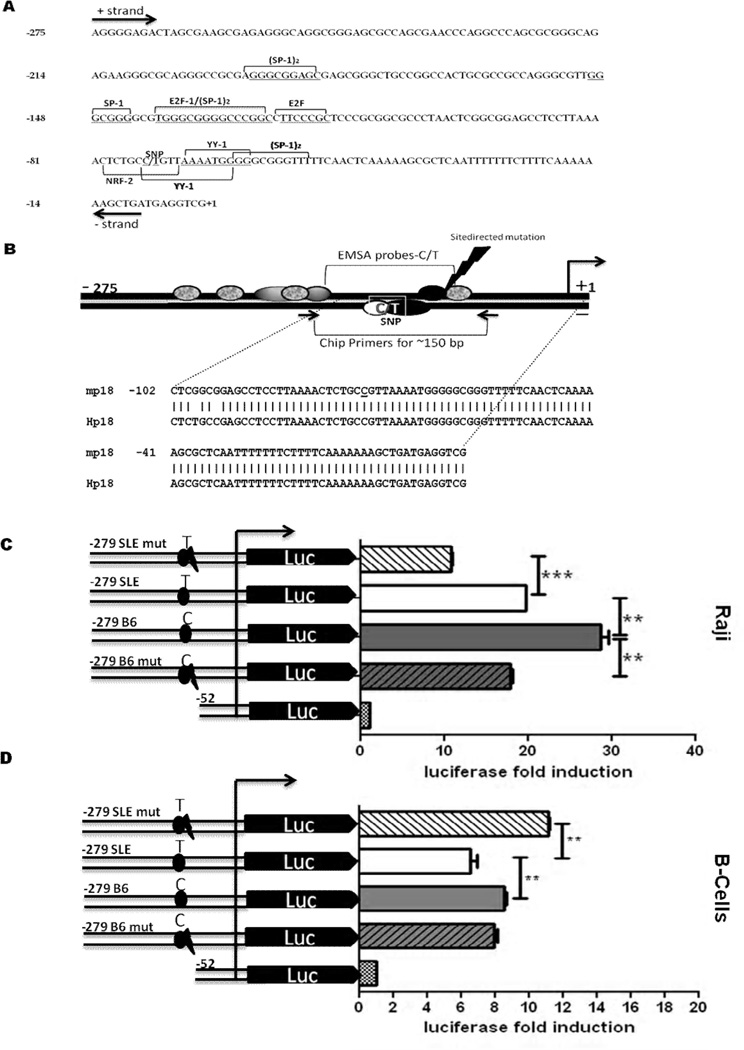
FIGURE 1.
The −74 C/T SNP is associated with a decreased activity of the p18 promoter. A, The 279 bp of the p18 promoter preceding the transcription initiation site are presented with the putative YY-1, E2F1,NRF-2 and SP-1 transcription factor-binding sites on the sense (+, above) or antisense (−, below) strand. B, Schematic representation of the p18 promoter. The arrow on the right indicates the position of the transcription initiation site, and the black ovals indicate the putative YY-1 binding sites, grey ovals indicate E2F1, granite ovals indicate SP-1 and the white oval indicates the position of the putative NRF-2 binding site on the antisense strand. The lightning bolt indicates where SDM deleted the common YY-1 binding site. The position and sequence (m for mouse and H for its human equivalent) of the oligonucleotide probe used in EMSA, as well as the position of the primers used in ChIP experiments are also depicted. C – D, Deletion and mutation analysis of the mouse p18 promoter using luciferase assays in Raji (C) and activated spB cells (D). Cells were transiently transfected with −279 deletion constructs carrying the SLE T or B6 C allele, with or without SDM (mut) of the common YY-1 binding site. The −52 construct was used as negative control. Results were expressed as luciferase fold induction by each construct over the expression induced by pGL4 basic. The graphs show means + SD of three independent experiments. **: p< 0.05; ***: p < 0.001.
Transient transfection, siRNA and luciferase assays
The activity of the p18 promoter was determined by measuring the expression of the luciferase reporter gene. Transient transfection assays in NIH3T3 cells were performed using Lipofectamine 2000 (Invitrogen) in serum free medium in 12-well plate at 80–90% cell confluence with 1.6 µg of reporter plasmids plus 160 ng of the Renilla luciferase plasmid pRL-TK (Promega) as an internal control for transfection efficiency. After 6 h of incubation of the cells with the Lipofectamine:DNA complexes, the medium was changed to DMEM supplemented with 10% FCS, and incubated for an additional 48 h at 37°C. Primary splenic B (spB) cells were isolated by negative selection from 8 – 11 week old B6 or B6.Sle2c1 mice using the Miltenyi B Cell Isolation kit. The purity of the preparation was typically about 95% CD19+ B cells. After 24 h stimulation with LPS (50 ug/ml), cells (3 × 106) were transfected with 2.5 µg of p18/Luc constructs along with 250 ng of pRL-TK Renilla by using the Mouse B Cell Nucleofector® Kit program Z-001 (Amaxa, LONZA). The transfection efficiency of spB cells with the pMax-GFP vector was > 90% of the non-adherent cells by fluorescent microscopy (data not shown). For in vitro siRNA transfection, 3 × 106 spB cells in 1ml media were incubated with 5 µM YY-1 siRNA (sc- 36864 Santa Cruz Biotech) in the Nucleofector transfection reagent at room temperature with gentle agitation for 30 min, with or without expression plasmids of YY-1. Transfected cells were incubated for 6–8 h before being subjected to the luciferase assays. Similarly, 2 × 106 Raji B cells were transiently transfected with 2.5 µg of p18/Luc constructs along with 250 ng of pRL-TK Renilla by using the Amaxa Nucleofector device (program V-01) with the Nucleofector Kit V. Transfected Raji cells were incubated for 18 h before being subjected to the luciferase assays. Transfection of SL2 cells was performed using 25 µl of Insectogene (Biontex) in serum-free medium with 2.5 µg of p18 promoter-containing plasmids and 250 ng of pRL-TK. After 4 h of incubation of the cells with the Insectogene : DNA complexes, the medium was changed to Schneider’s Drosophila medium supplemented with 10% FCS, and the cells were maintained at 24°C without supplemental CO2 for 48 h.
All transfected cells were collected by centrifugation and lysed in reporter lysis buffer (Promega). Cell extracts were prepared using the Dual Luciferase Assay System (Promega). Luciferase activity in cell extracts was measured using a Lumat LB 9507 luminometer (Berthold technologies). Firefly luciferase activity was normalized to Renilla luciferase activity. Results were expressed as luciferase fold induction by each construct over the expression induced by pGL4 basic. These experiments were repeated with three independent DNA preparations.
Quantitative RT-PCR and Western blot
cDNAs from transfected spB cells were prepared using the Promega reverse transcription kit. Quantitative RT-PCR was performed with TaqMan probes Mm00483243_m1 for Cdkn2c and Mm99999915_g1 for Gapdh (ABI) using the comparative Ct method normalized to control non-transfected spB cells. For Western blots, cells were lysed in lysis buffer (Promega) on ice for 60 min. After centrifugation, the supernatants were assayed for protein concentration (Bradford assay; Bio-Rad). Samples were run on 12 % SDS-PAGE gels. Proteins were electroblotted onto 0.45-µm pore size nitrocellulose filters, and the filters were blocked at room temperature with 5% nonfat milk in TBS and 1% Tween-20. Filters were then incubated overnight with rabbit polyclonal anti-YY-1 (sc-1703), or anti-IgG-HRP (Southern Biotech). The blots were washed three times with 0.1% Tween-20 in TBS and incubated with Donkey anti-rabbit HRP (Sigma). Blots were developed using an enhanced chemiluminescence detection system (ECL, Amersham).
