Abstract
There is accumulating evidence that the complement activation product, C5a, can orchestrate cellular immune functions. IL-27 (p28/EBI3) is an emerging key player essential for regulating inflammatory responses and T cells. Here, we report that C5a robustly suppressed IL-27(p28) gene expression and release in peritoneal macrophages. These cells from C57BL/6J mice abundantly produced IL-27(p28) after engagement of either the TLR3 (Poly I:C) or TLR4 (LPS) receptor. Genetic deficiency of either TLR4 or LPS-binding protein (LBP) completely incapacitated the ability of macrophages to secrete IL-27(p28) in response to LPS. IL-27(p28) producing macrophages also expressed the C5aR receptor, thus displaying an IL-27(p28)+F4/80+C5aR+ phenotype. C5a suppressed IL-27(p28) in LPS-stimulated macrophages via interactions with the C5aR receptor, rather than the C5L2 receptor. C5aR−/− mice after endotoxemia displayed higher plasma levels of IL-27(p28) when compared to C57BL/6J mice. C5a did not affect the release of IL-27(p28) or frequency of IL-27(p28)+F4/80+ macrophages after engagement of TLR3. Mechanistically, LPS activated both NFκB and the PI3K-Akt pathways, whereas C5a activated only the PI3K-Akt pathway. Engagement of PI3K-Akt was inhibitory for IL-27(p28) production, since PI3K-Akt pharmacologic blockade resulted in increased amounts of IL-27(p28) and reversed the suppressive effects of C5a. Blockade of PI3K-Akt in endotoxemic C57BL/6J mice resulted in higher generation of IL-27(p28). In contrast, for TLR3-mediated release of IL-27(p28), the PI3K-Akt pathway was not involved. These data provide new evidence on how complement activation may selectively interfere with production of T cell regulatory cytokines by antigen-presenting cells in the varying contexts of either bacterial (TLR4-pathway) or viral (TLR3-pathway) infection.
Keywords: C5aR, C5L2, p28, IL-30, PI3K, Akt, NFκB
Introduction
Activation of the complement cascade includes cleavage of the plasma glycoproteinC5 (190 kDa) by C5-convertases resulting in generation of C5b and the smaller C5a (~11 kDa) products. In contrast to C5b functioning to generate the microbicidal membrane-attack complex (C5b-C9), C5a is a tremendously active chemotactic molecule responsible for directing and orchestrating subsequent immune responses(1–3). In plasma, C5a is rapidly degraded by carboxypeptidases into C5adesArg and has a half-life of less than 5 min in vivo(4). Both C5a and with lower affinity C5adesArg bind to the G-protein-coupled membrane receptor C5aR (CD88)(5, 6). C5aR is expressed on a wide variety of cells not only on neutrophils and antigen-presenting cells but also T cells and even parenchymal cells such as alveolar type II epithelial cells(7–10). More recently, a second C5a receptor, C5L2 (GPR77) with high binding affinities for C5a and C5adesArg has been discovered(11, 12). The biological functions of the C5L2 receptor remain somewhat enigmatic, with suggestions describing its role as a non-signaling “decoy” receptor while other data suggest distinct biological responses(13–16). For example, C5a is critically involved in the immune pathophysiology during experimental sepsis via its interactions with both C5aR and C5L2(17). In cardiomyocytes the production of pro-inflammatory mediators in response to C5a is dependent on both receptors(18). Furthermore, C5L2 plays a crucial role during experimental allergic airway disease(19). Noteworthy, C5a not only regulates innate immune functions but also modulates adaptive T and B cell responses including T cell proliferation / differentiation and balance of the various T cell subsets (Th1/Th2/Th17)(20–22).
Heterodimeric Interleukin-27 (IL-27) is a recently described cytokine with major roles in regulating T cell functions(23). IL-27 is assembled by the two subunits IL-27(p28) and EBI3. Of note, EBI3 can also partner with p35 to form IL-35(24). Both EBI3 and IL-27(p28) are strongly induced during infections with both bacteria and viruses(25–29). Under such circumstances, the predominant cellular sources of IL-27 are macrophages and dendritic cells. These antigen-presenting cells can release IL-27 either after activation of the TLR4 receptor by LPS during bacterial infection or activation of the TLR3 receptor by ds RNA in the course of viral disease(30–33). IL-27 binds to the IL-27RA (WSX-1) and gp130 receptor complex, highly expressed on T cells. Whereas in most situations IL-27 appears not be required for immediate clearance of pathogens, it provides necessary inhibitory signals to confine T cell dependent immune responses and to prevent destructive and lethal chronic inflammation(34). For example, absence of IL-27RA signaling resulted in aggravated immune pathology of the central nervous system during infection or the EAE model(35, 36). One hallmark of IL-27 to constrain adaptive immune responses is the induction of IL-10 from T cells, e.g. involving STAT3 and STAT1 dependent mechanisms(37, 38). On the other hand, IL-27 can promote inflammation under at least some experimental circumstances such as inflammatory bowel disease and autoimmune kidney disease(39, 40). Although defining the biological functions of IL-27 is a still ongoing process, it seems that IL-27 may emerge as a novel key player of immunity(41).
Here, we describe the negative regulation of IL-27(p28) production by the complement component C5a via the C5aR receptor (but not the C5L2 receptor) in macrophages. Interestingly, such suppression occurred with preference to the nature of IL-27(p28) transcriptional activation and release. Whereas, C5a substantially inhibited IL-27(p28) after TLR4-activation, there were no such effects seen when IL-27(p28) was induced through TLR3, indicating a degree of specificity for the regulatory effects of C5a. These data provide further evidence how C5a may selectively influence immune functions by regulating the cytokine milieu in the varying context of either bacterial or viral infection.
Materials and Methods
Animals
All procedures were performed in accordance with the U.S. National Institutes of Health guidelines and the University Committee on Use and Care of Animals (UCUCA), University of Michigan. All mice were housed under specific pathogen free conditions. 10–12 week old, male C57BL/6J mice and TLR4−/− mice (B6.B10ScN-Tlr4lps-del/JthJ) were purchased from the Jackson Laboratories (Bar Harbor, Maine). C5aR−/− mice, C5L2−/− mice and LBP−/− mice were bred and genotyped at the facilities of the University of Michigan.
Peritoneal elicited macrophages
Mice were injected i.p. with 1.5 ml thioglycollate 2.4% (w/v) and 4 days later the peritoneal cavity was lavaged with 8 ml sterile HBSS (without calcium and magnesium). After centrifugation (650 rpm, 5 min, 4 ° C), the cells were counted, transferred to polystyrene plates (2×106 cells/ml) and incubated at 37°C, 5% CO2. The purity of cell preparations was evaluated by flow cytometry (>85% F4/80+CD11b+). The media used was RPMI 1640 supplemented with L-Glutamine, 25 mM HEPES, 0.1% BSA and 100 U/ml penicillin-streptomycin. At the end of experiments, the supernatant fluids were cleared from non-adherent cells by centrifugation (650 rpm, 5 min, 4°C) and stored at −80°C until further analysis.
In vivo models
Mice were injected with either LPS (E. coli, 0111:B4, 10 mg/kg bodyweight, i.p., Sigma-Aldrich) or Poly (I:C) (HMW, 10 mg/kg bodyweight, i.p., Invivogen). Bodyweight was measured on a PG802-S scale (Mettler Toledo). Blood plasma was collected in EDTA tubes (5–10 mM). For survival studies mice were monitored at least every 12 h for 10 days.
Quantification of proteins by immunodetection
IL-27(p28) in plasma and supernatant fluids was quantified by ELISA (R&D Systems). According to the manufacturer this assay has <5% cross-reactivity with recombinant mouse IL-27 (EBI3/p28 fusion). Samples were diluted in PBS plus 1%BSA to fit in the range of standards. Optical densities were measured on a SpectraMax 190 microplate reader (Molecular Devices). Detection of NFκB p65 activity in nuclear extracts was performed by DNA-binding ELISA according to manufacturer’s instructions (Active Motif). A bead-based assay (Millipore) was used to detect phosphorylated Akt (Thr308) in whole cell lysates and samples were analyzed on a Luminex instrument as described before(42). Levels of KC and IL-6 were quantified by bead-based assay (BioRad) in supernatant fluids in the same fashion.
