Austrobaileya has long served as a model for ancient angiosperm pollen structure. Its pollen germination is relatively rapid and requires < 10 % of the progamic phase. Extensive evidence suggests pollen germination underwent acceleration early in angiosperm history.
Abstract
Background and aims
The pollination to fertilization process (progamic phase) is thought to have become greatly abbreviated with the origin of flowering plants. In order to understand what developmental mechanisms enabled the speeding of fertilization, comparative data are needed from across the group, especially from early-divergent lineages. I studied the pollen germination process of Austrobaileya scandens, a perennial vine endemic to the Wet Tropics area of northeastern Queensland, Australia, and a member of the ancient angiosperm lineage, Austrobaileyales.
Methodology
I used in vivo and in vitro hand pollinations and timed collections to study development from late pollen maturation to just after germination. Then I compared the contribution of pollen germination timing to progamic phase duration in 131 angiosperm species (65 families).
Principal findings
Mature pollen of Austrobaileya was bicellular, starchless and moderately dehydrated—water content was 31.5 % by weight and volume increased by 57.9 % upon hydration. A callose layer in the inner intine appeared only after pollination. In vivo pollen germination followed a logarithmic curve, rising from 28 % at 1 hour after pollination (hap) to 97 % at 12 hap (R2 = 0.98). Sufficient pollen germination to fertilize all ovules was predicted to have occurred within 62 min. Across angiosperms, pollen germination ranged from 1 min to >60 h long and required 8.3 ± 9.8 % of the total duration of the progamic phase.
Significance
Pollen of Austrobaileya has many plesiomorphic features that are thought to prolong germination. Yet its germination is quite fast for species with desiccation-tolerant pollen (range: <1 to 60 h). Austrobaileya and other early-divergent angiosperms have relatively rapid pollen germination and short progamic phases, comparable to those of many insect-pollinated monocots and eudicots. These results suggest that both the pollen germination and pollen tube growth periods were marked by acceleration of developmental processes early in angiosperm history.
Introduction
Flowering plants are notable for having evolved rapid reproductive cycles relative to other seed plants (Stebbins 1976, 1992; Takhtajan 1976; Favre-DuChartre 1979; Willson and Burley 1983; Bond 1989; Friedman 1990; Doyle and Donoghue 1993; Taylor and Hickey 1996). An extreme abbreviation of their pollination to fertilization period (progamic phase) is hypothesized to have been the enabling step in the origin of rapid reproduction (Stebbins 1992; Williams 2008, 2009). Male gametophytes interact with maternal tissues and compete for fertilizations during the progamic phase. Hence, the question of how progamic phase duration evolves is intertwined with the question of how male gametophyte developmental rates evolve.
The progamic phase comprises two semi-independent phases of male gametophyte development: pollen germination and pollen tube growth from stigma to egg. The duration of pollen tube growth is most affected by how far and how fast pollen tubes grow. Angiosperm pollen tube pathways have been reconstructed as initially very short (Williams 2008), consistent with their earliest fossil record showing that carpels lacked prominent styles (Friis et al. 2010). However, pollen tube growth rates, even among the earliest-divergent angiosperm lineages, are orders of magnitude faster than those of other seed plants (Williams 2009, 2012). Thus, the speeding of pollen tube growth alone explains much about how the angiosperm progamic phase became abbreviated.
To put the role of pollen tube growth into perspective, one must also understand how pollen germination speed evolves and quantify its contribution to the total duration of the progamic phase. Since pollen germination comes before pollen tube growth, selection can act first on pollen germination speed, if there is heritable variation. If pollen tube growth rates were initially slow to evolve, as they seem to be in non-flowering seed plants (Williams 2009, 2012), pollen germination might have been the primary process affected by pollen competition among early angiosperms. Germination competition has been shown to be strong in a number of woody perennials within ancient angiosperm lineages. For example, hand-pollination experiments found high levels of pollen germination and tube growth on the stigma but few pollen tubes within stylar canals of Amborella trichopoda (Thien et al. 2003; Williams 2009), Austrobaileya scandens (Williams 2008) and Annona cherimola (Lora et al. 2010). In Amborella, the probability of reaching an ovule went from <1 % on the stigma to 50 % at the top of the stylar canal (Williams 2009). If maternal control over the form and/or intensity of competition differs between the stigma and the stylar canal or ovary, then pollen germination and pollen tube growth should evolve at different rates, especially if the two processes have some degree of modularity.
In this study, I report on the pollen germination process of Austrobaileya scandens White (Austrobaileyaceae is monospecific and hereafter I use the genus name for the taxon). Austrobaileya is the sister lineage to the rest of Austrobaileyales, an order that is itself sister to all angiosperms other than Nymphaeales and Amborella (Soltis et al. 2011 and references therein). It is a perennial vine endemic to the Wet Tropics area of northeastern Queensland, Australia (Endress 1980, 1983a). It flowers in the subcanopy and is thought to be insect pollinated (Endress 1980; Thien et al. 2009).
The goal of this study was to understand the contribution of pollen germination speed to the evolution of progamic phase duration in angiosperms. To that end, I first document the timing and duration of pollen germination in Austrobaileya, and aspects of pollen structure related to pollen germination speed. Previous studies have described Austrobaileya pollen morphology and development (Endress 1980, 1983b; Endress and Honegger 1980; Zavada 1984) and pollen tube growth patterns (Williams 2008). I place these data in context by quantifying the contribution of pollen germination to the total duration of the progamic phase across a broad sample of angiosperms. That analysis highlights some pathways and limitations to the evolution of pollen germination.
Methods
Pollen hydration
Pollen hydration status was quantified using flowers from a single plant that flowered in the greenhouse in March 2010. Flowers were collected in the morning, placed in a plastic bag and transported to the lab. Flowers were kept in the plastic bag until each was used (within 2h after flower collection). Pollen from dehiscing anthers was placed in immersion oil for determination of size and shape at presentation. Pollen from the same anther was at the same time placed in B-K medium (Brewbaker and Kwak 1963) with 2.5 % sucrose (which gave optimal germination percentage). Pollen on B-K medium reached maximum size within minutes, and was photographed at least 1h after immersion. Photomicrographs were made using differential interference contrast (DIC) microscopy at ×200 magnification with a Zeiss Axiocam camera at 3000 × 3900 pixel resolution and analysed using Axiovision, version 4.1 software (Carl Zeiss, Thornwood, NY, USA). Volume was calculated for 30 pollen grains in each treatment using the formula: V = (π × A × B2)/6, where A is the length of the major axis and B the minor axis. The volume (V) gain of pollen in its hydrated state relative to its size at anthesis was calculated as 100 × (Vhydrated− Vanthesis) ÷ Vhydrated (Nepi et al. 2001).
Water content was estimated by comparing pollen weight at anthesis and after drying. Fresh pollen was removed from open anthers with a toothpick, placed on weighing paper and weighed within 1–3 min after removal. Weighed pollen was then dried in an oven at 50 °C and reweighed afterwards until near constant weight was reached at 24, 48 or 72 h (Aylor 2002). Weights were determined on a Mettler-Toledo UMX2 (Columbus, OH, USA) microbalance with display resolution of 0.1 µg.
Pollen germination process
The pollen germination study was carried out on private land near the Millaa Millaa lookout, Queensland, Australia (17°31′15″S, 145°33′53″E). The site was clearcut in the 1950s but Austrobaileya vines are now very common in the c. 50- to 60-year-old secondary rainforest. Only seven of the many vines had enough flowers to use in a pollination study. The average temperature and relative humidity at this site during the study (13–24 September 2008) were 17.6 ± 4.3 °C and 86.1 ± 16.0 %, as measured hourly in the subcanopy (mean of 2 HOBO Pro series data loggers; Onset, Bourne, MA, USA).