EMSA
Nuclear extracts were prepared using a kit (Active Motif) according to the manufacturer’s instructions. Protein content was determined using the Bradford assay. All the forward strands of oligonucleotide probes (Table 1) were biotinylated (ID Technologies) for detection using a light-shift chemiluminescent EMSA kit (Pierce). After labeling, complementary strands were mixed together in an equimolar ratio and allowed to anneal for 1 h at 37 °C to form double-stranded probe. EMSAs were performed by incubating 0.5 ng of labeled probe with nuclear extract (5–10 µg) with or without competing unlabeled oligonucleotides at 10–100 fold excess in binding buffer (10 mm Tris-HCl, pH 7.5, 50 mm Nacl, 1 mM dithiothreitol, 1 mM EDTA, 5% glycerol, and 1 µg/µl poly(dI·dC) at room temperature for 30 min. Mixtures were size-fractionated on a non-denaturing 6% polyacrylamide gel followed by transfer to Zeta nitrocellulose membranes (Biorad) and detection by streptavidin-HRP/chemiluminescence for biotin-labeled probes. Supershift reactions were run as described above with the addition of 2 µg of rabbit polyclonal anti-YY-1 Ab (TRAPPC4), anti-E2F1 antibody (SC-193) or goat polyclonal anti-SP1 Ab, all from Santa Cruz Biotech. A Sox3 probe was used as an unrelated cold probe as control for binding specificity. Density values for each band were calculated relative to the band generated by the T-allele or C-allele oligonucleotide without competitor. Extracts from spB cells isolated from three B6 and three B6.Sle2c1 mice by negative selection (Myltenyi) were analyzed and each EMSA was performed at least three times.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed using the Millipore kit according to the manufacturer’s protocol with minor modifications. Briefly, formaldehyde was added to the culture medium to a final concentration of 1%. Cells were incubated for 10 min at 37°C and washed twice with ice-cold PBS containing a protease inhibitor cocktail (Sigma). After centrifugation, the pellets were resuspended in sonication buffer (1% SDS; 10 mM EDTA; 50 mM Tris-HCl, pH 8.1; protease inhibitor cocktail) and sonicated on ice to an average length of 500 to 1,000 bp with a Sonic Dismembrator (Fisher Scientific), then centrifuged at 12,000 rpm. The chromatin solution was diluted 10-fold with dilution buffer (0.01% SDS; 1.1% Triton X-100; 1.2 mM EDTA; 16.7 mM Tris-HCl, pH 8.1; 167 mM NaCl); one-third of which was reserved as a total input of chromatin. The diluted chromatin solution was precleared with salmon sperm DNA–protein A-agarose for 1 h and incubated with either anti-Nrf2 (C-20), anti-YY-1 Ab (sc-1703), non-specific rabbit IgG, or no Ab, for 18 h at 4°C with rotation. Immunoprecipitation, washing, and elution were carried out according to the manufacturer’s instructions. Cross-linked immunoprecipitates and total chromatin inputs were reversed, and samples were treated with proteinase K (Sigma) then extracted with phenol-chloroform-isoamyl alcohol (25:24:1). DNA was precipitated with ethanol and resuspended in water. Q-PCR was performed with the primers shown in Table 1 using 5 µl of template, with the Syber Green Real-Time PCR Detection System (Bio-Rad Laboratories) and analyzed using the CFX Manager Software v1.0 (Bio-Rad Laboratories).
Statistics
Statistical analyses were performed using the GraphPad Prism 5 Software. Two-tailed unpaired t tests were used, with multiple-test corrections as appropriate. Figures show means and SD, as well as statistical significance when p ≤ 0.05.
Results
Decreased transcriptional activity of the p18 promoter with the −74 T allele
The sequence of the p18 promoter from −1209 to +24 was analyzed with Mapper and TFSEARCH to find putative transcriptional elements. This analysis predicted that the −74 C/T transition results in the loss of binding sites for the NRF2 and Hunchback transcription factors, whereas it creates a binding site for YY-1 that is adjacent to the already existing YY-1 site (Fig. 1A and B). The first 150 bp of the promoter have a very high G + C content (Fig. 1A), which is a hallmark of several TATA-less promoters (27). Several putative binding sites for E2F1 and SP-1, which transactivate the human p18 promoter (8), were identified in the mouse proximal promoter (Fig. 1A). BLAST alignment revealed that the first 100 nucleotide of the mouse and human p18 promoter share 98% identity (Fig. 1B and data not shown). This high degree of conservation suggests that these elements are likely to play an important role in the regulation of p18 expression.
Using deletion constructs, we have determined that the −279 to −52 p18 region contains all elements that are necessary for basal transcription in mouse NIH3T3 and human Raji cells, and that the promoter construct carrying the −74 T variant was associated with a lower transcriptional activity (10). We extended these experiments to determine the functional significance of the −74 C → T transition in the p18 promoter. We confirmed that the transcriptional activity of the −279/+24 region with the C allele was significantly higher than that with the T allele in Raji human B cells (Fig. 1C), and mouse NIH3T3 fibroblasts (supplemental Fig. 1A). To validate the physiological relevance of these findings, we repeated the above experiments in primary spB cells and found the same result showing a higher transcriptional activity of the C allele than the T allele (Fig.1D).
To further confirm the role of YY-1 in driving the transcriptional activity of the p18 promoter, point mutants were created by SDM to eliminate the YY-1 binding site common to both alleles. The resulting mutant constructs carried either 0 (mutant C allele) or 1 (mutant T allele) YY-1 binding sites in the −74 region. The mutated T allele resulted in an increased promoter activity as compared to the unmutated construct in spB cells (Fig. 1D). We observed similar results in NIH3T3 cells (supplemental Fig. 1A and B). This validated the hypothesis that the presence of two YY-1 binding sites associated with the T allele results in transcription repression. In Raji cells however, the loss of the common YY-1 binding site resulted in a decrease of the promoter activity as compared to the respective unmutated constructs (Fig. 1C). Interestingly, the difference between the C and T alleles was maintained between the mutant constructs, suggesting that the remaining YY-1 binding site in the mutated T allele is functional. Overall, these data indicated that the −279 to −52 bp in the p18 promoter plays a vital regulatory role in the basal transcriptional activity of this gene, and that within this region, the −74 C/T variant may result in functional transcriptional consequences.