Real time PCR
Total RNA was isolated by guanidinium thiocyanate-phenol-chloroform extraction and quantified with a micro-volume spectrophotometer (NanoQuant Infinite M200, Tecan). To generate cDNA the reverse transcription was performed with TaqMan reagents (Applied Biosystems) in a GeneAmp 9700 thermocycler (Applied Biosystems; 25°C × 10 min, 48°C×30 min, 95°C × 5 min). For amplification the SYBR Green Mastermix (Applied Biosystems) was used and samples run on a 7500 real time PCR System (Applied Biosystems; 50°C × 2 min, 95°C × 10 min; 40 cycles: 95°C × 10 s, 60°C × 60 s, 72°C × 10 s followed by acquisition of melting curves). Calculation of relative quantitative results was done with the 2−ΔΔCt algorithm. Following primers were used (Invitrogen): mouse IL-27p28 5’-GGCCATGAGGCTGGATCTC-3’ (fo), mouse IL-27p28 5’-AACATTTGAATCCTGCAGCCA-3’ (re), mouse GAPDH 5′-TACCCCCAATGTGTCCGTCGTG-3′ (fo), mouse GAPDH 5′-CCTTCAGTGGGCCCTCAGATGC-3′ (re).
Flow cytometry
For detection of intracellular IL-27(p28), cells were incubated in the presence of Monensin (2µM) for ≤12 h. Fc receptors were blocked with anti-CD16/CD32 (Fc Block, BD Biosciences). Cells were stained for surface markers before fixation and permeabilization (Cytofix/CytopermPlusKit, BD Biosciences). For detection of phosphorylated Akt, the cells were immediately fixated and permeabilized with the harsh alcohol method (Perm Buffer III, BD Biosciences) before antibody staining. Antibodies (anti-mouse) were used together with matched fluorochrome labeled isotype controls: PE IL-27(p28) (clone MM27-7B1, eBioscience), APC F4/80 and efluor450 F4/80 (clone BM8, eBioscience), APC C5aRand PE C5aR (clone 20/70, BioLegend), PE Akt(Thr308) (clone J1-223.371, BD Biosciences). A minimum of 50,000 events were acquired on a BD LSR II flow cytometer with high throughput sampler (BD Biosciences) and further analyzed with Win List for Win 32 3.0 Software (Verity Software) or Flow Jo 7.6.4 Software. Histograms in the figures show ≥10,000 events.
Reagents
Following reagents were used: TLR2 (Zymosan from S. cerevisiae), TLR3 (Poly(I:C), HMW), TLR5 (Flagellin from B. subtilis), TLR9 (type A CpG oligonucleotide ODN1518 and ODN 1585 Control), Wortmannin and Bay 11-7082 (all from Invivogen); LPS (E. coli, 0111:B4), Monensin and FMLP (all from Sigma-Aldrich); recombinant mouse C5a (<1.0 EU endotoxin per 1 µg, R&D Systems); recombinant mouse C5adesArg (Hycult Biotech).
Statistical Analysis
We used the GraphPad Prism Version 5.04 software for statistical analysis. All values are expressed as mean and error bars represent s.e.m. Data sets were analyzed by two-tailed Student’s t-test and survival curves by the log-rank (Mantel-Cox) test. We considered differences significant when p< 0.05.
Results
Release of IL-27(p28) from macrophages after TLR3- and TLR4-activation
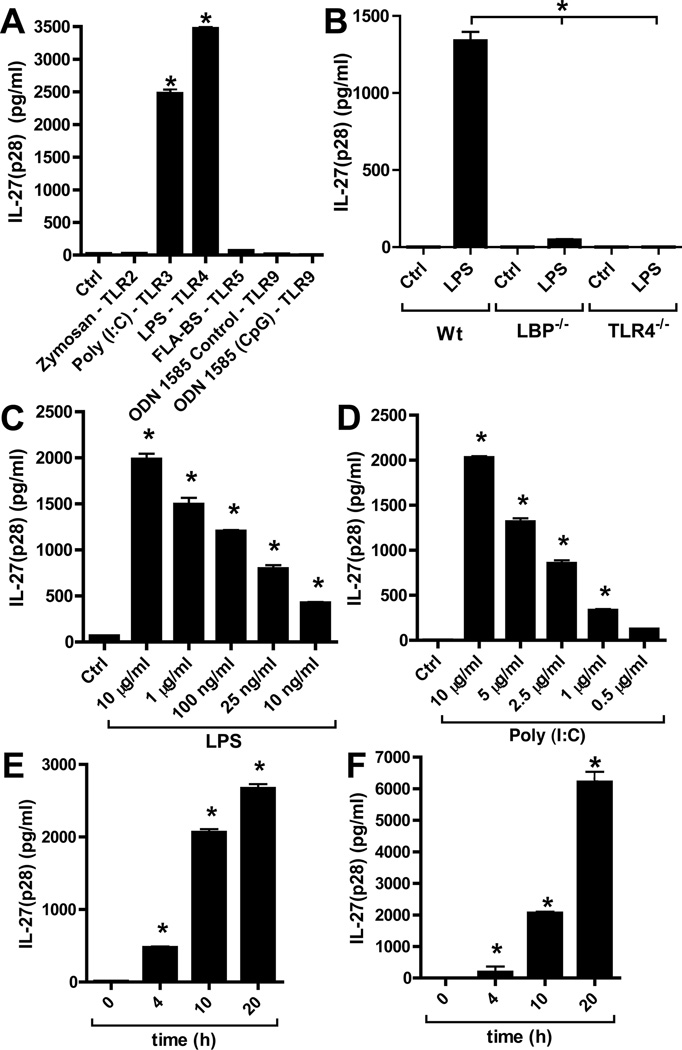
Thioglycollate elicited peritoneal macrophages (PEM) from C57BL/6J (Wt) mice were incubated for 10 h with agonists for several TLRs (Fig. 1A). After activation of TLR3 by Poly (I:C) or TLR4 by LPS, IL-27(p28) became abundantly detectable. On the other hand, activation of TLR2, TLR5 and TLR9 pathways did not induce IL-27(p28) under the conditions employed, despite an earlier report that activation of the TLR9 pathway can induce low levels of mRNA for IL-27(p28) in dendritic cells(43). All TLR agonists were fully biological active as indicated by induction of IL-6 and/or KC (Supplementary Figure 1A–B). When macrophages were isolated from TLR4−/− or LBP−/− mice, cells from both mouse strains, as expected, completely failed to secrete IL-27(p28) after LPS (Fig. 1B). Dose responses of macrophages incubated with either LPS (10 µg/ml – 10 ng/ml) or Poly (I:C) (10 µg/ml – 0.5 µg/ml) are shown in Fig. 1C, 1D, respectively. Time course studies indicated abundant presence of IL-27(p28) with incubation periods longer than 4 h (Fig. 1E, 1F). Overall, these data are consistent with previous reports on the production of heterodimeric IL-27 from antigen-presenting cells after LPS or Poly (I:C)(30–33).