Pollen germination was assessed using hand pollinations of newly receptive flowers on seven vines in the wild or of flowers from cuttings from these vines that were placed in water and kept outdoors, nearby in a similar climate. Cuttings were used because Austrobaileya flowers had to be accessed individually in the tree canopy, and each vine produced only a few receptive flowers each day. A comparison of flowers from cuttings vs. wild flowers showed no difference in germination percentage (paired two-tailed t-test, P = 0.28, n =5 time classes). Crosses were done among the seven vines, and I attempted to maximize the number of cross combinations per timepoint from among the available male and female phase flowers on each day. Self (geitonogamous)pollinations were also included since self pollen germination was equivalent to outcross germination (J. H. Williams, unpublished data). In both wild flowers and those from cuttings, pollen was transferred directly from living anthers of a single cut flower to a stigma by a toothpick, thus pollen was only exposed to the dispersal environment for a few seconds.
To get a minimum estimate of duration of male phase, six cut flowers were monitored from anther opening until either all anthers or the whole flower abscised. Pollen viability was then assessed under a field microscope at the end of male phase of each flower as the percentage of pollen germinated 9–12 h after placing pollen on B-K medium as above (4 samples × 50 pollen grains each per flower).
Gynoecia were fixed in formalin-acetic acid-alcohol (FAA) at 1, 2, 3, 4, 6 and 12 hours after pollination (hap), and either hand sectioned directly or embedded in glycol methacrylate and serial sectioned. Sectioned material was viewed with DIC microscopy or with fluorescence microscopy using aniline blue (AB) or 4′,6-diamidino-2-phenylindole (DAPI) stains (methods in Williams 2009). The starch content of pollen was assessed using iodine–potassium iodide (IKI) stain, whereas alcian blue, toluidine blue or ruthenium red were used to detect pectins (Ruzin 1999). Images were processed with Adobe Photoshop version 7 (Adobe, San Jose, CA, USA).
For timing of pollen germination, two carpels from each flower were cut from the apocarpous gynoecium. Since all the stigmas in an Austrobaileya gynoecium are united by a massive stigmatic secretion, only a random portion of the secretion and total pollen load was represented in each of these subsamples. All pollen grains were then categorized as either germinated (i.e. with pollen tube longer than pollen grain width) or ungerminated. If no pollen was present, another carpel was taken, until two were found with pollen. The percentage of pollen germinated was averaged from two subsamples of each cross before averaging over crosses at each timepoint (i.e. the cross was the experimental unit). Pollen number did not affect the probability of pollen germination, as indicated by a post hoc linear regression on the data (P= 0.746; R2= 0.021, n= 244).
Pollen germination literature survey
A previous literature survey identified data on the time between pollination and fertilization (Williams 2008). I attempted to find data on the in vivo time between pollination and pollen germination (emergence of the pollen tube) for as many species in that survey as possible. Data on pollen germination for each taxon were either provided within the same study or in another by the same authors, or in a few cases data on germination from a different author were used. In vitro pollen germination data were not used. Few studies provided quantitative data on average time to germination. Most provided data on when germination first began and/or the time when substantial pollen germination had occurred. Thus, I attempted to use values for the earliest time that a substantial (relative to the number of ovules) portion of the pollen load had germinated. The species data were averaged over genera, since multiple species within a genus often had similar germination times and this also prevented skewing of results towards a few intensively studied genera.
Results
Pollen ontogeny from flower opening to pollination and germination
Flowers of Austrobaileya first opened at any time of day or night, with receptive stigmas. Anthers did not open until 1–2 (up to 3) days after flower opening in the wild. Pollen from newly dehiscent anthers was bright yellow and sticky, tending to clump when handled (Fig. 1A). On cuttings, pollen viability was 75.9 ± 30.1 % initially, and 51.1 ± 32.1 % at the end of male phase (measurements taken 66.1 ± 11.9 h after onset of male phase). Pollen germinates on a massive secretion formed from the many individual stigmas at carpel tips and tubes grow through the secretion to reach open stylar canals (Fig. 1B).
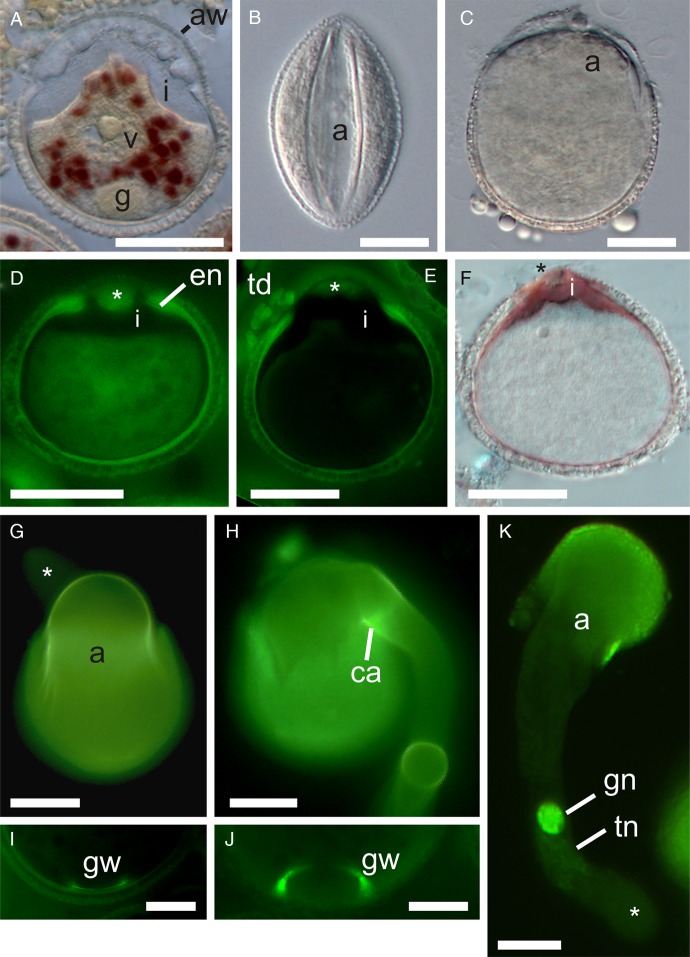
Fig. 1.
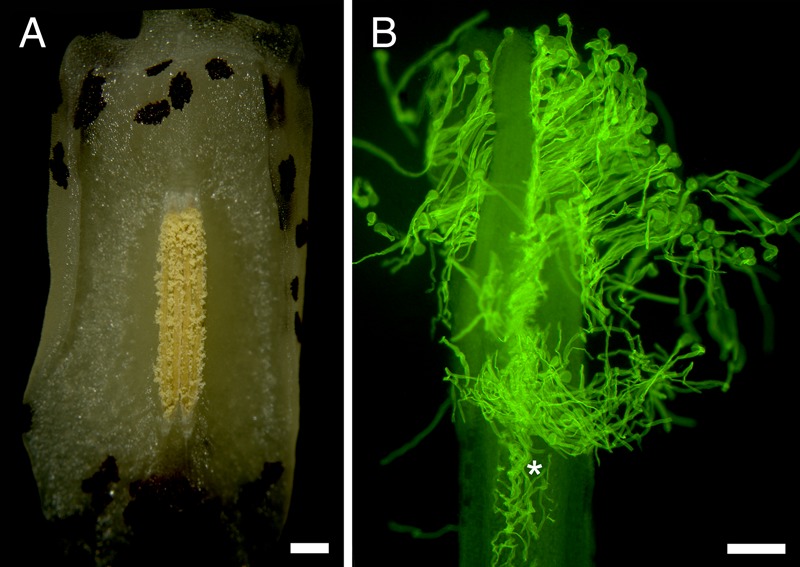
The anther and stigma of A. scandens. (A) Fully dehiscent anther. Scale bar = 500 µm. (B) Germinated pollen with pollen tubes growing through stigmatic secretion to open stylar canal (asterisk) of a single carpel. Scale bar = 200 µm.