YY-1 is a critical transcription factor regulating the function of the C and T alleles
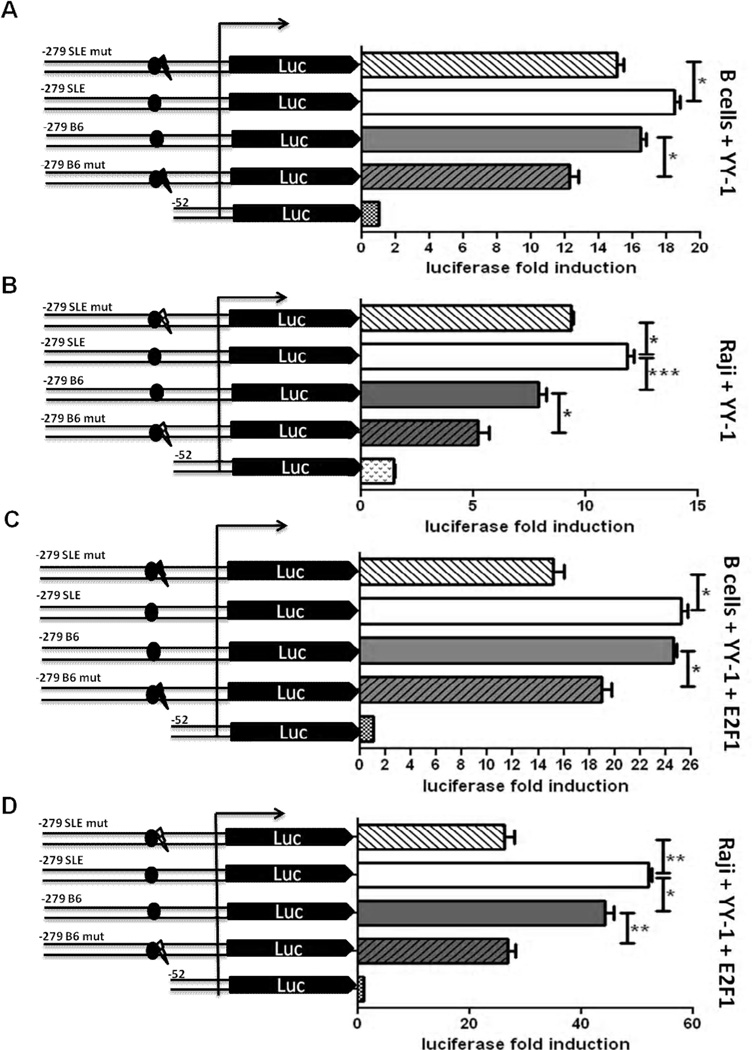
To determine whether the additional putative YY-1 site created by the −74 C → T transition has a positive or negative role in driving the transcriptional activity of p18 promoter, we co-transfected the −279 -C and T constructs with a YY-1 expression plasmid in spB, Raji and NIH3T3 cells. In all three cell types, YY-1 over-expression reversed (spB and Raji cells, Fig. 2A and B) or nullified (NIH3T3 cells, supplemental Fig. 1C) the differential transcriptional activity between the T and C alleles. Moreover, when YY-1 overexpression was transactivated with E2F1, the C and T alleles showed a similar transcriptional activity in spB cells (Fig. 2C), and the T allele showed a higher transactivation than the C allele in Raji cells (Fig. 2D). This suggested that the additional YY-1 binding site in the T allele increases binding for YY-1, and thus positively regulates the p18 promoter activity in conditions in which YY-1 and E2F1 are both overexpressed, as opposed to basal conditions with limiting amount of YY-1 in which the T allele is associated with a lower transcriptional activity. Furthermore, YY-1 knock-down by siRNA transfection of either B6 or B6.Sle2c1 spB cells lowered p18 transcriptional activity to equivalent levels of all four constructs that differed by their number of YY-1 binding sites (Fig. 3A and B and data not shown).
FIGURE 2.
YY-1 overexpression affects p18 transcription. Luciferase assays in activated spB (A) or Raji (B) cells transfected with mutated or intact p18 promoter constructs and a YY-1 expression plasmid. Transactivation of p18 activity by co-expression of exogenous YY-1 and E2F1 in spB (C) or Raji (D) cells. The results show means + SD of three independent experiments. *: p < 0.05; **: p < 0.01, ***: p < 0.001.
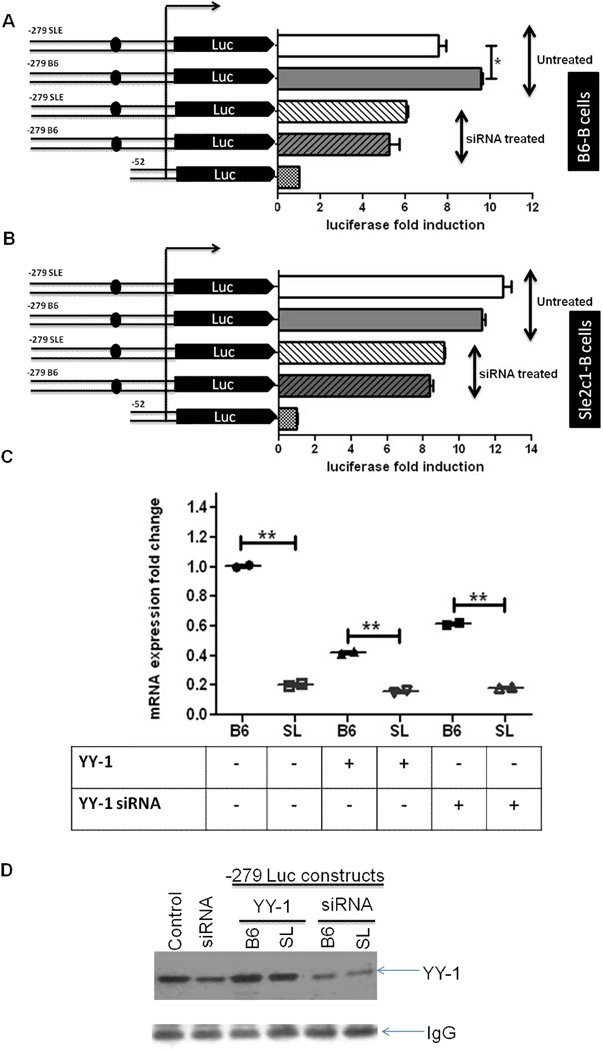
FIGURE 3.
p18 expression is affected by YY-1 level and number of binding sites. Luciferase activity in activated B6 (A) and B6.Sle2c1 (B) spB cells transfected with p18 promoter–luciferase constructs in the presence or absence of siRNA-YY-1. C. Endogenous p18 gene expression in B6 and B6.Sle2c1 (SL) spB cells transfected with the B6 p18 promoter–luciferase construct alone, in the presence of YY-1 or with siRNA-YY-1. The results were normalized to the expression of B6 cell transfected with the p18 promoter construct alone. **: p < 0.01. D. Western blot analysis of YY-1 expression B6 and B6.Sle2c1 (SL) spB cells transfected with or without p18 promoter–luciferase construct, and/or YY-1 expression plasmid or siRNA-YY-1. IgG levels are shown as controls.