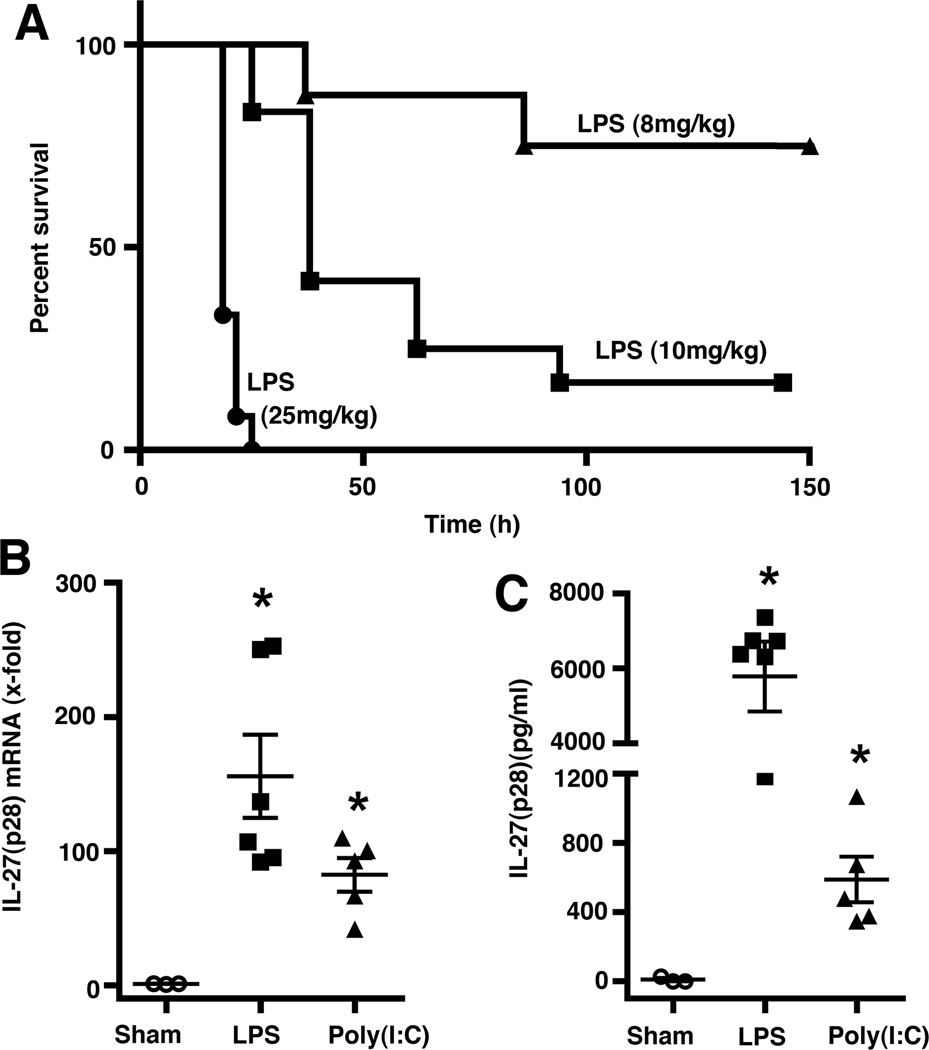
Figure 1.
Characterization of IL-27(p28) release from macrophages after activation of TLR3 or TLR4. (A) Peritoneal elicited macrophages (PEM) from C57BL/6J (Wt) mice were incubated with agonists for various TLR-agonists (all 1 µg/ml) and secreted IL-27(p28) detected by ELISA after 10h. (B) Macrophages from Wt mice, LBP−/− mice and TLR4−/− mice were incubated with LPS (50ng/ml) for 10 h before detection of IL-27(p28). (C) Dose response studies of IL-27(p28) release by PEM after TLR4-activation by LPS (10 µg/ml – 10 ng/ml), 10 h. (D) Dose response curve of IL-27(p28) production after TLR3-activation by Poly (I:C) (10 µg/ml – 500 ng/ml), 10 h. (E) Time course of IL-27(p28) release after LPS (1 µg/ml). (F) Time course of IL-27(p28) production after Poly (I:C) (10 µg/ml). All experiments shown were done with thioglycollate elicited PEM from C57BL/6J (Wt) mice. Data are representative of at least 3 independent experiments. *p < 0.05, error bars represent s.e.m.
IL-27(p28) production in vitro is suppressed by C5a
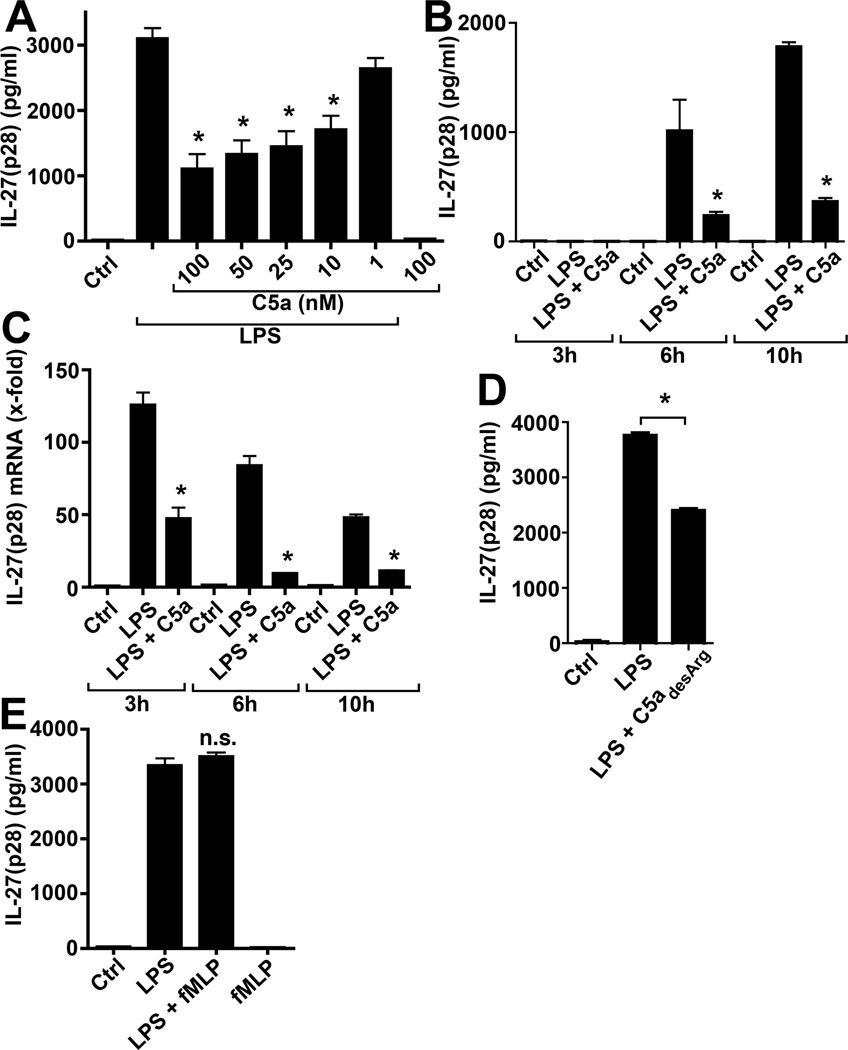
To evaluate for effects of C5a on IL-27(p28), PEM from Wt mice were incubated with LPS alone or in combination with increasing concentrations of recombinant mouse C5a. As shown in Fig. 2 A, the co-presence of C5a in concentrations between 10–100 nM suppressed the release of IL-27(p28) from LPS-activated macrophages. This C5a-relatedinhibition of secreted IL-27(p28) was observed at all the time points studied (Fig. 2B) and was accompanied by C5a-dependent profound suppression of mRNA for IL-27(p28) (Fig. 2C). The natural degradation product of C5a, C5adesArg (100 nM), also suppressed IL-27(p28) when added to cultures of LPS-activated PEM (Fig. 2D). On the other hand, other chemotactic peptides for phagocytic cells, such as bacterial fMLP, did not appear to negatively regulate macrophage-derived IL-27(p28) production, when fMLP was used alone or in combination with LPS (Fig. 2E).
Figure 2.