In the unopened anther during female phase, pollen was most often in the early two-celled stage (bicellular pollen 1 stage of Blackmore et al. 2007), and the vegetative cell cytoplasm stained for abundant starch grains (Fig. 2A). Mature pollen in newly opened anthers did not stain for starch (not shown). In mature pollen, the generative cell was positioned adjacent to the tube nucleus within the centre of the vegetative (tube) cell. At this stage, a remnant callose wall was often seen at the proximal pole where the generative cell had initially formed, sequestered from the microspore wall and vegetative cell cytoplasm (Fig. 2I and J). Upon germination, the generative cell nucleus is seen in close proximity to the tube cell nucleus as they pass into the pollen tube, within ∼50 µm of the growing tip (Fig. 2K). Sperm were first observed in pollen tubes at 4 hap and most pollen tubes contained sperm at 6 hap.
Fig. 2.
Pollen germination of A. scandens. (A) Two-celled pollen from female phase flower (closed anther) with IKI-stained granules in vegetative (tube) cell cytoplasm. (B) Pollen from open anther in immersion oil, showing dehydrated state at presentation (DIC). (C) In vitro hydrated pollen, just before germination (DIC). (D) Immature pollen (female phase of flower) with AB staining of extra-apertural endexine (en) and isolated endexine in aperture wall (asterisk). (E) Mature pollen from open anther (AB). Note AB stain in a thin layer of endexine which is thickened at the aperture edge, absent in its margins and present in the centre of the apertural wall (asterisk). Clumps of AB-stained material (of tapetal origin) are associated with the outer apertural wall. Note also the bulging of intine through aperture, probably caused by partial hydration of pollen during fixation. (F) Pollen from open anther showing intine stained by ruthenium red. (G) In vitro germinated pollen showing AB stain in inner pollen wall and its continuity with the emerging inner tube wall. Note that the tube tip (asterisk) in the background lacks AB staining. (H) Emergence of pollen tube has ruptured part of pollen wall and pushed aside aperture covering. Note strong AB staining of a presumably callose annulus (ca) at base of tube. (I, J) A ring of AB-stained material is prominent at the proximal pole of many mature and germinated pollen grains, marking the location of the generative cell wall (gw). Scale bars = 10 µm. (I) Callose wall of generative cell inside of intine. (J) Remnants of callose wall of generative cell. (K) In vivo germinated pollen (3 hap) with faintly stained tube nucleus (tn) in association with the generative cell near the young pollen tube tip (asterisk) (DAPI). Pollen from (G), (H), (J) and (K) was fixed and stained 2 h after innoculation on growth medium. (A), (D–F) and (I) are from methacrylate sections. a, aperture; aw, aperture wall; g, generative cell; gn, generative cell nucleus; i, intine; td, tapetal deposits; v, vegetative cell nucleus. Scale bars = 20 µm, except where noted.
At maturity, pollen is anasulcate (as observed at the tetrad stage) and the long and wide aperture has prominent margins (Fig. 2B). It has a globose-euprolate shape (Walker and Doyle 1975) at presentation in open anthers (Fig. 2B, see also Fig. 1A). Pollen assumes a nearly spherical shape in aqueous B-K medium (and after fixation in FAA), and abundant hydrophobic droplets are released from the outer wall (Fig. 2C). In unopened anthers, pollen has a very thin AB-staining endexine which is considerably thickened at the aperture margin (Fig. 2D). Mature pollen in newly opened anthers displays the same AB-staining endexine (Fig. 2E). In both immature and mature pollen there is a very thick mass of AB-staining material in the inner aperture wall, isolated from but corresponding in position to the extra-apertural endexine (Fig. 2D and E). Also in both, the extra-apertural intine is very thin, but becomes massive in the apertural area (Fig. 2A and D–F). Strong staining of the intine with ruthenium red indicates that pectins are abundant (as also confirmed with alcian blue and toluidine blue) (Fig. 2F).
Fully hydrated pollen shows a swelling of the vegetative (tube) cell in the distal apertural zone (Fig. 2C), and then a bulging of the tube cell through the sulcus (Fig. 2E–H). Aperture deposits are pushed aside and remain conspicuous long after pollen tube growth commences (Fig. 2E, H and K). The pollen wall sometimes tears as the bulging tube cell emerges (Fig. 2H). The intine of mature pollen lacks AB staining (Fig. 2E), but in germinating pollen it stains brightly with AB and is continuous with the pollen tube wall (Fig. 2G and H). AB is not seen at the tube tip (Fig. 2G). The cylindrical shape of the pollen tube is formed as a small offshoot from the large bulging tube cell outside of the aperture (Fig. 2G, H and K). Note that the reduction to a small and uniform tube diameter can be gradual (Fig. 2K), or quite abrupt in cases where the tube initiates from the side of the bulge (Fig. 2G and H), the more common situation.
Pollen hydration
At presentation, pollen in oil had a mean (±s.d.) length × width of 51.8 ± 2.8 × 32.5 ± 1.6 μm (Fig. 2B; Table 1). Hydrated pollen from the same anthers was nearly spherical with a mean length × width of 53.2 ± 2.9 × 49.4 ± 2.0 μm (Fig. 2C). Pollen volume at presentation was 42.1 % of that of hydrated pollen (Table 1). On liquid B-K medium, individual pollen grains reached ≥90 % of their maximum volume within 3min (three flowers, mean of 16.5 pollen grains/timepoint).
Table 1.
Austrobaileya pollen volume changes after hydration. Values are means ± 1 s.d.
| Flower ID | Volume at anthesis (μm3) | Volume after hydration (μm3) | % volume increase |
|---|---|---|---|
| S | 24 293 ± 5216 | 58 050 ± 11 902 | 57.07 |
| T | 28 039 ± 5020 | 59 695 ± 21 411 | 53.03 |
| U | 32 213 ± 8321 | 75 154 ± 13 188 | 57.14 |
| X | 28 920 ± 7167 | 74 037 ± 16 432 | 60.94 |
| AA | 29 003 ± 9198 | 83 315 ± 27 022 | 65.19 |
| BB | 28 117 ± 4416 | 71 231 ± 15 887 | 60.53 |
| CC | 31 857 ± 7796 | 65 661 ± 65 661 | 51.48 |
| Mean | 57.91 ± 4.75 |
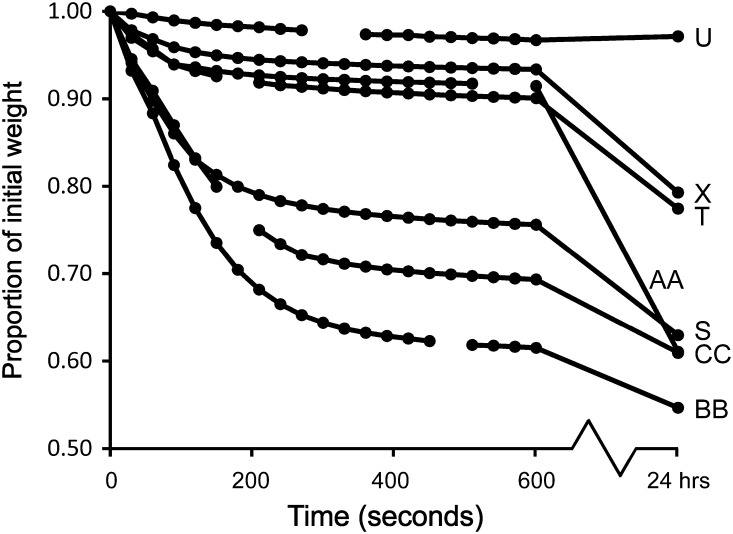
Fresh pollen had a mean (±s.d.) weight loss of 31.52 ± 16.02 % after drying (n= 13 flowers). Pollen in most replicates underwent a rapid initial weight loss, within the first 5 min after removal from open anthers (Fig. 3). Since Austrobaileya pollen is sticky, some of this rapid weight loss may have been a result of rapid drying of substances in the pollen coating. The mean weight loss of pollen from the four oldest flowers (including U and X in Fig. 3) was 15.71 % and pollen in old flowers was also notably less sticky (and turning light yellow).