We also tested the effect of YY-1 levels on the transcription of endogenous p18 in B6 or B6.Sle2c1 spB cells transfected with the luciferase constructs by Q-RT PCR (Fig. 3C). Overexpression of YY-1 decreased p18 expression significantly in the B6 allele while maintaining the significant difference between the C allele than T allele. YY-1 knock-down also decreased p18 expression in the B6 allele while maintaining the difference between the two alleles. This level of p18 expression with YY-1 siRNA was however statistically higher (p <0.0013) than with YY-1 overexpression conditions in both alleles. The fact that endogenous p18 expression in the Sle2c1 allele was largely not affected by either overexpression or knock-down of YY-1 may be related to the fact that endogenous level of YY-1 protein was about 1.5 fold higher in the Sle2c1 than B6 B cells. Moreover, although the siRNA knock-down was successful, a substantial residual YY-1 expression remained (Fig. 3D). Attempts to increase the amount of transfected siRNA above 5 uM resulted in substantiall cytotoxicity. One might interpret these results as output efficiency of the p18 promoter being regulated by the balance between the number of YY-1 binding sites and YY-1 availability. YY-1 knockdown eliminates the difference between YY-1 binding site numbers on the high copy number luciferase constructs, and lowers p18 expression in the B6 endogenous allele. The Sle2c1 enodogenous allele however is largely unaffected either due to higher intrinsic levels of YY-1 or to the existence of two YY-1 binding sites that allow for a better competition for its ligand. Steady states levels of YY-1 results in lower transcription from the T allele in both the endogenous promoters and the luciferase constructs. Finally, YY-1 over expression decreases the expression by the endogenous promoters while maintaining allelic differences, but increased the expression of the T allele on the high copy number luciferase constructs. Overall, this data demonstrates that p18 expression is greatly affected by YY-1 levels and binding capacity.
YY-1 binds to p18 promoter in vitro and in vivo
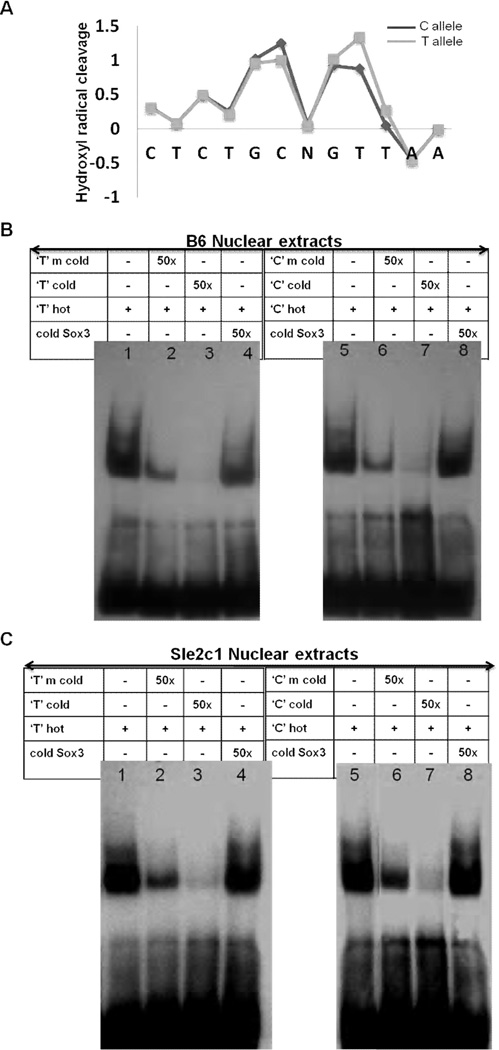
The Mapper and TFSEARCH programs predicted that YY-1 binds to putative sites in the p18 promoter, which is in agreement with our reporter construct experiments. Besides the primary sequence, a feature that can influence transcription factor binding specificity is the DNA backbone conformation (28). In fact, topography-informed evolutionarily constrained DNA regions correlate better with functional non-coding elements than those predicted by primary sequence-constraint algorithms (29). The solvent accessible surface area of the DNA backbone is well-reflected by its hydroxyl radical cleavage pattern (30). Strong differences in the predicted hydroxyl radical cleavage pattern were found between the C and T alleles in the region surrounding the −74C/T SNP (Fig. 4A), suggesting global differences in protein binding affinity. The lower score of the T-allele distal to the mutation reflects narrowing of the DNA backbone which can act as an electrostatic groove to enhance protein-DNA interaction (28).
FIGURE 4.
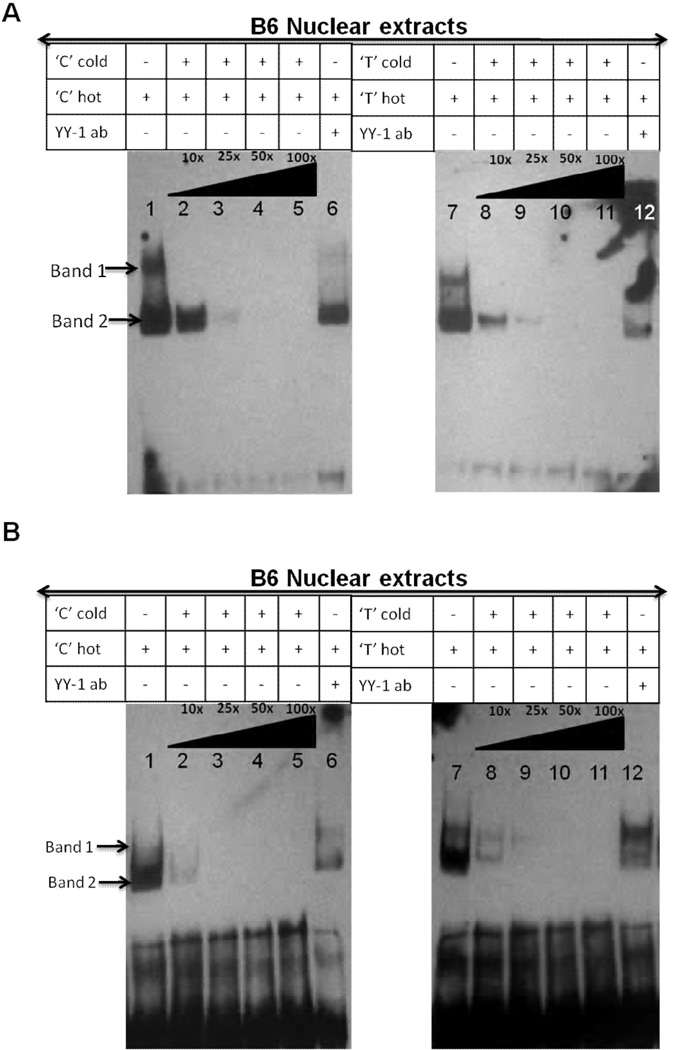
Analysis of protein binding on the 18 promoter. A, Allelic difference in DNA surface structure in the 12 nucleotide region surrounding the −74C/T SNP (represented by N) by using predicted hydroxyl radical cleavage patterns. Each nucleotide is shown on the X-axis. Grey line = T allele, black line = C allele. Representative EMSAs performed with B6 (B) and B6.Sle2c1 (C) spB cell nuclear extracts showing that the elimination of the common YY-1 binding site by SDM (m cold probes, lanes 2 and 6) results in lower DNA-protein binding for both the C and T alleles. The content of each lane indicated in the table is common to both types of nuclear extracts. A cold probe from the Sox3 promoter was used as a control for binding specificity.
