Abstract
Recent studies show that electrophysiological markers of auditory processing such as the cortical 100ms response (M100) and the mismatch field (MMF), derived from magnetoencephalography (MEG), might be used to identify children with autism spectrum disorders - M100 peak latency - and to stratify children with autism according to the degree of language impairment – mismatch field peak latency.
The present study examined the latencies of right superior temporal gyrus M100 and mismatch field in a cohort of children and young adolescents with specific language impairment (n=17), in comparison to age and non-verbal IQ matched typically developing controls (n=21). Neither group showed symptoms associated with autism.
Whereas M100 latency (reflecting early auditory processing) did not distinguish controls from children with specific language impairment, the later “change detection” mismatch field response was significantly delayed (by >50ms) in the specific language impairment group. Linear discriminant analysis confirmed the role of mismatch field latency (92%) but not M100 latency (8%) in distinguishing groups.
Present results add support to the claim that a delayed M100 is specific to autism spectrum disorders (with relative independence of degree of language impairment) and that a delayed mismatch field reflects an abnormality more generally associated with language impairment, suggesting that mismatch field delay in the present specific language impairment group and previously reported in autistic children with language impairment may be indicative of a common neural system dysfunction.
Keywords: Language impairment, magnetoencephalography, mismatch field
Introduction
Recent studies suggest that magnetoencephalography (MEG) measures of auditory processing distinguish children with autism spectrum disorders (ASD) from typically developing controls (TD) and, within the ASD spectrum, degree of language impairment (LI) [1–3]. In particular, delayed auditory M100 superior temporal gyrus responses to simple tone stimuli have been associated with autism spectrum disorders, whereas delayed superior temporal gyrus magnetic mismatch field (MMF) latencies, although prolonged in autism in general, show prolongation in the presence of clinically significant language impairment [1, 4]. Mismatch negativity (MMN), the electrical analog of magnetic mismatch field, has also been explored as an index of auditory and speech processing in specific language impairment (see [5] for a review). However, with low statistical power and methodological variation between studies, published results in this area have been inconsistent. In addition, whether these auditory abnormalities are specific for autism spectrum disorders or are instead indicative of neural system dysfunction shared across diagnoses (“common neural pathways”) is debated, as most published studies do not include clinical control groups [1, 3–5].
The present study examined the latency of superior temporal gyrus M100 and mismatch field responses in a cohort of children with specific language impairment free of symptoms associated with autism. Based on Roberts et al. [2], the first hypothesis was that M100 latency would not distinguish children with specific language impairment from controls. Based on Roberts et al. [1], the second hypothesis was that mismatch field latency (induced by tone or vowel changes in the auditory stream) would distinguish children with specific language impairment from controls, proportional to the degree of language impairment. Further, based on Naatanen and Kujala [6], we explored the degree to which observed latencies could be accounted for by general intelligence, indexed by a non-verbal IQ measure, the Perceptual Reasoning Index from the Wechsler Intelligence Scale for Children – 4th Edition.
Materials and Methods
All studies were performed with approval from the institutional review board and written informed consent was obtained from the participants’ parents and assent from the children.
Subjects
Seventeen children with specific language impairment (mean age 10±3yrs, 10M, 7F) were recruited from the Center for Childhood Communication at the Children’s Hospital of Philadelphia. Language and neuropsychological evaluations were performed by child neuropsychologists and speech-language pathologists and included the Clinical Evaluation of Language Fundamentals – 4th edition (CELF-4)[7] the Wechsler Intelligence Scale for Children – 4th edition (WISC-IV) [8], the Wechsler Individual Achievement Test – 2nd edition (WIAT-II) [9], and the Comprehensive Test of Phonological Processing (CTOPP) [10]. A cohort of 21 age-matched controls (mean age 10±3 yrs, 9M, 12F; ages did not differ between groups, p>0.05) had received identical MEG scanning protocols with successful recording of evaluable M100 and mismatch field responses. M100 and mismatch field measures from some but not all of the control subjects were previously reported in Roberts et al. (11 of 21) [2] and/or Roberts et al. (20 of 21)[1].