Fig. 3.
Timing of initial weight loss from fresh Austrobaileya pollen. On the y-axis, 1.00 represents standardized initial weight of pollen just after removal from anthers.
In vivo pollen germination timing
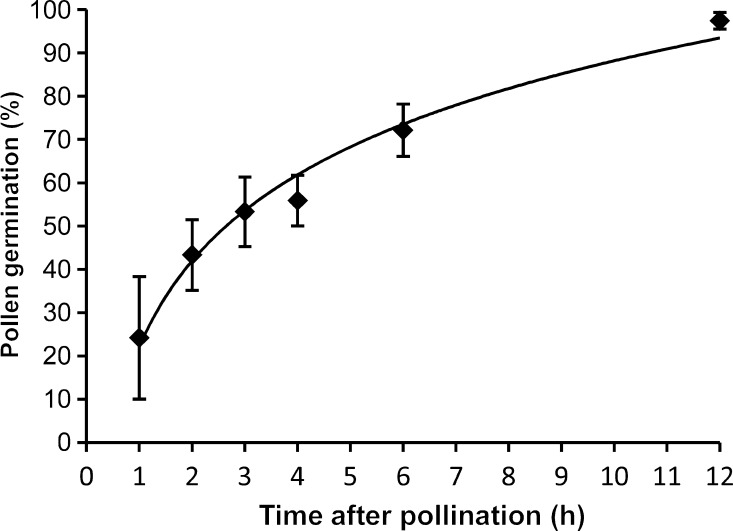
Pollen germination began within 1 hap and reached its maximum between 6 and 12 hap (Fig. 4). Pollen germination progression best fit a logarithmic curve: y = 0.2872×ln(x) + 0.2204 (R2= 0.981). The mean (±s.d.) number of pollen grains per carpel was 37.8 ± 26.3, and there was a mean of 8.70 ± 0.57 ovules per carpel in flowers from these vines (N= 6 vines; mean of 7.2 flowers/vine). The predicted time needed for 8.7 of 37.8 pollen grains, or 23 %, of the pollen load to germinate is 62 min (Fig. 4).
Fig. 4.
In vivo pollen germination timing in Austrobaileya. Bars are standard errors. N =7, 13, 13, 13, 10 and 6 hand pollinations from 1–12 hap, respectively.
Pollen germination literature survey
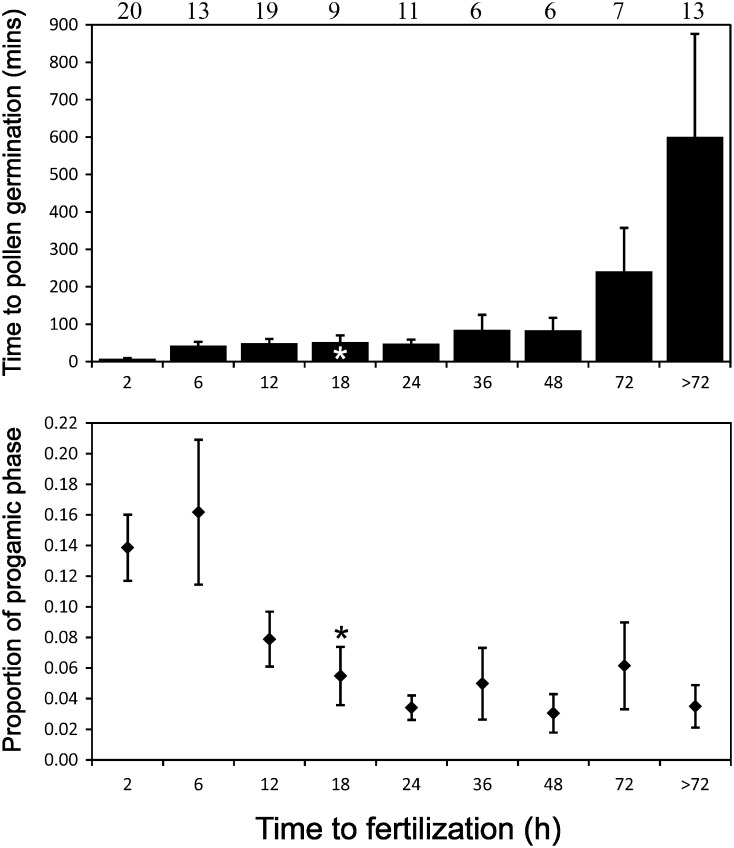
There were data on both time to pollen germination and the duration of the progamic phase for 131 species in 104 genera (65 families). A pollen germination time of 1.5 h was used to represent Austrobaileya (consistent with methodology used in the literature review), which is 8.33 % of its 18-h-long progamic phase (Williams 2008). The time from pollination until pollen germination ranged from <1 min to 60 h, whereas the time between pollination and fertilization in the same set of species ranged from 15 min to 13 months (Fig. 5A). There were 55 genera with ‘fast’ pollen germination of 30 min or less (Nepi et al. 2001), all with progamic phases of <60 h. Thirty-six genera had ‘slow’ germination of 1 h or more. Pollen germination required a mean (±s.d.) of 8.3 ± 9.8 % of the total duration of the progamic phase. However, pollen germination required a significantly greater proportion of time in genera with very short progamic phases (≤6 h; 33 genera) than in genera with longer times of >6 h (mean ± 2s.e.= 14.8 ± 4.4 vs. 5.3 ± 1.4 %, respectively; Fig. 5B).
Fig. 5.
In vivo pollen germination timing in angiosperms. (A) Time reported for pollen germination to occur (range: 1 min to 60 h). (B) Time to pollen germination as a proportion of the total time from pollination to fertilization (progamic phase). In both graphs, classes on the x-axis represent the number of hours (≤h) reported for the minimum duration of the progamic phase. The number of genera in each class is shown at the top. Asterisks represent Austrobaileya. Bars are standard errors.
Discussion
Pollen germination process in Austrobaileya
In female phase (within at least a day of anther opening), Austrobaileya pollen is at a bicellular stage. At this stage, a conspicuous callose wall was often seen completely surrounding the generative cell just inside of the intine, as also shown by J. Heslop-Harrison and Y. Heslop-Harrison (1997). Some time later in female phase, but before anther opening (onset of male phase), the generative cell migrates to a central position in the vegetative (tube) cell, in close association with the tube cell nucleus. The callose wall remnants seem to be left in place at the proximal pole, as also shown by Boavida et al. (2005), whereas Tanaka (1988) found that the free generative cell in the cytoplasm may also have a callose wall.
In both immature and mature pollen, there is a conspicuous AB-stained layer beneath the foot layer of the exine, corresponding to the endexine in the transmission electron micrographs of Austrobaileya pollen in Endress and Honegger (1980). The extra-apertural endexine is considerably thickened at the edge of the aperture. Endexine is absent from the aperture wall margin, but in the main aperture wall, a very thick but slightly more diffusely stained mass, corresponding in position to the extra-apertural endexine, is present beneath the much reduced exine. Aniline blue staining indicates that there is callose, or perhaps mixed glucans, in the endexine, which is unusual but has previously been reported in Helianthus (Vithanage and Knox 1979).