This in silico prediction of altered protein-DNA interaction in the −74C/T region was validated by EMSAs using nuclear extracts prepared from either B6 or B6.Sle2c1 spB cells. With both extracts, incubation of C and T allele probes spanning from −89 to −60 (containing both YY-1 sites and the NRF-2 site) gave rise to the formation of DNA-protein complexes (Fig. 4B and C lanes 1 and 5). We showed the specificity of the binding by competing with unrelated cold Sox3 probe, which could not compete at all (Fig. 4B and C, lanes 4 and 8). Mutated C or T probes in which the common YY-1 site has been removed could not compete as efficiently as the unmutated C or T probes (Fig. 4B and C, lanes 2–3 and 6–7). These results further showed the functionality of the common YY-1 binding site.
The DNA-protein complexes could be competed off by the addition of graded excess amount of unlabelled C or T probes (Fig. 5, lanes 1–5 and 8–11). Similar results were obtained with YY-1 consensus commercial probes (data not shown). With B6 nuclear extracts, the faster migrating specific complex (band 2) was more intense than band 1 and appeared to comprise multiple complexes with similar electromobilities. Addition of an YY-1 Ab to the binding reaction caused the disappearance of the specific DNA/protein complex band 2 (Fig. 5, lanes 6 and 12, and Supplementary Fig. 2, lanes 2 and 7). Densitometric analysis of band 2 revealed that the T-allele probe showed an about 50% weaker protein binding than the C probe and was competed off more efficiently than the C-allele probe (data not shown). With the B6.Sle2c1 nuclear extracts, the C probe did not show a distinct band 1 (Fig. 5B lane 1) and was competed off more efficiently than the T probe, with which two bands were clearly visible (Fig. 5B lane 7). Densitometry analysis showed weaker protein binding for the C probe (data not shown), suggesting that the T-variant increases protein binding affinity in B6.Sle2c1 extracts. These results indicate that the YY-1 binding sites within the p18 proximal promoter region are capable of binding with transcription factor YY-1 in vitro, and this binding differs between the C and T alleles. The differences between the Sle2c1 and B6 B cell nuclear extracts may reflect the higher amount of YY-1 in Sle2c1 B cells, and/or feedback regulatory mechanisms resulting from the differential expression of p18 in these cells. The multiple DNA binding complexes also suggested the presence of other transcriptional active proteins such as E2F1 and SP1 in addition to YY-1. E2F1 and SP1 Abs partially inhibited both complexes formed with either the C or T probes, indicating that E2F1 and SP1 are included in the complex of proteins that binds to this sequence (Supplementary Fig. 2, lanes 3–4 and 9–10).
FIGURE 5.
YY-1 binds to the p18 promoter in a C/T allele dependent fashion. Representative EMSAs performed with B6 (A) or B6.Sle2c1 (B) spB cell nuclear extracts showing DNA-protein complexes (bands 1 and 2) captured more strongly by C than the T allele biotin-labeled oligonucleotide probes. Specificity of this interaction was demonstrated by adding increasing amounts of unlabeled “cold” C or T-allele oligonucleotides as competitors or anti-YY-1 Ab. The arrow on the right of lane 12 indicates the supershift created by the anti YY-1 Ab. The content of each lane indicated in the table is common to both types of nuclear extracts.
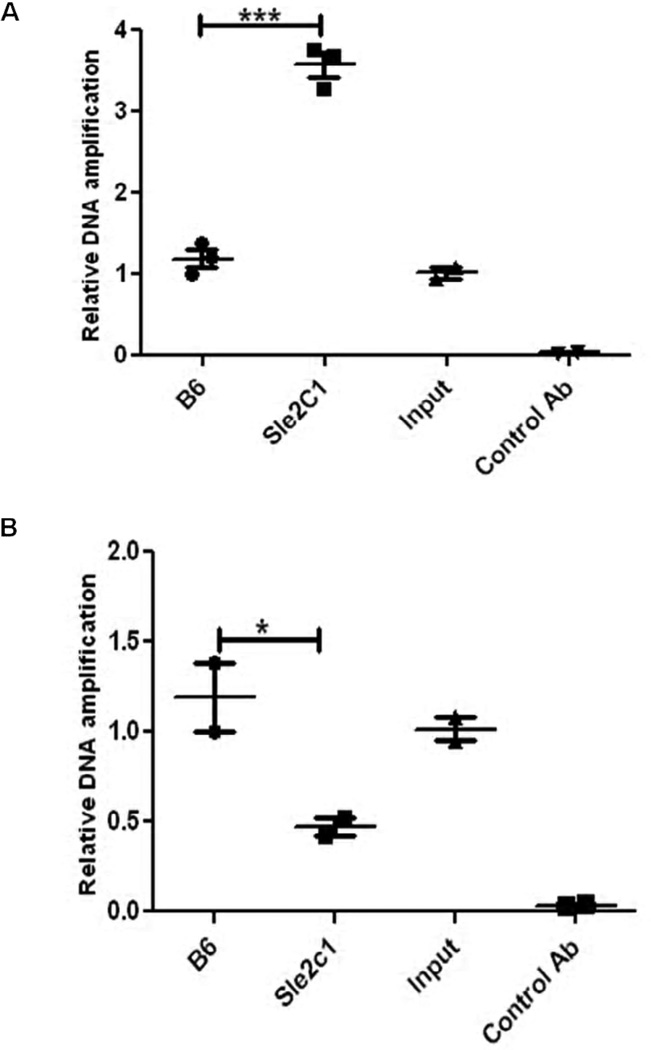
To further determine whether YY-1 binds to the mouse p18 promoter in vivo in an allele-specific fashion, we performed ChiP analysis in spB cells from B6 and B6.Sle2c1 mice, in which we found a differential p18 gene expression (10). A 150 bp DNA fragment was strongly amplified from the B6.Sle2c1 B cell extracts precipitated by the YY-1 Ab, but not from control B6 B cells or from negative control immunoprecipitations using either no Ab or rabbit IgG. The identity of the amplified fragment was confirmed by sequencing. The possibility of non-specific binding of the YY-1 antibody was ruled out by the lack of PCR amplification of the −52 to +123 region of the p18 gene in all the precipitated chromatins (data not shown). Quantitative ChIP PCR showed a significantly stronger YY-1 binding in B6.Sle2c1 than B6 B cells (Fig. 6A). Conversely, the amount of p18 promoter bound to NRF-2 was two folds lower with the Sle2c1 allele than the B6 allele (Fig. 6B). These results indicate that YY-1 specifically binds to the p18 promoter in vivo, and support the prediction that the T mutation creates a second YY-1 binding site to the expense of the NRF-2 binding site.
FIGURE 6.
The p18 promoter binds significantly more YY-1 and less NRF-2 in B6.Sle2c1 than in B6 B cells in vivo. ChIP analyses performed with chromatin extracted from B6 and Sle2c1 spB cells and immunoprecipitated with anti-YY-1 (A) or anti-NRF-2 (B) Ab. Input B6 chromatin without immunoprecipitation or with control Ab are shown as negative controls. The results show the mean + SD of 2–3 replicates per strain. *: p < 0.05; ***: p < 0.001.