C5a mediates inhibition of IL-27(p28) from TLR4-activated macrophages. (A) ELISA of IL-27(p28) levels in supernatants from macrophages after incubation with LPS alone, the combination of LPS and different concentrations of recombinant mouse C5a (100-10 nM) or C5a alone (100 nM), 10 h.(B) Suppression of IL-27(p28) production from LPS-activated macrophages by co-incubation with C5a (100 nM) at different time points. (C) RT-PCR of mRNA levels for IL-27(p28) in macrophages after LPS alone or in combination with C5a (100 nM). (D) Effects of recombinant mouse C5adesArg (100 nM) on IL-27(p28) secretion by LPS-activated macrophages, 10h. (E) IL-27(p28) release from macrophages after co-stimulation with LPS and formyl-methionyl-leucyl-phenylalanine (FMLP, 4 µM) compared to LPS alone, n.s. denotes not significant. All experiments shown were done with PEM from C57BL/6J (Wt) mice and LPS was used as 1 µg/ml. Data are representative of at least 3 independent experiments. *p < 0.05, error bars represent s.e.m.
Suppression of IL-27(p28) by C5a is mediated via the C5aR receptor rather than the C5L2 receptor
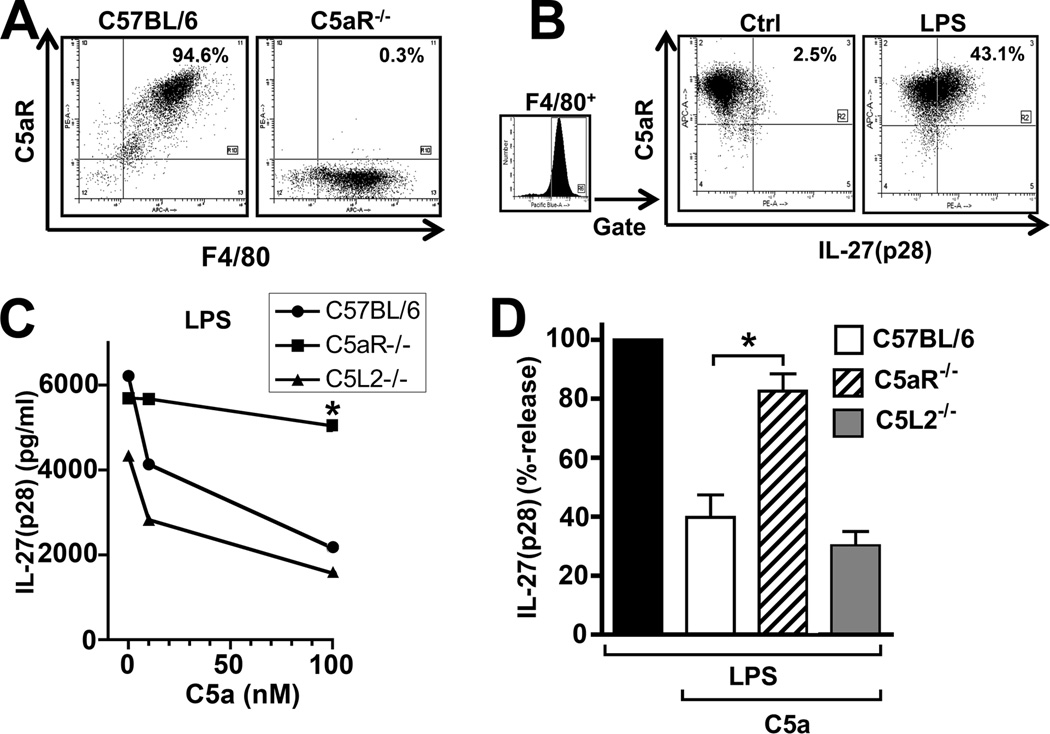
Resting peritoneal macrophages from Wt mice abundantly expressed the receptor C5aR (CD88) as evaluated by flow cytometry(Fig. 3A, left panel). On macrophages from C5aR−/− mice, there was virtually noC5aR detectable (Fig. 3A, right panel), thereby confirming both, the phenotype of the C5aR−/− mice and specificity of the anti-mouse C5aR antibody. Next, PEM from Wt mice were left as untreated controls or stimulated with LPS, both in the presence of the protein transport inhibitor Monensin. After 12 h, cells were analyzed by flow cytometry for intracellular IL-27(p28) and surface C5aR with dot-plots gated on F4/80+ macrophages (Fig. 3B). In preparations of resting cells, the frequency of IL-27(p28)+C5aR+F4/80+ cells was 2.5%. After LPS, intracellular IL-27(p28) became abundantly detectable, as indicated by presence of 43.1% IL-27(p28)+C5aR+F4/80+ cells. All macrophages that produced IL-27(p28) also expressed C5aR, indicating that IL-27(p28) producing cells respond to C5a via this receptor. Staining results with isotype controls for anti-C5aR and anti-IL-27(p28) are shown in Supplementary Fig. 1C–E. There was also some evidence that macrophages can express the C5L2 receptor as detected by RT-PCR (data not shown). To further investigate the relative contribution of C5aR and C5L2 in regulating IL-27(p28) production, peritoneal macrophages from Wt mice, C5aR−/− mice and C5L2−/− mice were isolated and incubated with LPS alone or in combination with either 10 nM C5a or 100 nM C5a (Fig. 3C). In PEM from C5L2−/− mice, C5a was fully active to suppress IL-27(p28) production. However, in PEM with deficiency of C5aR, we observed a nearly complete loss of the activity of C5a to inhibit IL-27(p28). Analysis of data from several independent experiments, showed that LPS-induced production of IL-27(p28) was reduced by C5a to 40% in Wt macrophages, 30% in C5L2−/− macrophages while production remained at 83% in C5aR−/− macrophages (Fig. 3D). These data suggest that C5aR is the predominant receptor facilitating the ability of C5a to suppress IL-27(p28) release in LPS-stimulated PEM.
Figure 3.
C5a suppresses IL-27(p28) via the C5aR but not the C5L2 receptor. (A) Flow cytometry analysis of the C5aR receptor together with the macrophage surface marker F4/80 on PEM from Wt mice or C5aR−/− mice. (B) PEM were left as resting controls (Ctrl) or stimulated with LPS (1 µg/ml) for 12 h, both in the presence of Monensin (2 µM). Cells were then analyzed for F4/80, C5aR and intracellular IL-27(p28) by flow cytometry. Dot-plots were gated to show only F4/80+ events. (C) PEM of the strains C57BL/6J, C5aR−/− and C5L2−/− were incubated with LPS and increasing concentrations of C5a (0, 10, 100 nM) for 10 h. (D) Relative inhibition of LPS-induced IL-27(p28) release in macrophages from C57BL6/J, C5aR−/− or C5L2−/− mice by C5a (100 nM). Levels of IL-27(p28) with LPS alone were used as 100% for each individual strain. All experiments were done with thioglycollate elicited macrophages (PEM) and LPS was used as 1 µg/ml. Data are representative of at least 3 independent experiments. *p < 0.05, error bars represent s.e.m.