In immature and mature pollen, the intine stained strongly for pectins in both the thin extra-apertural and the massive apertural areas. Callose was not detected in the inner layer of the intine at these stages, but appeared only later during pollen hydration and germination. Endress and Honegger (1980, fig. 4) showed an inner, electron-translucent intine layer in hydrated Austrobaileya pollen, consistent with the occurrence of callose in my material. Thus, the callose wall layer of the intine is secreted after pollination, not before. The pollen tube itself is organized outside of the pollen grain from the wall of the large bulging tube cell, usually at one of the narrow extremes of the sulcus (see also Y. Heslop-Harrison and J. Heslop-Harrison 1992). The callose layer of the intine is continuous with the new wall of the cylindrical pollen tube, and a thick callose annulus is present at the base of the tube.
Pollen of Austrobaileya undergoes moderate dehydration before anther opening. That an active process of dehydration occurs is indicated by the prolate shape and relatively low water content of mature pollen, and by the >50 % increase in volume to a spheroidal shape upon hydration. Moderately dehydrated pollen, with >23 % water content, is characterized as ‘drought tolerant’ by Hoekstra et al. (2001), but further work would be needed to determine whether Austrobaileya pollen is tolerant of more severe desiccation (viable with ≤23 % water content). The starchless condition of mature pollen suggests some degree of desiccation tolerance, because in pollen with desiccation tolerance starch is converted to sucrose during dehydration (Speranza et al. 1997), and sucrose is especially important for membrane protection in dehydrated cells (Hoekstra et al. 1989, 2001).
The starchless condition is associated with insect pollination (Baker and Baker 1979), and entomophily is also indicated by the stickiness of pollen, which causes clumping. The abundant oily droplets that emerged from hydrated pollen in aqueous solutions suggest that some form of pollenkitt is the cause of pollen stickiness (Pacini and Hesse 2005), as also suggested by Zavada (1984) from electron microscopy. Pollenkitt has not been reported in pollen of extant non-flowering seed plants (Pacini and Hesse 2005), but has been found in at least one extant early-divergent angiosperm, Cabomba caroliniana (Osborn et al. 1991). The angiosperm fossil record indicates that pollen clumping was common by the mid-Cretaceous (Hu et al. 2008).
Consequences of pollen structure and development for germination speed
Austrobaileya pollen has a number of character states that are thought to be plesiomorphic for angiosperms (Sampson 2000). Most of these have at one time or another been hypothesized to prolong pollen germination. Briefly, germination is said to be slower in: monosulcate vs. multi-aperturate pollen (Dajoz et al. 1991; Furness and Rudall 2004); thick- vs. thin-walled pollen (Heslop-Harrison 1979a; Nepi et al. 2001); starchless, oil-rich vs. starchy pollen (Baker and Baker 1979; Franchi et al. 1996); bicellular vs. tricellular pollen (Torabinejad et al. 1998); pollen initiating synthesis of callose intine layer after pollination vs. prior to pollination (Heslop-Harrison 1979b; J. Heslop-Harrison and Y. Heslop-Harrison 1992; Nepi and Pacini 1999; Pacini 2000); dehydrated vs. hydrated pollen (Nepi et al. 2001; Franchi et al. 2002); pollen with underdeveloped vs. mature mitochondria (Hoekstra and Bruinsma 1978, 1979; Hoekstra 1979; Rounds et al. 2011); and pollen with low vs. high metabolic rate (Hoekstra and Bruinsma 1975; Hoekstra 1979). These traits mediate germination speed either passively by affecting the speed of pollen hydration (e.g. aperture and pollen wall composition and sizes) or actively via the metabolic state of mature pollen at pollination. Pollen that does not undergo dehydration prior to dispersal is usually desiccation sensitive (i.e. ‘partially hydrated’, Franchi et al. 2011). It is well hydrated and metabolically very active, and hence germination proceeds rapidly (often within minutes) (Franchi et al. 2002), but it is generally very short lived (Dafni and Firmage 2000). Active dehydration of pollen before dispersal confers greater longevity but slower germination—developmental and structural characteristics of mature desiccation-tolerant pollen, as well as the lower metabolic rate of germinating pollen, result in great variation in the duration of the pollen germination process.
Austrobaileya pollen germinated within 1h, but full germination was not reached until after 6 h. Both the delay in germination and the variation are consistent with its moderately dehydrated status at maturity, as well as with its single aperture and the need to initiate synthesis of the callose layer in the inner wall after pollination (Nepi and Pacini 1999; Nepi et al. 2001). Among angiosperm pollens with some degree of desiccation tolerance, however, germination within 1h is considered fairly rapid (Franchi et al. 2002). In Austrobaileya, relatively rapid hydration of pollen occurs in part because of the humid environment and the aperture structure. The likely route for initial hydration of the pollen grain is along the edge of the aperture, where the pectinaceous intine is most exposed to stigmatic secretions due to the highly reduced apertural exine and lack of an endexine layer (J. Heslop-Harrison and Y. Heslop-Harrison 1991).
Pollen longevity was not measured in this study, but it is likely to be at least 3 days, since pollen from late male-phase flowers still had >50 % in vitro germination. Austrobaileya vines grow in a wet tropical rainforest environment, and though they flower in the subcanopy during the driest month of the year (September), the relative humidity during this study averaged 86 %. Since pollen dispersal via insects may take only a few minutes and occurs in humid conditions, one might wonder why Austrobaileya pollen undergoes dehydration at all. In the populations I studied most flowers did not set fruit in each of three years, and most flowers that abscised received no pollen (Thien et al. 2009). If insect pollination is rare, then pollen limitation may explain why a moderately long period of pollen presentation has been maintained in Austrobaileya (Ashman 2004; Harder and Aizen 2010). Because pollen presentation lasts 3 days or more, individual pollens must maintain viability for at least a few days, and Austrobaileya pollen from old flowers was viable. Thus, the pollination ecology of Austrobaileya favours some degree of developmental arrest via dehydration in the face of pollinator uncertainty; and the humid environment allows weak desiccation protection mechanisms and hence fairly rapid germination.
Evolution of pollen germination timing and the origin of angiosperms
Among seed plants, the intensity of pollen competition is thought to have become greatly magnified in angiosperms (Mulcahy 1979). If so, then one prediction is that extant angiosperms should have faster pollen germination and faster pollen tube growth rates than other seed plants. Hoekstra (1983) used in vitro experiments to show that a broad sample of phylogenetically derived angiosperms had faster pollen germination and faster pollen tube growth rates than a number of species of conifers and Gnetales. Williams (2008, 2009) showed that in vivo pollen tube growth rates within some early-divergent lineages of angiosperms were faster than in vivo rates of representatives from all major non-flowering seed plant groups, but slower than or comparable to those of some monocots and eudicots. However, patterns of in vivo pollen germination timing in angiosperms are less well known.
A review of studies of in vivo pollen germination supports the prediction that both early-divergent and derived angiosperms should have shorter pollen germination times than non-flowering seed plants. In Austrobaileya, substantial in vivo pollen germination occurred within about an hour and a half. This is considered quite rapid relative to that of non-flowering seed plants, in which in vivo germination times range from 1 to 72 h in Gnetales, from 0.5 to 9 months in conifers (Williams 2009) and several days or more among cycads (Pettitt 1982; Choi and Friedman 1991). Nor is Austrobaileya unusual: among early-divergent angiosperms pollen germination occurs within 1–3 h in the woody perennials A. trichopoda (Williams 2009) and Illicium floridanum (Koehl et al. 2004; Williams 2009), and in 15 min or less in aquatics of the order Nymphaeales, such as C. caroliniana and Brasenia schreberi (Taylor and Williams 2009), Nymphaea odorata (Williams et al. 2010) and Trithuria austinensis and T. submersa (Taylor and Williams 2012). Many aspects of pollen structure of extant early-divergent angiosperms are similar to those of early Cretaceous fossil pollens (Endress and Honegger 1980; Zavada 1984; Hughes 1994; Sampson 2000; Hesse 2001; Doyle 2005), and furthermore, because of habitat tracking, many of these species still occupy environments similar to those present early in angiosperm history (Feild et al. 2009). Seen in this light, pollen germination timing of extant early-divergent angiosperms is a reasonable model for that of an ancestor of extant angiosperms.