YY-1 regulates the transcriptional activity of the p18 promoter in Drosophila SL2 cells in a −74C/T mutation dependent fashion
To directly test the role of YY-1 and Sp family members in mediating the transcriptional activity through the −74 polymorphic residue, we performed p18 promoter transient transfection assays in the SL-2 cell line, which is deficient of YY and Sp transcription factor family members (31). YY-1 expression in SL2 cells induced a significantly higher transcriptional activity in the 279/+24 p18 promoter T allele with two putative YY-1 sites than in the C allele (Fig. 7A). We also observed an increase in promoter activity associated with SP-1 activity. Both alleles of the−1209/+24 constructs, which contain 13 putative SP-1 sites, showed a higher promoter activity in the presence of an SP-1 expression plasmid than the 279/+24 constructs, which contains 7 putative SP-1 sites (Supplemental Fig. 1D). As with spB and Raji cells (Fig. 1), the mutation of the common YY-1 binding site significantly decreased transcription from both alleles, but maintained the difference between alleles (Fig. 7A). This resulted in a YY-1 binding site dose-dependent transcriptional activity, with the highest activity produced by the Sle2c1 promoter (2 binding sites), and the lowest produced by the mutated B6 promoter (0 binding sites), and equivalent activities between the B6 and mutated Sle2c1 promoters (1 binding site each). E2F1 over-expression resulted in 10 fold transactivation with similar significant differences between the T and C alleles, although a lesser effect of the mutations (Fig. 7B). Moreover, YY-1 expression maintained a higher transcriptional activity for the T allele in SL2 cells co-transfected with SP-1 (Fig. 7C), or the combination of SP-1 and E2F1 expression vectors (Fig. 6D). In all these co-transfection experiments, the mutations of the shared YY-1 binding site was associated with a significant decrease in transcriptional activity, which occurred to a greater extent in the C allele in which no YY-1 site was left (Fig. 7C and D). These experiments clearly showed that YY-1 binding to the p18 promoter plays a major role in its transcriptional activity, and that the −74C to T transition affects significantly this activity in a manner consistent with the in silico prediction of the creation of an additional YY-1 binding site.
FIGURE 7.
p18 transcriptional activity is affected by the −74 C/T SNP in SL2 insect cells. The activity of p18 promoter constructs was tested in SL2 cells co-transfected with expression vectors for YY-1 singly (A) or in combination with E2F1 (B) and/or SP-1 (C and D). Data are presented as fold activation relative to the expression of the p18-luciferase constructs in the absence of any mammalian transcription factors. The results show the means + SD of three separate transfections. *: p < 0.05; **: p < 0.01.
Discussion
B1a cell expansion is the major phenotype of the Sle2c1 lupus susceptibility locus (9). We have previously showed that this phenotype segregated with a markedly reduced (~80%) expression of the Cdkn2c gene encoding for p18, as well as with an increased spontaneous proliferation of the B1a cells, leading to their age-dependent accumulation (10). We have identified a novel −74 C/T polymorphism in the p18 promoter, with the T allele derived from the lupus prone strains NZM2410 and NZB associated with a reduced Cdkn2c expression (10). We have sequenced the entire p18 promoter and this polymorphism was the only difference between the B6 and Sle2c1 allele (10). There is no evidence that p18 transcription is regulated by downstream elements (8) and the p18 protein is very stable with a very low turnover (7). These results combined with our previously published luciferase reporter assays (10) suggested that the −74 C/T mutation was responsible for the difference in p18 expression between the B6 and B6.Sle2c1 B cells. The present study was conducted to functionally characterize the region surrounding the −74C/T SNP relative to the p18 promoter activity, to test the in silico prediction that the mutation affects YY-1 binding and to identify if there is, besides YY-1, any other transcription factor binding to this region, regulating the transcriptional activity of the p18 promoter in vitro and in vivo. We first showed that the luciferase activity of truncated reporter constructs was significantly lower for the T allele than for the C allele in mouse spB cells, human Raji-B cells and mouse NIH3T3 cells. This indicated that −74C/T variant regulates the p18 promoter activity in a non-cell- or non-species-specific pattern that involved ubiquitous transcriptional factors. The limiting effect of YY-1 binding on the p18 promoter function was demonstrated by overexpression of YY-1 that eliminated the difference between the C and T alleles in spB, Raji, SL-2 and NIH3T3 cells, most likely by compensating for the varying number of binding sites by unrestrained availability of the transcription factor. YY-1 overexpression resulted in a greater promoter activity with the T allele in three (spB, Raji, SL-2) out of the four cell types examined in this study, indicating that YY-1 availability was rate limiting for the presence of two YY-1 binding sites. The fact that YY-1 overexpression eliminated but did not reverse the difference between the C and T alleles in NIH3T3 cells is likely due to a higher endogenous YY-1 expression level, which corresponds to a relatively smaller difference between alleles in the absence of YY-1 expression plasmid in NIH3T3 as compared to the other cells types. Elimination of the YY-1 binding site common to both alleles by SDM clearly showed a transcriptional activity of the p18 promoter was significantly affected in both alleles. Moreover, siRNA knock-down experiments showed that a low YY-1 expression was also associated with equivalent transcriptional activity of all four alleles expression 0 to 4 YY-1 binding sites. In insect cells in which YY-1 activity can be tested without transactivation by endogenous co-factors such as SP-1 and E2F1, exogenous YY-1 activated the p18 promoter proportionally to the number of predicted YY-1 binding sites. Overall, these results demonstrated that YY-1 by itself regulates the p18 promoter activity and that the −74T mutation created a novel YY-1 binding site. Unfortunately, these in vitro results cannot be readily validated in vivo, as both B cell-specific YY-1 knock-out (32) and overexpression of YY-1 in hematopoietic stem cells (33) eliminate mature B cells.
YY-1 is a ubiquitous factor that mediates both transcriptional activation and repression depending both on the sequence and cellular contexts (13). The comparison of sequences surrounding the YY-1 binding site between promoters that are repressed and promoters that are activated by YY-1 has determined consensus repressor and activator sequences (13). Accordingly, the −74 T polymorphism creates a YY-1 repressor binding site, which fits with the results that we have obtained with spB and Raji cells. The variable effects of YY-1 on the transcription of a same gene in different cell types have been clearly demonstrated for the human IL-13 gene. The YY-1 binding site created by a C to T mutation increased IL-13 expression in TH2 T cells due to STAT1 and STAT6 transactivation, had no effect in non-polarized CD4+ T cells due to the binding of NFAT-2, and repressed IL-13 transcription in Jurkat T cells, due to the absence of STAT-6 and the binding of OCT-1 (23). These complex gene-environment interactions can explain some of our results which showed different effects of the −74 C/T SNP in spB and Raji B cells, NIH3T3 fibroblasts, and SL2 insect cells. The results obtained in the spB and Raji B cells are probably the most informative for p18 transcriptional regulation in B cells, and the repressive effect of the −74 T mutation in these cells corresponds indeed to what we have observed in mouse B cells (10).