C5a inhibits TLR4-dependent but not TLR3-dependent production of IL-27(p28)
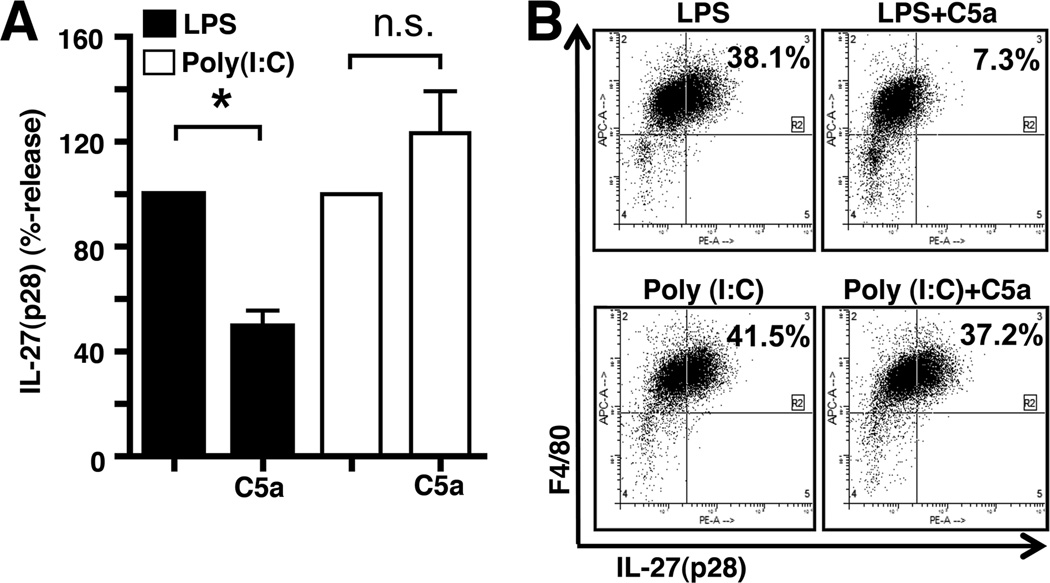
PEM (Wt) were stimulated with LPS or Poly (I:C) both in the absence or co-presence of recombinant mouse C5a. Surprisingly, C5a did not seem to suppress IL-27(p28) production, when IL-27(p28) was induced via the TLR3-pathway (Fig. 4A). Similar results were observed by flow cytometry studies (Fig. 4B). In resting macrophages, the frequency of IL-27(p28)+F4/80+ cells was 3.1% with regions set according to staining with isotype controls (data not shown). After stimulation with LPS, 38.1% of PEM were IL-27(p28)+F4/80+ cells. This number was reduced to 7.3% with the combination of LPS plus C5a (100 nM). With Poly (I:C) alone, the frequency of IL-27(p28)+F4/80+ macrophages increased to 41.5%, this number was little affected when C5a was present together with Poly (I:C) (37.2%; Fig. 4B, right lower plot).
Figure 4.
C5a inhibition of IL-27(p28) is specific for the TLR4- but not the TLR3-pathway. (A) Relative effects of C5a on IL-27(p28) release by macrophages (PEM) after activation by either LPS or Poly (I:C), 20 h, ELISA. Levels of IL-27(p28) were set to 100% for LPS and Poly (I:C) alone, respectively, n.s. indicates not significant. (B) Flow cytometry analysis of intracellular IL-27(p28) and surface F4/80 in PEM after 12 h incubation with either LPS and Poly (I:C) alone or in combination with C5a. All experiments shown were done with thioglycollate elicited macrophages (PEM) from C57BL/6J mice. LPS and Poly (I:C) were both used as 1 µg/ml and C5a as 100 nM. Data are representative of at least 3 independent experiments. *p < 0.05, error bars represent s.e.m.
Evidence for Complement-dependency of IL-27(p28) release in vivo
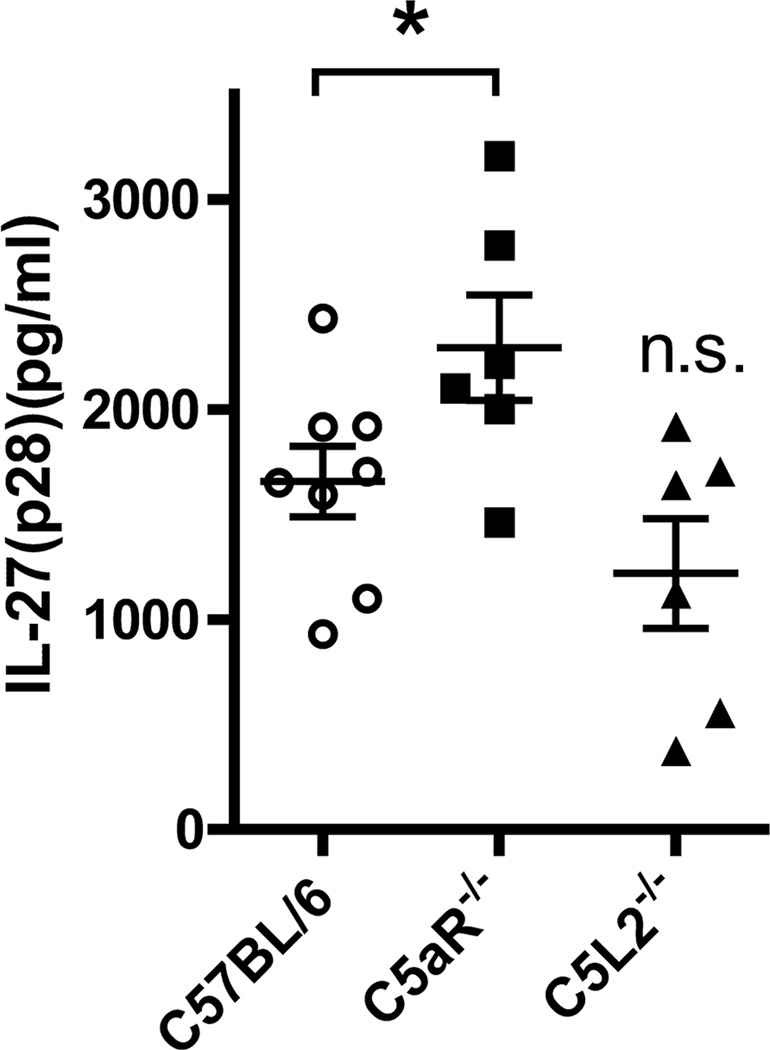
Fig. 5A shows survival curves of male C57BL/6J (Wt) mice (12 weeks old) after challenge by intra-peritoneal injection with different doses of LPS (E. coli, 0111:B4). A dose of 10 mg/kg BW of LPS resulted in a survival rate of 17% and this dose was used for further experiments. As expected, IL-27(p28) mRNA was detectable in spleen homogenates 6 h after injection of mice with LPS or Poly (I:C) (Fig. 5B). In plasma samples from the same mice, IL-27(p28) was present in higher concentrations after LPS compared to Poly (I:C) (Fig. 5C). The up-regulation of IL-27(p28) after systemic administration of LPS or Poly (I:C) in mice has also been reported before by others(44). Given the results indicating that C5a via C5aR sharply and specifically reduced IL-27(p28) levels after TLR4-activation, we determined if absence of the C5a receptors would affect IL-27(p28) levels after endotoxemia. In mice with genetic deficiency of C5aR, plasma levels of IL-27(p28) were significantly increased by an average of 39% when compared to responses of C57BL/6J (Wt) mice (Fig. 6). Collectively, the absence of C5aR leading to higher levels of IL-27(p28) in endotoxemic mice fits with the data that C5a interacting with C5aR both in vitro and in vivo results in reduced production of IL-27(p28).
Figure 5.
LPS and Poly (I:C) mediate release of IL-27(p28) in vivo. (A) Survival curves of C57BL/6J (Wt) mice (n=8–12 per group) after i.p. injection with different doses of LPS from E. coli (0111:B4). (B) RT-PCR of IL-27(p28) gene expression in spleen homogenates from Wt mice 6 h after administration of either LPS (10 mg/kg BW i.p., n=6) or Poly (I:C) (10 mg/kg BW i.p., n=5). Sham mice (n=3) received an i.p. injection with PBS. (C) Levels of circulating plasma IL-27(p28) as detected by ELISA after 6 h from the same experiment as shown in (B). *p < 0.05, error bars represent s.e.m.
Figure 6.
Absence of the C5aR receptor correlates with increased IL-27(p28) levels in vivo. C57BL/6J (n=8), C5aR−/− (n=6) and C5L2−/− (n=6) mice were injected with LPS (10 mg/kg BW i.p.). Plasma was collected after 12 h and IL-27(p28) detected by ELISA.*p < 0.05, error bars represent s.e.m. and n.s. denotes not significant compared to C57BL/6J.