Among monocots and eudicots, in vivo pollen germination in less than 30 min is considered rapid (Nepi et al. 2001; Franchi et al. 2002) and very rapid germinators (1–15 min) almost always have desiccation-sensitive pollen at maturity (Nepi et al. 2001). As noted above, all Nymphaeales studied to date are rapid germinators. Because desiccation-sensitive pollen has a very short lifespan (Dafni and Firmage 2000), pollen that germinates in >1 h is increasingly likely to have undergone some degree of dehydration before maturity. Among angiosperm pollens with some degree of desiccation tolerance, germination can last up to 60 h (Fig. 5A) or even 3 days (references in Nepi et al. 2001). Thus, pollen germination of Austrobaileya and other early-divergent woody angiosperms with desiccation-tolerant pollen is near the fastest possible (Nepi et al. 2001). These data from extant early-divergent lineages suggest that an acceleration of pollen germination occurred prior to or during the origin of extant angiosperms. As such, an evolutionary transition to a very short progamic phase in an ancestor of extant flowering plants (as reconstructed in Williams 2008) involved accelerations of both the pollen germination and pollen tube growth processes.
An interesting observation brought up by the literature review is that pollen germination requires such a consistent proportion of a progamic phase that varies from 30 min to over a year in duration (Fig. 5). This implies an evolutionary association between pollen germination speed and the duration of pollen tube growth, even though germination and tube growth are physiologically semi-autonomous (Addicott 1943). Furthermore, pollen hydration is a largely passive process and its duration is completely independent of one aspect of pollen tube growth duration: the distance tubes have to grow. The apparent correlated evolution of these traits is also surprising given that causes of evolution might be quite different on the stigma than within the reproductive tract.
There have been previous studies supporting the correlated evolution of germination speed and pollen tube growth rate: for example, it has often been noted that species with tricellular pollen have faster pollen germination and faster initial pollen tube growth rates than species with bicellular pollen (Brewbaker and Majumder 1961; Hoekstra and Bruinsma 1979; Hoekstra 1983; Mulcahy and Mulcahy 1983). An underlying cause of that observation is that tricellular pollen is usually desiccation sensitive and metabolically active at dispersal, whereas bicellular pollen is often desiccation tolerant and developmentally delayed. Thus, a more relevant test for evolutionary dissociation would be among pollens with some degree of desiccation tolerance and among those that are desiccation sensitive. Outside of model system taxa, few studies of pollen tube growth provide data on pollen hydration status, and virtually none on either water potential or metabolic rates of mature pollen (Heslop-Harrison 1979c; Taylor and Hepler 1997; Aylor et al. 2005; Rounds et al. 2011). Clearly, some aspects of pollen germination speed and pollen tube growth rate should be similar due to their shared metabolic machinery. However, if pollen competition has been important in a lineage over a long period, one might expect both processes to have approached their limits of acceleration, erasing indicators of evolutionary dissociation.
Despite these caveats, there are indications from the literature survey that pollen tube growth rates and pollen germination speed have evolved independently (future work will consider correlations in light of phylogenetic relationships). First, there are cases of long germination/short pollen tube growth periods and vice versa. In Talinum teretefolium pollen germination takes up 67 % of its 3-h-long progamic phase, whereas pollen tubes grow for only an hour at a very fast rate of 2–4 mm h−1 (Dubay 1981). Conversely, some species with very rapid germination of desiccation-tolerant pollen (e.g. Erythronium grandiflorum) or desiccation-sensitive pollen (e.g. A. cherimola) have very slow tube growth rates (<0.300 mm h−1) (Thomson 1989; Cruzan 1990; Lora et al. 2009, 2010).
Secondly, in groups with very short progamic phases (≤6 h), pollen germination required an almost 3-fold higher percentage of the progamic phase (14.8 %) than it did in all other groups (5.3 %) (Fig. 5). The species in the former group include grasses and asterids, which have some of the fastest known pollen tube growth rates in angiosperms. This suggests that in the group most likely to have been selected for rapid reproductive cycles, pollen tubes have had a great capacity to evolve ever faster growth rates even after pollen germination speed has approached a limit.
Finally, as noted earlier, pollen germination speeds in early-divergent angiosperms are not only faster than those of almost all non-flowering seed plants, but are already near the maxima reported for both desiccation-sensitive and desiccation-tolerant pollens of monocots and eudicots. Pollen tube growth rates of these same species are also much faster than those of any non-flowering seed plant, but such rates are near the minima, not the maxima, reported for monocots and eudicots (Williams 2009). These observations suggest that with the evolution of novel features of angiosperm pollen, germination speed evolved rapidly, approaching its maximum limit early in history, whereas pollen tube growth rates have evolved slowly, relative to their maximum potential. Nevertheless, pollen tube growth rates in Nymphaeales are 2–10 times faster than those of Amborella, Austrobaileya and other ancient woody perennials. Together, these observations suggest that the rapid progamic phases of early angiosperms evolved via accelerations of both germination and tube growth simultaneously but independently, as shown by patterns of dissociation that are present even among extant early lineages.
Conclusions and forward look
The progamic phase is a life history period whose duration is determined by interactions between male and female tissues. Flowering plants, from Amborella, Nymphaeales and Austrobaileyales to monocots and eudicots have remarkably short progamic phases, implying that the evolution of novel pollen–carpel interactions was involved in the speeding of fertilization. This study indicates that the pollen germination process of Austrobaileya is relatively rapid, and comparable in duration to those of other insect-pollinated early-divergent angiosperms as well as monocots and eudicots. Because pollen germination precedes pollen tube growth, pollen germination speed may have responded rapidly to the stronger pollen competition regimes brought on by the early origin(s) of insect pollination and larger pollen loads. We still know little about the evolutionary developmental relationship between germination speed and early and late pollen tube growth rates.
Sources of funding
This work was supported by National Science Foundation award IOS 1052291 to J.H.W.
Contributions by the authors
J.H.W. conceived of and carried out the work, and authored the article.
Conflict of interest statement
None declared.
Acknowledgements
I am grateful to Andrew Moffatt for sectioning pollen and taking several photographs (Fig. 2D–F), and to Matthew Lettre for lab assistance. I thank Taylor Feild for providing flowers from his greenhouse-grown Austrobaileya plant and the owner of private land near McHugh lookout, Millaa Millaa, for access to the population. I especially thank Bruce Sampson, Brian Murray and two anonymous reviewers for insightful comments. I greatly appreciate the academic support provided by Anne Gaskett and the School of Biological Sciences, University of Auckland, during the writing of this manuscript.