In vitro and in vitro assays confirmed that YY-1 binds to the −74 region of the p18 promoter. EMSA with B cell nuclear extracts produced multimeric complexes, which most likely reflects multimer formation of YY-1 with other proteins like E2F2 and SP1. Support for the concept that the interactions of YY1 with gene promoters involves ternary complexes comes from studies on the IL-13 promoter where YY-1 forms a complex with STAT-1 and a variable number of other factors (23), and on the IFN-γ promoter that involves interaction between YY-1 and NFAT (34). In the current study, a fast migrating complex (band 2) is an integral part of the YY-1/p18 promoter complexes, based on competitions with excess unlabeled probes, YY-1 consensus probes, C or T mutant probes, and supershift assays. Band 2 was strongly bound by the −74 C probe as compared to the −74 T probe with the B6 extracts, and the reverse was observed with Sle2c1 extracts. The fact that opposite results was obtained between the two strains may be a direct consequence of their different levels of p18 expression. This may lead to different physiological states through a dysregulated cell cycle, and different milieus of transcription factors that transactivate the p18 promoter on the YY-1 binding site in these cells, in a similar fashion of what has been described for the IL-13 promoter between non-polarized and TH2 CD4+ T cells (23). This hypothesis needs to be formally tested, by comparing the respective amounts of SP-1, NRF-2 and potentially Hunchback available in B cells between the two strains. Additional and yet unidentified binding factors that may also play a role in p18 transcription may be expressed at different levels depending on p18 expression.
Finally, in vivo binding of YY-1 to the p18 promoter was detected by ChIP assay, with significantly more binding to the T allele in B6.Sle2c1 spB cells than to the C allele in spB B6 cells. ChIP assays also verified the prediction that the −74C/T SNP caused the loss of NRF-2 binding site in T allele. NRF-2 is an antioxidant-activated transcription factor, and interestingly, the Nrf2 targeted deletion has been associated with a multiorgan autoimmune inflammatory syndrome which includes lupus-like manifestations (35–37). Moreover, a polymorphism that affects Nrf-2 expression level has been associated with lupus (38). Nrf-2 deficiency affects the expression of a large number of genes besides the antioxidant pathway (36), but it has not been reported to regulate p18 expression. It is intriguing that the loss of an Nrf-2 binding site is also associated with lupus susceptibility in the Sle2c1 locus, and suggesting that the cell cycle regulation in B cells and the size of the B1a cell compartment should be examined in Nrf2-deficient mice.
YY-1 has been implicated in the onset of the S phase (39) and in the regulation of p16INK4a expression (40). Our results demonstrate that YY-1 plays a major role in regulating p18INK4c expression, and that alterations in the number of its binding site on the p18 promoter are associated with substantial consequences on B cell homeostasis. Furthermore, our results suggest that the dominant binding of YY-1 created by the −74T element in combination with SP-1 plays a stronger negative regulatory role on p18 promoter activity than the binding of NRF-2 in combination with SP-1 that occurs with the C allele. Although the −74 region on the p18 promoter is identical between mice and humans, the −74 C/T SNP has not been reported in humans. This does not mean that it does not exist, since the mouse mutation in the NZM2410/NZB genome that we have found was also novel. It probably indicates, however, that it may exist at a much lower frequency than in the IL-10, IL-13, TGF-B and TBX21 genes, in which it has been found by multiple groups. We believe that our findings have provided not only significant insights on the regulation of the p18 promoter activity, but also have identified a causative variant responsible for the decreased p18 expression in the Sle2c1 lupus susceptibility locus. Analysis of the ever increasing amount of genomic data should reveal if the mouse SNP exists in the human p18 promoter, and allow to test whether it is associated with the size of the newly identified human B1 cell compartment (41), which is expanded in lupus patients (42) as in many mouse models of lupus (43).
Supplementary Material
Acknowledgments
We thank Prof. Yuki Yamaguchi (PRESTO, Japan), Prof. Yasumasa Iwasaki (Kochi University, Japan), and Dr. Shiwu Li (University of Florida) for their generous gifts of expression plasmids.
This work was supported by grant RO1 AI068965 from the National Institutes of Health (LM).
Abbreviations used in this article
- CDK
cyclin dependent kinase
- CDKI
cyclin dependent kinase inhibitor
- p18
p18Ink4c
- SLE
systemic lupus erythematosus
- SNP
single nucleotide polymorphism
- YY-1
Ying-Yang 1
- B6
C57BL/6J
- SL2
Schneider line 2
- SDM
site-directed mutagenesis
- ChIP
chromatin immunoprecipitation
References
- 1.Sherr CJ. The ins and outs of RB: coupling gene expression to the cell cycle clock. Trends Cell. Biol. 1994;4:15–18. doi: 10.1016/0962-8924(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 3.Schrantz N, Beney GE, Auffredou MT, Bourgeade MF, Leca G, Vazquez A. The expression of p18INK4 and p27kip1 cyclin-dependent kinase inhibitors is regulated differently during human B cell differentiation. J. Immunol. 2000;165:4346–4352. doi: 10.4049/jimmunol.165.8.4346. [DOI] [PubMed] [Google Scholar]
- 4.Morse L, Chen D, Franklin D, Xiong Y, Chen-Kiang S. Induction of cell cycle arrest and B cell terminal differentiation by CDK inhibitor p18 INK4c and IL-6. Immunity. 1997;6:47–56. doi: 10.1016/s1074-7613(00)80241-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhou JN, Linder S. Expression of CDK inhibitor genes in immortalized and carcinoma derived breast cell lines. Anticancer Res. 1996;16:1931–1935. [PubMed] [Google Scholar]
- 6.Suzuki T, Narita T, Uchida-Toita M, Yoshida M. Down-regulation of the INK4 family of cyclin-dependent kinase inhibitors by tax protein of HTLV-1 through two distinct mechanisms. Virol. 1999;259:384–391. doi: 10.1006/viro.1999.9760. [DOI] [PubMed] [Google Scholar]
- 7.Forget A, Ayrault O, den Besten W, Kuo ML, Sherr CJ, Roussel MF. Differential post-transcriptional regulation of two Ink4 proteins, p18 Ink4c and p19 Ink4d. Cell Cycle. 2008;7:3737–3746. doi: 10.4161/cc.7.23.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blais A, Monté D, Pouliot F, Labrie C. Regulation of the human cyclin-dependent kinase inhibitor p18INK4c by the transcription factors E2F1 and Sp1. J. Biol. Chem. 2002;277:31679–31693. doi: 10.1074/jbc.M204554200. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Duan B, Croker BP, Wakeland EK, Morel L. Genetic dissection of the murine lupus susceptibility locus Sle2 : contributions to increased peritoneal B-1a cells and lupus nephritis map to different loci. J. Immunol. 2005;175:936–943. doi: 10.4049/jimmunol.175.2.936. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Potula HH, Vallurupalli A, Perry D, Baker H, Croker BP, Dozmorov I, Morel L. Cyclin-dependent kinase inhibitor Cdkn2c regulates B cell homeostasis and function in the NZM2410-derived murine lupus susceptibility locus Sle2c1. J. Immunol. 2011;186:6673–6682. doi: 10.4049/jimmunol.1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. Biochim. Biophys. Acta. 1997;1332:F49–F66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 12.Thomas MJ, Seto E. Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key? Gene. 1999;236:197–208. doi: 10.1016/s0378-1119(99)00261-9. [DOI] [PubMed] [Google Scholar]