Assessment of concomitant autism spectrum symptoms, an exclusion criterion for both groups in the present study, included direct observation with the Autism Diagnostic Observation Schedule (ADOS) [11], the Krug Asperger’s Disorder Index (KADI)[12], and parent report on the Social Communication Questionnaire (SCQ) [13]. All subjects scored below ADOS, KADI, and SCQ cutoffs. Inclusion criteria for specific language impairment included clinically significant language impairment: CELF-4 core language index < 85 or either (a) a discrepancy of at least 1.5 standard deviations between their nonverbal IQ (WISC-IV Perceptual Reasoning Index) and their CELF-4 Core Language Index score, and/or (b) scores on two or more language-based subtests (including measures of language-based academic achievement on the WIAT-II, phonological processing on the CTOPP, and receptive and expressive language on the CELF-4) at least 1 SD below the mean. All controls had a CELF-4 Core Language Index >85. All subjects had a Full Scale IQ > 75.
MEG Scanning and Analysis
Recordings were performed at the Lurie Family Foundations’ MEG Imaging Center of the Department of Radiology at Children’s Hospital of Philadelphia in a magnetically shielded room using a whole-cortex 275-channel MEG system (VSM MedTech Inc., Coquitlam, British Columbia, Canada). At the start of the session, three head-position indicator coils were attached to the scalp to provide continuous specification of the position and orientation of the MEG sensors relative to the head. Foam wedges were inserted between the side of each subject’s head and the inside of the MEG dewar to increase subject comfort and ensure that the head remained in the same place in the dewar across recording sessions. To minimize fatigue and encourage an awake state, subjects viewed (but did not listen to) a movie projected on to a screen positioned at a comfortable viewing distance. To aid in the identification of eye-blink activity, the electro-oculogram (bipolar oblique, upper right and lower left sites) was collected. Electrodes were also attached to the left and right collarbone for electrocardiogram recording. After a band-pass filter (0.03 –150 Hz), electro-oculogram, electrocardiogram, and magnetoencephalogram signals were digitized at 1200 Hz with third order gradiometer environmental noise reduction for the magnetoencephalographic data.
To measure M100 latency, subjects were presented 105 500Hz sinusoidal tones (300ms duration, 900–1100ms inter-stimulus interval). To measure mismatch field, subjects were administered two oddball paradigms, using either 300 and 700Hz tones or /u/ and /a/ vowel stimuli, presented at 750ms inter-stimulus-interval (onset to onset). Auditory stimuli were presented using Eprime v1.1 (Psychology Software Tools Inc., Pittsburgh, Pennsylvania, USA), delivered via a sound pressure transducer and sound conduction tubing to the subject’s peripheral auditory canal via eartip inserts (ER3A, Etymotic Research, Elk Grove Village, Illinois, USA). Prior to the magnetoencephalography exam, each participant’s hearing threshold was determined, and the auditory stimuli were presented 45 dB above threshold (45 dB SL).
All analyses were performed blind to participant group, and have been previously described [1, 2]. Briefly, epochs 500ms pre- and post-stimulus for the 500Hz tone responses, and 130ms pre-stimulus to 470ms post-stimulus for the oddball paradigms were defined from the continuous recording. Artifact correction was applied to remove eye-blink and cardiac activity using BESA™ 5.2 (see methods in Roberts et al. [1,2]). Epochs with artifacts other than blinks and heartbeats were rejected on the basis of amplitude and gradient criteria (amplitude >1200fT/cm, gradients >800fT/cm/sample). Artifact-free epochs were then averaged according to stimulus type and filtered using a 1 Hz (6dB/octave, forward) to 40 Hz (48dB/octave, zero-phase) band-pass.
M100 source localization and latencies were determined as detailed in Roberts et al. [2]. Briefly, a standard multiple-dipole source model, including bilateral sources in the superior temporal gyri (as well as additional regional sources to account for artifacts and other activity) was used in BESA™ 5.2 to transform each subject’s raw MEG surface activity into brain source space (MEG data co-registered to the Montreal Neurologic Institute (MNI) averaged brain). Each subject’s final source model included eye-blink and heartbeat vectors. Left and right hemisphere dipoles were oriented at the maximum of M100 to optimize the orientation of the standard superior temporal gyrus source for each subject.
M100 latencies were determined from identification of the peak response with M100 topography between 90 and 180ms post stimulus in the right superior temporal gyrus 500 Hz source waveform (Fig. 1). Mismatch field peak latencies were determined as described in Roberts et al. [1]. Briefly, for each oddball pair of tone (300 and 700Hz) or vowel stimuli (/u/ and /a/), responses were obtained with each token as the standard (85%) or deviant (15%) stimulus. Subtraction of standard from deviant responses for each token created difference waves from which the mismatch field was identified (Fig. 1). As described in Roberts et al.[1], mismatch field latencies from each token were averaged across stimuli and hemispheres for each subject to yield a single mismatch field response measure.
Figure 1.