C5a mediates suppression of IL-27(p28) production via activation of the PI3K-Akt pathway
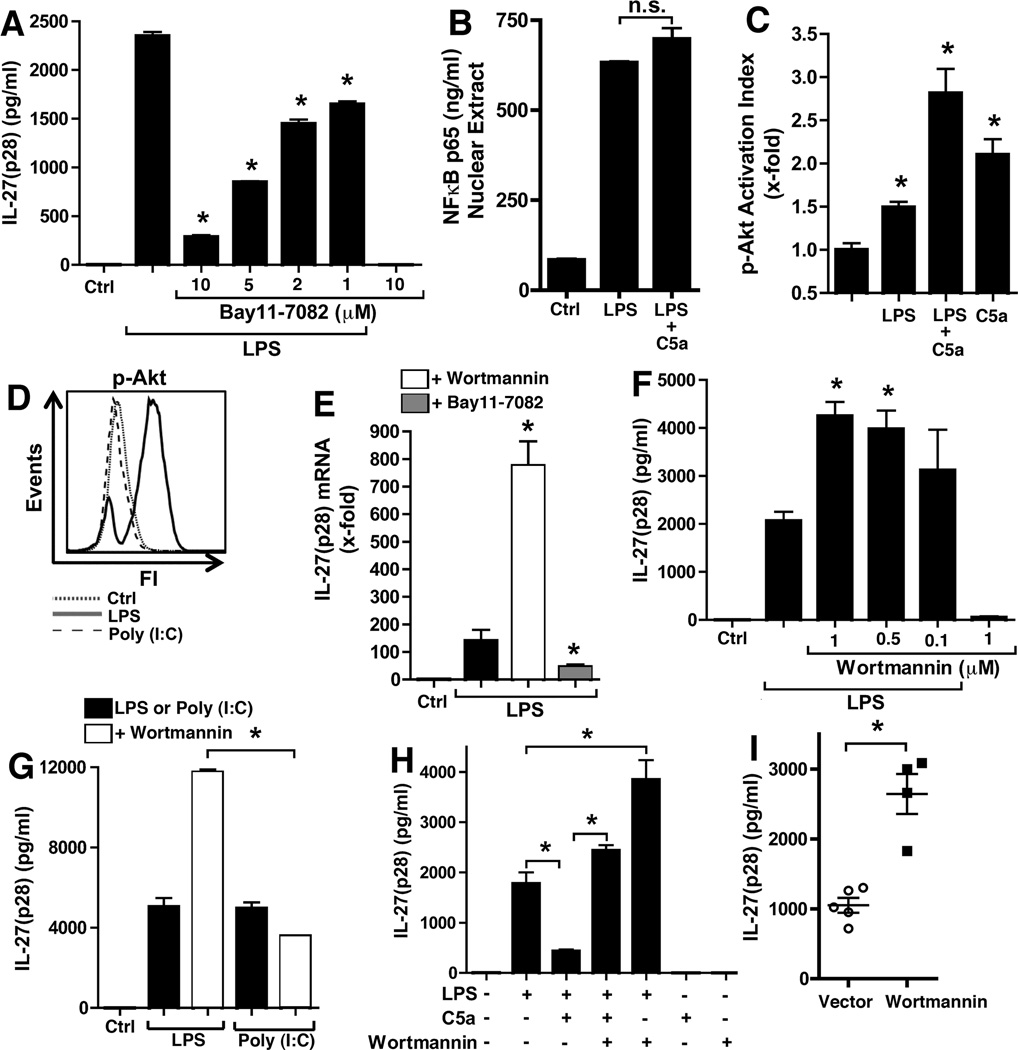
IL-27(p28) gene expression in LPS-stimulated macrophages has been reported to involve the NFκB pathway(31). Indeed, when macrophages were pre-incubated with Bay11–7082 (which prevents proteolysis of IκB and thereby NFκB activation) before addition of LPS, considerably lower amounts of IL-27(p28) were produced in a dose-dependent manner (Fig. 7A). To determine, if C5a regulates IL-27(p28) production by preventing full NFκB activation, nuclear extracts from LPS or LPS plus C5a treated macrophages were evaluated for DNA-binding activity of NFκB for its consensus sequence (5’-GGGACTTTCC-3’, Fig. 7B). No evidence was found that C5a blocks (or significantly increases) NFκB in macrophages from Wt mice. On the other hand, we have recently characterized the synergistic recruitment of the PI3K-Akt pathway by C5a and LPS in such cells(45, 46). Here, we used a novel bead-based assay with antibodies specific for Akt phosphorylation at amino acid residue threonine 308 to confirm these effects (Fig. 3C). After Poly (I:C) no phosphorylation of Akt was observed using this assay (data not shown). The selective activation of the PI3K-Akt pathway by LPS but not Poly (I:C) was also studied by flow cytometry with an antibody specific for Akt phosphorylation at threonine 308 (Fig. 7D).
Figure 7.
C5a suppression of LPS-induced IL-27(p28) is related to differential roles of the NFκB and PI3K-Akt signaling pathways. (A) Effects of different doses of the IκB-inhibitor Bay11-7082 on IL-27(p28) production from Wt macrophages after LPS, 6h, ELISA.(B) Wt macrophages were stimulated for 2 h with LPS alone, the combination of LPS and C5a (100 nM) or left as untreated controls (Ctrl). Nuclear lysates were prepared an analyzed for NFκB activity for binding of its DNA consensus sequence (5’-GGGACTTTCC-3’) by immunodetection. (C) Bead-based assay for phospho-Akt (threonine 308). Wt macrophages were stimulated for 30 min with LPS and C5a (100 nM) alone or in combination, or left as un stimulated controls. (D) Macrophages were stimulated with either LPS, Poly (I:C) or left as un stimulated controls. Cells were fixated after 60 min and phosphorylation of Akt (threonine 308) was analyzed by flow cytometry. Histograms were gated to only show F4/80+ cells. (E) RT-PCR of mRNA levels for IL-27(p28) from macrophages treated with LPS alone or in combination with wortmannin (1 µM) or Bay11-7082 (10 µM), 6h.(F) Effects of specific inhibition of the PI3K-Akt pathway by wortmannin (1–0.1 µM) for the release of IL-27(p28) from LPS-activated Wt macrophages, ELISA, 6 h. (G) Differential effects of wortmannin (1 µM) on production of IL-27(p28) when induced by either LPS or by Poly (I:C), 20 h. (H) PI3K-Akt blockade by wortmannin reverses C5a-mediated suppression of IL-27(p28) release from macrophages after LPS, 8 h, ELISA.(I) Plasma levels of IL-27(p28) in C57BL/6J Wt mice 8 h after endotoxemia. Mice had been injected with wortmannin (1 mg/kg BW i.p., n=4) or vehicle (n=5) 1 h before LPS (10 mg/kg BW i.p.). All data shown except (I) were done with thioglycollate elicited macrophages from C57BL/6J (Wt) mice. Wortmannin and Bay11-7082 were added 1 h before LPS (1 µg/ml), Poly (I:C) (1 µg/ml) or C5a (100 nM). Data are representative of 3 independent experiments or were performed with the numbers of mice as indicated. *p < 0.05, error bars represent s.e.m.
Wortmannin is a specific pharmacologic small molecule inhibitor of the PI3K-Akt pathway. Treatment of PEM with wortmannin before addition of LPS resulted in substantially higher levels of mRNA for IL-27(p28) (Fig. 7E). On the other hand, Bay11–7082 (see above) reduced levels of IL-27(p28) mRNA compared to LPS alone (Fig. 7E). Wortmannin increased levels of secreted IL-27(p28) in a dose-dependent manner more than 2-fold, if added before LPS (Fig. 7F). In contrast, such effects were not observed, when wortmannin was used in combination with Poly (I:C) (Fig. 7G). This is consistent with the fact that no activation of Akt occurred with Poly (I:C) in macrophages (Fig. 7D). When macrophages were treated with the combination of LPS and C5a in the co-presence of wortmannin, C5a was unable to suppress IL-27(p28) production (Fig. 7H). Finally, when Wt mice were given wortmannin (1 mg/kg BW i.p.) before induction of endotoxemia, this resulted in much higher levels of IL-27(p28) in plasma (Fig. 7I). In conclusion, LPS activated both NFκB and PI3K-Akt, but whereas NFκB appeared to be an activating pathway for IL-27(p28) gene expression, PI3K-Akt was a negative regulator. C5a suppressed IL-27(p28) by amplified up-regulation of PI3K-Akt activity.