- 13.Shrivastava A, Calame K. An analysis of genes regulated by the multi-functional transcriptional regulator Yin Yang-1. Nucleic Acids Res. 1994;22:5151–5155. doi: 10.1093/nar/22.24.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austen M, Lüscher B, Lüscher-Firzlaff JM. Characterization of the transcriptional regulator YY1. The bipartite transactivation domain is independent of interaction with the TATA box-binding protein, transcription factor IIB, TAFII55, or cAMP-responsive element-binding protein (CPB)-binding protein. J. Biol. Chem. 1997;272:1709–1717. doi: 10.1074/jbc.272.3.1709. [DOI] [PubMed] [Google Scholar]
- 15.Seto E, Lewis B, Shenk T. Interaction between transcription factors Sp1 and YY1. Nature. 1993;365:462–464. doi: 10.1038/365462a0. [DOI] [PubMed] [Google Scholar]
- 16.Hobbs K, Negri J, Klinnert M, Rosenwasser LJ, Borish L. Interleukin-10 and transforming growth factor-beta promoter polymorphisms in allergies and asthma. Am. J. Respir. Crit. Care Med. 1998;158:1958–1962. doi: 10.1164/ajrccm.158.6.9804011. [DOI] [PubMed] [Google Scholar]
- 17.Pulleyn LJ, Newton R, Adcock IM, Barnes PJ. TGFbeta1 allele association with asthma severity. Hum. Genet. 2001;109:623–627. doi: 10.1007/s00439-001-0617-y. [DOI] [PubMed] [Google Scholar]
- 18.de Souza AP, Trevilatto PC, Scarel-Caminaga RM, de Brito RB, Line SR. Analysis of the TGF-beta1 promoter polymorphism (C-509T) in patients with chronic periodontitis. J. Clin. Periodontol. 2003;30:519–523. doi: 10.1034/j.1600-051x.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- 19.Li JR, Li JG, Deng GH, Zhao WL, Dan YJ, Wang YM, Chen S. A common promoter variant of TBX21 is associated with allele specific binding to Yin-Yang 1 and reduced gene expression. Scand. J. Immunol. 2011;73:449–458. doi: 10.1111/j.1365-3083.2011.02520.x. [DOI] [PubMed] [Google Scholar]
- 20.You Y, Zhao W, Chen S, Tan W, Dan Y, Hao F, Deng G. Association of TBX21 gene haplotypes in a Chinese population with systemic lupus erythematosus. Scand. J. Rheumatol. 2010;39:254–258. doi: 10.3109/03009740903347983. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Zhao W, Tan W, Xu B, Dan Y, Mao Q, Kuang X, Wang Y, Deng G. Association of TBX21 T-1993C polymorphism with viral persistence but not disease progression in hepatitis B virus carriers. Hepatol. Res. 2009;39:716–723. doi: 10.1111/j.1872-034X.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- 22.Suttner K, Rosenstiel P, Depner M, Schedel M, Pinto LA, Ruether A, Adamski J, Klopp N, Illig T, Vogelberg C, Schreiber S, von Mutius E, Kabesch M. TBX21 gene variants increase childhood asthma risk in combination with HLX1 variants. J. Allergy Clin. Immunol. 2009;123:1062–1068. doi: 10.1016/j.jaci.2009.02.025. 1068.e1061-1068. [DOI] [PubMed] [Google Scholar]
- 23.Cameron L, Webster RB, Strempel JM, Kiesler P, Kabesch M, Ramachandran H, Yu L, Stern DA, Graves PE, Lohman IC, Wright AL, Halonen M, Klimecki WT, Vercelli D. Th2 cell-selective enhancement of human IL13 transcription by IL13-1112C>T, a polymorphism associated with allergic inflammation. J. Immunol. 2006;177:8633–8642. doi: 10.4049/jimmunol.177.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su K, Li X, Edberg JC, Wu J, Ferguson P, Kimberly RP. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. II. Differential binding of GATA4 and Yin-Yang1 transcription factors and correlated receptor expression and function. J. Immunol. 2004;172:7192–7199. doi: 10.4049/jimmunol.172.11.7192. [DOI] [PubMed] [Google Scholar]
- 25.Greenbaum JA, Pang B, Tullius TD. Construction of a genome-scale structural map at single-nucleotide resolution. Genome Res. 2007;17:947–953. doi: 10.1101/gr.6073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marinescu VD, Kohane IS, Riva A. MAPPER: a search engine for the computational identification of putative transcription factor binding sites in multiple genomes. BMC Bioinform. 2005;6:79. doi: 10.1186/1471-2105-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azizkhan JC, Jensen DE, Pierce AJ, Wade M. Transcription from TATA-less promoters: dihydrofolate reductase as a model. Crit. Rev. Eukaryot. Gene Expr. 1993;3:229–254. [PubMed] [Google Scholar]
- 28.Joshi R, Passner JM, Rohs R, Jain R, Sosinsky A, Crickmore MA, Jacob V, Aggarwal AK, Honig B, Mann RS. Functional specificity of a Hox protein mediated by the recognition of minor groove structure. Cell. 2007;131:530–543. doi: 10.1016/j.cell.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker SC, Hansen L, Abaan HO, Tullius TD, Margulies EH. Local DNA topography correlates with functional noncoding regions of the human genome. Science. 2009;324:389–392. doi: 10.1126/science.1169050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balasubramanian B, Pogozelski WK, Tullius TD. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. U S A. 1998;95:9738–9743. doi: 10.1073/pnas.95.17.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courey AJ, Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988;55:887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21:1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan X, Jones M, Jiang J, Zaprazna K, Yu D, Pear W, Maillard I, Atchison ML. Increased Expression of PcG Protein YY1 Negatively Regulates B Cell Development while Allowing Accumulation of Myeloid Cells and LT-HSC Cells. PLoS ONE. 2012;7:e30656. doi: 10.1371/journal.pone.0030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soutto M, Zhang F, Enerson B, Tong Y, Boothby M, Aune TM. A minimal IFN-gamma promoter confers Th1 selective expression. J. Immunol. 2002;169:4205–4212. doi: 10.4049/jimmunol.169.8.4205. [DOI] [PubMed] [Google Scholar]
- 35.Ma Q, Battelli L, Hubbs AF. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am. J. Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol. Genom. 2004;18:261–272. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 37.Yoh K, Itoh K, Enomoto A, Hirayama A, Yamaguchi N, Kobayashi M, Morito N, Koyama A, Yamamoto M, Takahashi S. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney. Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 38.Córdova EJ, Velázquez-Cruz R, Centeno F, Baca V, Orozco L. The NRF2 gene variant, −653G/A, is associated with nephritis in childhood-onset systemic lupus erythematosus. Lupus. 2010;19:1237–1242. doi: 10.1177/0961203310367917. [DOI] [PubMed] [Google Scholar]
- 39.Palko L, Bass HW, Beyrouthy MJ, Hurt MM. The Yin Yang-1 (YY1) protein undergoes a DNA-replication-associated switch in localization from the cytoplasm to the nucleus at the onset of S phase. J. Cell. Sci. 2004;117:465–476. doi: 10.1242/jcs.00870. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Feng Y, Xu L, Chen Y, Zhang Y, Su D, Ren G, Lu J, Huang B. YY1 restrained cell senescence through repressing the transcription of p16. Biochim. Biophys. Acta. 2008;1783:1876–1883. doi: 10.1016/j.bbamcr.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J. Exp. Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffin DO, Rothstein TL. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med. 2011;208:2566–2569. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun. Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.