References
- Addicott FT. Pollen germination and pollen tube growth, as influenced by pure growth substances. Plant Physiology. 1943;18:270–279. doi: 10.1104/pp.18.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman TL. Flower longevity. In: Nooden LD, editor. Cell death in plants. London, UK: Elsevier; 2004. [Google Scholar]
- Aylor DE. Settling speed of corn (Zea mays) pollen. Journal of Aerosol Science. 2002;33:1601–1607. [Google Scholar]
- Aylor DE, Baltazar BM, Schoper JB. Some physical properties of teosinte (Zea mays subsp. parviglumis) pollen. Journal of Experimental Botany. 2005;56:2401–2407. doi: 10.1093/jxb/eri232. [DOI] [PubMed] [Google Scholar]
- Baker HG, Baker I. Starch in angiosperm pollen grains and its evolutionary significance. American Journal of Botany. 1979;66:591–600. [Google Scholar]
- Blackmore S, Wortley AH, Skvarla JJ, Rowley JR. Pollen wall development in flowering plants. New Phytologist. 2007;174:483–498. doi: 10.1111/j.1469-8137.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- Boavida LC, Vieira AM, Becker JD, Feijo JA. Gametophyte interaction and sexual reproduction: how plants make a zygote. International Journal of Developmental Biology. 2005;49:615–632. doi: 10.1387/ijdb.052023lb. [DOI] [PubMed] [Google Scholar]
- Bond WJ. The tortoise and the hare—ecology of angiosperm dominance and gymnosperm persistence. Biological Journal of the Linnean Society. 1989;36:227–249. [Google Scholar]
- Brewbaker JL, Kwak BH. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany. 1963;50:859–865. [Google Scholar]
- Brewbaker JL, Majumder SK. Cultural studies of the pollen population effect and the self-incompatibility inhibition. American Journal of Botany. 1961;48:457–464. [Google Scholar]
- Choi JS, Friedman WE. Development of the pollen tube of Zamia furfuracea (Zamiaceae) and its evolutionary implications. American Journal of Botany. 1991;78:544–560. [Google Scholar]
- Cruzan MB. Pollen-pollen and pollen-style interactions during pollen tube growth in Erythronium grandiflorum (Liliaceae) American Journal of Botany. 1990;77:116–122. [Google Scholar]
- Dafni A, Firmage D. Pollen viability and longevity: practical, ecological and evolutionary implications. Plant Systematics and Evolution. 2000;222:113–132. [Google Scholar]
- Dajoz I, Till-Bottraud I, Gouyon PH. Evolution of pollen morphology. Science. 1991;253:66–68. doi: 10.1126/science.253.5015.66. [DOI] [PubMed] [Google Scholar]
- Doyle JA. Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. Grana. 2005;44:227–251. [Google Scholar]
- Doyle JA, Donoghue MJ. Phylogenies and angiosperm diversification. Paleobiology. 1993;19:141–167. [Google Scholar]
- Dubay DT. Atlanta, GA: 1981. Interspecific differences in the effect of sulfur dioxide on angiosperm sexual reproduction. PhD Dissertation. [Google Scholar]
- Endress PK. The reproductive structures and systematic position of the Austrobaileyaceae. Botanische Jahrbücher für Systematik. 1980;101:393–433. [Google Scholar]
- Endress PK. Dispersal and distribution in some small archaic relic angiosperm families (Austrobaileyaceae, Eupomatiaceae, Himantandraceae, Idiospermoideae-Calycanthaceae) Sonderbände des Naturwissenschaftlichen Vereins in Hamburg. 1983a;7:201–217. [Google Scholar]
- Endress PK. The early floral development of Austrobaileya. Botanische Jahrbücher für Systematik. 1983b;103:481–497. [Google Scholar]
- Endress PK, Honegger R. The pollen of the Austrobaileyaceae and its phylogenetic significance. Grana. 1980;19:177–182. [Google Scholar]
- Favre-Ducharte M. Time relations and sexual reproduction in Cichorium and other angiosperms as compared with Archegoniates. Phytomorphology. 1979;29:166–178. [Google Scholar]
- Feild TS, Chatelet DS, Brodribb TJ. Ancestral xerophobia: a hypothesis on the whole plant ecophysiology of early angiosperms. Geobiology. 2009;7:237–264. doi: 10.1111/j.1472-4669.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- Franchi GG, Bellani L, Nepi M, Pacini E. Types of carbohydrate reserves in pollen: localization, systematic distribution and ecophysiological significance. Flora. 1996;191:143–159. [Google Scholar]
- Franchi GG, Nepi M, Dafni A, Pacini E. Partially hydrated pollen: taxonomic distribution, ecological and evolutionary significance. Plant Systematics and Evolution. 2002;234:211–227. [Google Scholar]
- Franchi GG, Piotto B, Nepi M, Baskin CC, Baskin JM, Pacini E. Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal, and survival. Journal of Experimental Botany. 2011;62:5267–5281. doi: 10.1093/jxb/err154. [DOI] [PubMed] [Google Scholar]
- Friedman WE. Sexual reproduction in Ephedra nevadensis (Ephedraceae): further evidence of double fertilization in a nonflowering seed plant. American Journal of Botany. 1990;77:1582–1598. [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. Diversity in obscurity: fossil flowers and the early history of angiosperms. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:369–382. doi: 10.1098/rstb.2009.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness CA, Rudall PJ. Pollen aperture evolution—a crucial factor in eudicot success? Trends in Plant Science. 2004;9:154–158. doi: 10.1016/j.tplants.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Harder LD, Aizen MA. Floral adaptation and diversification under pollen limitation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:529–543. doi: 10.1098/rstb.2009.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M. Pollen characters of Amborella trichopoda (Amborellaceae): a reinvestigation. International Journal of Plant Sciences. 2001;162:201–208. [Google Scholar]
- Heslop-Harrison J. Pollen walls as adaptive systems. Annals of the Missouri Botanical Garden. 1979a;66:813–829. [Google Scholar]
- Heslop-Harrison J. The grass pollen grain. Annals of Botany. 1979b;44(Suppl. 1):1–47. [Google Scholar]
- Heslop-Harrison J. Interpretation of the hydrodynamics of pollen. American Journal of Botany. 1979c;66:737–743. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Structural and functional variation in pollen intines. In: Blackmore S, Barnes SH, editors. Pollen and spores. Oxford, UK: Clarendon Press; 1991. Systematics Association Special Volume No. 44. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Germination of monocolpate angiosperm pollen: effects of inhibitory factors and the Ca2+-channel blocker, nifedipine. Annals of Botany. 1992;69:395–403. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Intracellular motility and the evolution of the actin cytoskeleton during development of the male gametophyte of wheat (Triticum aestivum L.) Philosophical Transactions of the Royal Society Series B: Biological Sciences. 1997;352:1985–1993. [Google Scholar]
- Heslop-Harrison Y, Heslop-Harrison J. Germination of monocolpate angiosperm pollen—evolution of the actin cytoskeleton and wall during hydration, activation and tube emergence. Annals of Botany. 1992;69:385–394. [Google Scholar]
- Hoekstra FA. Mitochondrial development and activity of binucleate and trinucleate pollen during germination in vitro. Planta. 1979;145:25–36. doi: 10.1007/BF00379924. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA. Physiological evolution in angiosperm pollen: possible role of pollen vigor. In: Mulcahy DL, Ottaviano E, editors. Pollen: biology and implications for plant breeding. Amsterdam, The Netherlands: Elsevier Science; 1983. [Google Scholar]
- Hoekstra FA, Bruinsma J. Respiration and vitality of binucleate and trinucleate pollen. Physiologia Plantarum. 1975;34:221–225. [Google Scholar]
- Hoekstra FA, Bruinsma J. Reduced independence of male gametophyte in angiosperm evolution. Annals of Botany. 1978;42:759–762. [Google Scholar]