Discussion
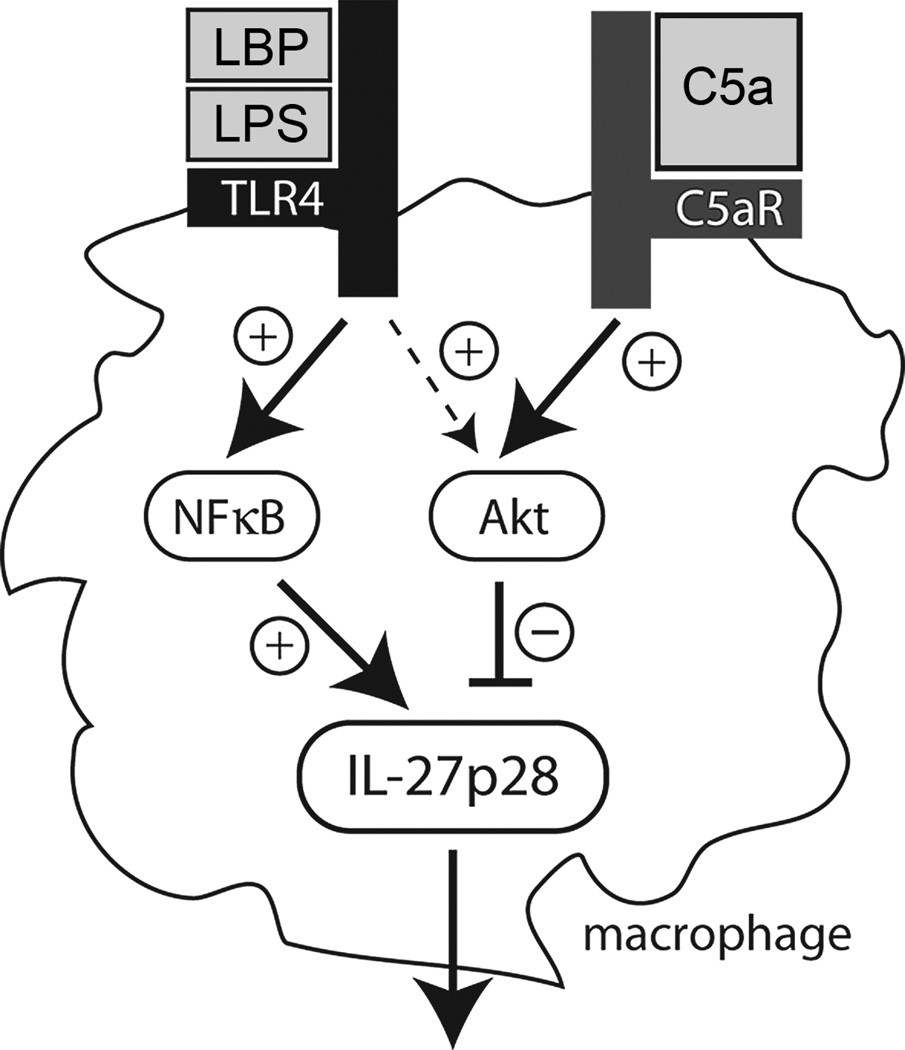
The data presented above characterize the negative regulation of IL-27(p28) gene expression by the complement activation product C5a. The effects of C5a were related to its interactions with C5aR rather than with C5L2. This is consistent with other reports suggesting that for macrophages the C5L2 receptor plays only a minor role when production of mediators is used as the endpoint(45–47). In contrast, C5a interactions with C5L2 are essential during other experimental models such as sepsis or asthma(17, 19). We and others have previously described the activation of the PI3K-Akt pathway by C5a via C5aR in several different cell types such as neutrophils, macrophages and lymphocytes(21, 45, 46, 48, 49). As shown above, pharmacologic blockade of Akt phosphorylation resulted in enhanced production of IL-27(p28) in LPS-stimulated macrophages. Interestingly, LPS at the same time appeared to activate both stimulatory and inhibitory signaling pathways for IL-27(p28) transcription (Fig. 8). The net result was abundant release of IL-27(p28). However, when the PI3K-Akt was blocked, hyperproduction of IL-27(p28) occurred both in vitro and in vivo because of loss of this inhibitory pathway. C5a via C5aR shifts the balance of positive and negative regulatory pathways towards PI3K-Akt without affecting NFκB activation, thereby antagonizing IL-27(p28) release (Fig. 8). This also explains the specificity of C5a for selective inhibition of IL-27(p28) only after activation of TLR4. TLR4 ligation with the LBP/LPS-complex resulted in phosphorylation of Akt, whereas TLR3 activation did not. Blockade of PI3K-Akt did not cause increased IL-27(p28) levels after Poly (I:C). Although C5a may still induce phosphorylation of Akt in the presence of Poly (I:C), this may not be sufficient to exceed a necessary activation threshold for this pathway.
Figure 8.
IL-27(p28) is suppressed by C5a. The proposed simplified scheme summarizes the interactions of C5a and IL-27(p28) in macrophages, highlighting the differential roles of the intracellular signaling pathways involved.
There is growing evidence that C5a regulates mediator production from innate immune cells. For example, C5a profoundly suppressed LPS-induced production of IL-12 from human monocytes and murine macrophages(47, 50). For the release of TNFα, the effects of C5a appear to be cell type specific, depending on whether neutrophils or macrophages are studied(51). Two earlier reports have mentioned regulation of mRNA for IL-27(p28) by C5a in human monocytes and murine macrophages, which is consistent with our findings(47, 52). Furthermore, we have recently reported that C5a modulates MyD88-dependent levels of the isoforms IL-17A and IL-17F(45, 46). In addition, C5a can act anti-inflammatory by augmentation of early (3–12 h) release of IL-10 from macrophages(46). C5a-dependent effects on immune cell functions may be beneficial for pathogen clearance or in the context of sepsis associated with inflammatory dysregulation and poor outcome(51, 53). In general, the complement system can be viewed as an essential arm of immune defense providing surveillance of the extracellular compartments against bacteria and parasites rather than intracellular viruses. It seems plausible to argue why C5a rather interferes with the IL-27 system after sensing of extracellular bacteria (TLR4) but not intracellular viruses (TLR3). Generation of C5a may signal ongoing bacterial infection to macrophages and prevent abundant release of IL-27 because IL-27 helps to terminate and curtail inflammation via IL-10 induction from T cells in the chronic state of inflammation(37, 38). Noteworthy, recombinant IL-27 did not induce early IL-10 production in cultures of macrophages within 24 h (data not shown), consistent with the idea that IL-27 mainly acts on lymphocytes. The termination of chronic inflammation is only desirable after pathogen clearance is achieved as would be indicated by subsiding complement activation, resulting in falling levels of C5a. The short in vivo half-life of C5a compared to other molecules (e.g. LPS) may be a key factor in this simplified hypothesis. However, the story may actually be more complex. It has been known for decades that complement consumption also occurs during some viral diseases with the classic example being hemorrhagic dengue fever(54). In principle, any anti-viral immune response resulting in production of antibodies binding to extracellular viral particles or viral proteins expressed on host cells can trigger the classical pathway of complement activation. However, this may not always be sufficient to ensure complement-dependent virolysis, in part due to viral immune evasive strategies(55).
In summary, the T cell regulatory cytokine IL-27 appears to be crucially involved during several viral, bacterial and protozoic infections. Our data provide a paradigm of how innate humoral factors such as C5a may accomplish to selectively influence subsequent adaptive immune cell functions.
Supplementary Material
Acknowledgments
We thank Norman F. Russkamp, Florence Pache, Fabien Meta, Vinay R. Patel and Rachel Voight for technical assistance and Ron Craig for advice regarding the flow cytometry studies. We cordially thank Beverly Schumann, Sue Scott and Robin Kunkel for assistance in the preparation of the manuscript.
This work was supported by grants from the National Institutes of Health, USA (GM-29507, GM-61656 to P.A.W.) and the Deutsche Forschungsgemeinschaft (Project 571701, BO 3482/1-1 to M.B.)
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annual Review of Immunology. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 2.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature immunology. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster RO, Larsen GL, Henson PM. In vivo clearance and tissue distribution of C5a and C5a des arginine complement fragments in rabbits. The Journal of clinical investigation. 1982;70:1177–1183. doi: 10.1172/JCI110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerard C, Gerard NP. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annual Review of Immunology. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 6.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 7.Wetsel RA. Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cells. Immunol Lett. 1995;44:183–187. doi: 10.1016/0165-2478(94)00212-a. [DOI] [PubMed] [Google Scholar]
- 8.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, Guo RF, Gao H, Sarma JV, Zetoune FS, Ward PA. Attenuation of IgG immune complex-induced acute lung injury by silencing C5aR in lung epithelial cells. FASEB J. 2009;23:3808–3818. doi: 10.1096/fj.09-133694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riedemann NC, Guo RF, Sarma VJ, Laudes IJ, Huber-Lang M, Warner RL, Albrecht EA, Speyer CL, Ward PA. Expression and function of the C5a receptor in rat alveolar epithelial cells. J Immunol. 2002;168:1919–1925. doi: 10.4049/jimmunol.168.4.1919. [DOI] [PubMed] [Google Scholar]
- 11.Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74) Journal of Biological Chemistry. 2002;277:7165–7169. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- 12.Ohno M, Hirata T, Enomoto M, Araki T, Ishimaru H, Takahashi TA. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol Immunol. 2000;37:407–412. doi: 10.1016/s0161-5890(00)00067-5. [DOI] [PubMed] [Google Scholar]
- 13.Okinaga S, Slattery D, Humbles A, Zsengeller Z, Morteau O, Kinrade MB, Brodbeck RM, Krause JE, Choe HR, Gerard NP, Gerard C. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42:9406–9415. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- 14.Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, Lee T, Baribault H, Tian H, Yeh WC. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- 15.Gao H, Neff TA, Guo R-F, Speyer CL, Sarma JV, Tomlins S, Man Y, Riedemann NC, Hoesel LM, Younkin E, Zetoune FS, Ward PA. Evidence for a functional role of the second C5a receptor C5L2. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1003–1005. doi: 10.1096/fj.04-3424fje. [DOI] [PubMed] [Google Scholar]
- 16.Bosmann M, Ward PA. Role of c3, c5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv Exp Med Biol. 2012;946:147–159. doi: 10.1007/978-1-4614-0106-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nature Medicine. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atefi G, Zetoune FS, Herron TJ, Jalife J, Bosmann M, Al-Aref R, Sarma JV, Ward PA. Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J. 2011;25:2500–2508. doi: 10.1096/fj.11-183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J. A Critical Role for C5L2 in the Pathogenesis of Experimental Allergic Asthma. Journal of Immunology. 2010;185:6741–6752. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]
- 20.Carroll MC. The complement system in regulation of adaptive immunity. Nature Immunology. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 21.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, Whitsett JA, Gerard C, Sfyroera G, Lambris JD, Wills-Karp M. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. The Journal of clinical investigation. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 24.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat Immunol. 2011;12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzzo C, Hopman WM, Che Mat NF, Wobeser W, Gee K. Impact of HIV infection, highly active antiretroviral therapy, and hepatitis C coinfection on serum interleukin-27. AIDS. 2010;24:1371–1374. doi: 10.1097/QAD.0b013e3283391d2b. [DOI] [PubMed] [Google Scholar]
- 28.Smits HH, van Beelen AJ, Hessle C, Westland R, de Jong E, Soeteman E, Wold A, Wierenga EA, Kapsenberg ML. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34:1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 29.Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 30.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 31.Liu JG, Guan XQ, Ma XJ. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. Journal of Experimental Medicine. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, Willems F, Goldman M, Goriely S. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. Journal of Immunology. 2007;178:7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 33.Schuetze N, Schoeneberger S, Mueller U, Freudenberg MA, Alber G, Straubinger RK. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int Immunol. 2005;17:649–659. doi: 10.1093/intimm/dxh247. [DOI] [PubMed] [Google Scholar]
- 34.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 35.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 36.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature Immunology. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 37.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 38.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 39.Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. Journal of Experimental Medicine. 2011;208:115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, Danilenko DM, Caplazi P, Wong M, Fulcher DA, Cook MC, King C, Tangye SG, de Sauvage FJ, Ghilardi N. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. Journal of Molecular Medicine. 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 42.Bosmann M, Russkamp NF, Patel VR, Zetoune FS, Sarma JV, Ward PA. The Outcome of Polymicrobial Sepsis is Independent of T and B Cells. Shock. 2011 doi: 10.1097/SHK.0b013e3182295f5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 44.Molle C, Goldman M, Goriely S. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J Immunol. 2010;184:1784–1792. doi: 10.4049/jimmunol.0902005. [DOI] [PubMed] [Google Scholar]
- 45.Bosmann M, Patel VR, Russkamp NF, Pache F, Zetoune FS, Sarma JV, Ward PA. MyD88-dependent production of IL-17F is modulated by the anaphylatoxin C5a via the Akt signaling pathway. FASEB J. 2011 doi: 10.1096/fj.11-191205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosmann M, Sarma JV, Atefi G, Zetoune FS, Ward PA. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB J. 2011 doi: 10.1096/fj.11-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Riedemann NC, Guo RF, Gao H, Sun L, Hoesel M, Hollmann TJ, Wetsel RA, Zetoune FS, Ward PA. Regulatory role of C5a on macrophage migration inhibitory factor release from neutrophils. J Immunol. 2004;173:1355–1359. doi: 10.4049/jimmunol.173.2.1355. [DOI] [PubMed] [Google Scholar]
- 49.Guo RF, Sun L, Gao H, Shi KX, Rittirsch D, Sarma VJ, Zetoune FS, Ward PA. In vivo regulation of neutrophil apoptosis by C5a during sepsis. Journal of Leukocyte Biology. 2006;80:1575–1583. doi: 10.1189/jlb.0106065. [DOI] [PubMed] [Google Scholar]
- 50.Wittmann M, Zwirner J, Larsson VA, Kirchhoff K, Begemann G, Kapp A, Gotze O, Werfel T. C5a suppresses the production of IL-12 by IFN-gamma-primed and lipopolysaccharide-challenged human monocytes. J Immunol. 1999;162:6763–6769. [PubMed] [Google Scholar]
- 51.Riedemann NC, Guo RF, Bernacki KD, Reuben JS, Laudes IJ, Neff TA, Gao H, Speyer C, Sarma VJ, Zetoune FS, Ward PA. Regulation by C5a of neutrophil activation during sepsis. Immunity. 2003;19:193–202. doi: 10.1016/s1074-7613(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 52.la Sala A, Gadina M, Kelsall BL. G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and JNK. J Immunol. 2005;175:2994–2999. doi: 10.4049/jimmunol.175.5.2994. [DOI] [PubMed] [Google Scholar]
- 53.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nature Reviews. Immunology. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bokisch VA, Muller-Eberhard HJ, Dixon FJ. The role of complement in hemorrhagic shock syndrome (dengue) Trans Assoc Am Physicians. 1973;86:102–110. [PubMed] [Google Scholar]
- 55.Doepper S, Kacani L, Falkensammer B, Dierich MP, Stoiber H. Complement receptors in HIV infection. Curr Mol Med. 2002;2:703–711. doi: 10.2174/1566524023361826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.