- Hoekstra FA, Bruinsma J. Protein synthesis of binucleate and trinucleate pollen and its relationship to tube emergence and growth. Planta. 1979;146:559–566. doi: 10.1007/BF00388832. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Crowe LM, Crowe JH. Differential desiccation sensitivity of corn and Pennisetum pollen linked to their sucrose contents. Plant, Cell and Environment. 1989;12:83–91. [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. Mechanisms of plant desiccation tolerance. Trends in Plant Science. 2001;6:431–438. doi: 10.1016/s1360-1385(01)02052-0. [DOI] [PubMed] [Google Scholar]
- Hu SS, Dilcher DL, Jarzen DM, Taylor DW. Early steps of angiosperm-pollinator coevolution. Proceedings of the National Academy of Sciences of the USA. 2008;105:240–245. doi: 10.1073/pnas.0707989105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes NM. The enigma of angiosperm origins. Cambridge, UK: Cambridge University Press; 1994. [Google Scholar]
- Koehl V, Thien LB, Heij EG, Sage TL. The causes of self-sterility in natural populations of the relictual angiosperm, Illicium floridanum (Illiciaceae) Annals of Botany. 2004;94:43–50. doi: 10.1093/aob/mch113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lora J, Testillano PS, Risueno MC, Hormaza JI, Herrero M. Pollen development in Annona cherimola Mill. (Annonaceae). Implications for the evolution of aggregated pollen. BMC Plant Biology. 2009;9:129. doi: 10.1186/1471-2229-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lora J, Hormaza JI, Herrero M. The progamic phase of an early-divergent angiosperm, Annona cherimola (Annonaceae) Annals of Botany. 2010;105:221–231. doi: 10.1093/aob/mcp276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy DL. The rise of the angiosperms—a genecological factor. Science. 1979;206:20–23. doi: 10.1126/science.206.4414.20. [DOI] [PubMed] [Google Scholar]
- Mulcahy GB, Mulcahy DL. A comparison of pollen tube growth in bi- and trinucleate pollen. In: Mulcahy DL, Ottaviano E, editors. Pollen: biology and implications for plant breeding. Amsterdam, The Netherlands: Elsevier Science; 1983. [Google Scholar]
- Nepi M, Pacini E. What may be the significance of polysiphony in Lavatera arborea? In: Clement C, Pacini E, Audran J-C, editors. Anther and pollen: from biology to biotechnology. Berlin: Springer; 1999. [Google Scholar]
- Nepi M, Franchi GG, Pacini E. Pollen hydration status at dispersal: cytophysiological features and strategies. Protoplasma. 2001;216:171–180. doi: 10.1007/BF02673869. [DOI] [PubMed] [Google Scholar]
- Osborn JM, Taylor TN, Schneider EL. Pollen morphology and ultrastructure of the Cabombaceae—correlations with pollination biology. American Journal of Botany. 1991;78:1367–1378. [Google Scholar]
- Pacini E. From anther and pollen ripening to pollen presentation. Plant Systematics and Evolution. 2000;222:19–43. [Google Scholar]
- Pacini E, Hesse M. Pollenkitt—its composition, forms and functions. Flora. 2005;200:399–415. [Google Scholar]
- Pettitt JM. Ultrastructural and immuno-cytochemical demonstration of gametophytic proteins in the pollen tube wall of the primitive gymnosperm, Cycas. Journal of Cell Science. 1982;57:189–213. doi: 10.1242/jcs.57.1.189. [DOI] [PubMed] [Google Scholar]
- Rounds CM, Winship LJ, Hepler PK. Pollen tube energetics: respiration, fermentation and the race to the ovule. AoB PLANTS 2011. 2011 doi: 10.1093/aobpla/plr019. plr019; doi:10.1093/aobpla/plr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. New York, NY: Oxford University Press; 1999. [Google Scholar]
- Sampson FB. Pollen diversity in some modern magnoliids. International Journal of Plant Sciences. 2000;161:S193–S210. [Google Scholar]
- Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF, Refulio-Rodriguez NF, Walker JB, Moore MJ, Carlsward BS, Bell CD, Latvis M, Crawley S, Black C, Diouf D, Xi ZX, Rushworth CA, Gitzendanner MA, Sytsma KJ, Qiu YL, Hilu KW, Davis CC, Sanderson MJ, Beaman RS, Olmstead RG, Judd WS, Donoghue MJ, Soltis PS. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany. 2011;98:704–730. doi: 10.3732/ajb.1000404. [DOI] [PubMed] [Google Scholar]
- Speranza A, Calzoni GL, Pacini E. Occurrence of mono- or disaccharides and polysaccharide reserves in mature pollen grains. Sexual Plant Reproduction. 1997;10:110–115. [Google Scholar]
- Stebbins GL. Seeds, seedlings, and the origin of angiosperms. In: Beck CB, editor. Origin and early evolution of angiosperms. New York, NY: Columbia University Press; 1976. [Google Scholar]
- Stebbins GL. Comparative aspects of plant morphogenesis—a cellular, molecular, and evolutionary approach. American Journal of Botany. 1992;79:589–598. [Google Scholar]
- Takhtajan A. Neoteny and the origin of flowering plants. In: Beck CB, editor. Origin and early evolution of angiosperms. New York, NY: Columbia University Press; 1976. [Google Scholar]
- Tanaka I. Isolation of generative cells and their protoplasts from pollen of Lilium longiflorum. Protoplasma. 1988;142:68–73. [Google Scholar]
- Taylor DW, Hickey LJ. Evidence for and implications of an herbaceous origin for angiosperms. In: Taylor DW, Hickey LJ, editors. Flowering plant origin, evolution and phylogeny. New York, NY: Chapman & Hall; 1996. [Google Scholar]
- Taylor LP, Hepler PK. Pollen germination and tube growth. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Williams JH. Consequences of pollination syndrome evolution for postpollination biology in an ancient angiosperm family. International Journal of Plant Sciences. 2009;170:584–598. [Google Scholar]
- Taylor ML, Williams JH. Pollen tube development in two species of Trithuria (Hydatellaceae) with contrasting breeding systems. Sexual Plant Reproduction. 2012 doi: 10.1007/s00497-012-0183-6. doi:10.1007/s00497-012-0183-6. [DOI] [PubMed] [Google Scholar]
- Thien LB, Sage TL, Jaffre T, Bernhardt P, Pontieri V, Weston PH, Malloch D, Azuma H, Graham SW, McPherson MA, Rai HS, Sage RF, Dupre JL. The population structure and floral biology of Amborella trichopoda (Amborellaceae) Annals of the Missouri Botanical Garden. 2003;90:466–490. [Google Scholar]
- Thien LB, Bernhardt P, Devall MS, Chen ZD, Luo YB, Fan JH, Yuan LC, Williams JH. Pollination biology of basal angiosperms (ANITA grade) American Journal of Botany. 2009;96:166–182. doi: 10.3732/ajb.0800016. [DOI] [PubMed] [Google Scholar]
- Thomson JD. Germination schedules of pollen grains—implications for pollen selection. Evolution. 1989;43:220–223. doi: 10.1111/j.1558-5646.1989.tb04219.x. [DOI] [PubMed] [Google Scholar]
- Torabinejad J, Caldwell MM, Flint SD, Durham S. Susceptibility of pollen to UV-B radiation: an assay of 34 taxa. American Journal of Botany. 1998;85:360–369. [PubMed] [Google Scholar]
- Vithanage H, Knox RB. Pollen development and quantitative cytochemistry of exine and intine enzymes in sunflower, Helianthus annuus L. Annals of Botany. 1979;44:95–106. [Google Scholar]
- Walker JW, Doyle JA. Bases of angiosperm phylogeny—palynology. Annals of the Missouri Botanical Garden. 1975;62:664–723. [Google Scholar]
- Williams JH. Novelties of the flowering plant pollen tube underlie diversification of a key life history stage. Proceedings of the National Academy of Sciences of the USA. 2008;105:11259–11263. doi: 10.1073/pnas.0800036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH. Amborella trichopoda (Amborellaceae) and the evolutionary developmental origins of the angiosperm progamic phase. American Journal of Botany. 2009;96:144–165. doi: 10.3732/ajb.0800070. [DOI] [PubMed] [Google Scholar]
- Williams JH. Pollen tube growth rates and the diversification of flowering plant reproductive cycles. International Journal of Plant Sciences. 2012 in press. [Google Scholar]
- Williams JH, McNeilage RT, Lettre MT, Taylor ML. Pollen tube growth and the pollen-tube pathway of Nymphaea odorata (Nymphaeaceae) Botanical Journal of the Linnean Society. 2010;162:581–593. [Google Scholar]
- Willson MF, Burley N. Mate choice in plants: tactics, mechanisms, and consequences. Princeton, NJ: Princeton University Press; 1983. [Google Scholar]
- Zavada MS. Pollen wall development of Austrobaileya maculata. Botanical Gazette. 1984;145:11–21. [Google Scholar]