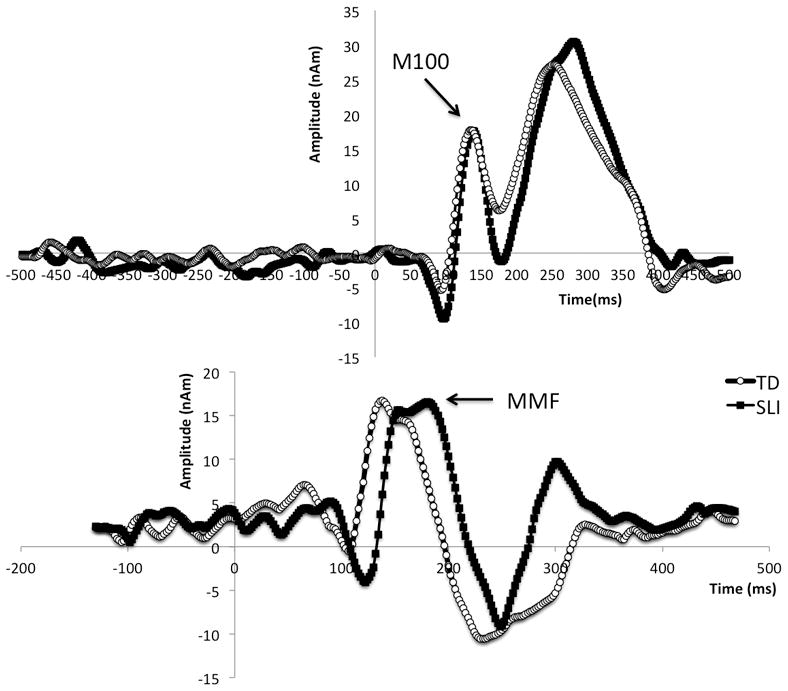
Representative waveforms from control subjects (open circles) and children with specific language impairment (solid dots) illustrating near-equivalent M100 response latencies (upper panel) and delayed mismatch field (MMF) in specific language impairment (lower panel).
Dependent variables, based on Roberts et al. [1, 2], were the right hemisphere 500 Hz superior temporal gyrus M100 latency and the average mismatch field latency. Given two tests, a Bonferroni correction was applied, providing a familywise corrected p-value of 0.025.
Statistical Analysis
For each dependent variable (M100 and mismatch field peak latencies), an age-covaried ANOVA was performed with diagnostic category as the between-subjects factor. As a secondary analysis, correlations were computed between mismatch field peak latency and CELF-4 core language index for the specific language impairment group. To assess the possible confounding effect of general intelligence, correlations were also examined after removing variance associated with the Perceptual Reasoning Index. Finally, to establish the relative contribution of M100 and mismatch field peak latencies in distinguishing specific language impairment and controls, a linear discriminant analysis was performed, incorporating both factors and using 5-fold cross validation, and the relative weighting of M100 and mismatch field peak latencies in the optimum solution were determined. Note, in n-fold cross-validation, the original sample is randomly partitioned into n subsamples (in this case 5) with each fold containing the same proportions of the two types of class labels. A fold is retained for testing / validation while the remaining n-1 folds are used to train the linear discriminant analysis function. This is repeated n times, and the results from these multiple runs are averaged to produce a single estimation.
Results
Specific language impairment and control groups did not differ on Perceptual Reasoning Index scores (controls: 109.5±3.2; specific language impairment: 100.8±3.6, p>0.05). As expected CELF-4 Core Language Index scores were significantly higher in controls than specific language impairment (controls: 108.4±2.4; specific language impairment: 81.8±2.6, p<0.001). A chi-squared analysis showed that the gender composition did not differ between groups (p>0.3).
M100 peak latency did not differ between the specific language impairment and control groups (age-corrected marginal means: controls: 133.7±3.0ms; specific language impairment: 141.0±3.6ms; p>0.1). After co-varying for Perceptual Reasoning Index (despite marginal although non-significant differences between groups), M100 latency still did not resolve specific language impairment from control groups (p>0.05). Mismatch field responses were later in the specific language impairment than control group (age-corrected marginal means: controls: 175.2±4.3ms; specific language impairment: 230.4±4.9ms; p<0.001) (Fig. 2). After co-varying for Perceptual Reasoning Index, MMF latency continued to differentiate the control and specific language impairment groups (p<0.001). Although correlations showed a negative relationship between mismatch field peak latency and CELF-4 Core Language Index in the specific language impairment group, this association was not significant. This is consistent with observations in Roberts et al. [1] for the language impaired autism spectrum disorder group.
Figure 2.
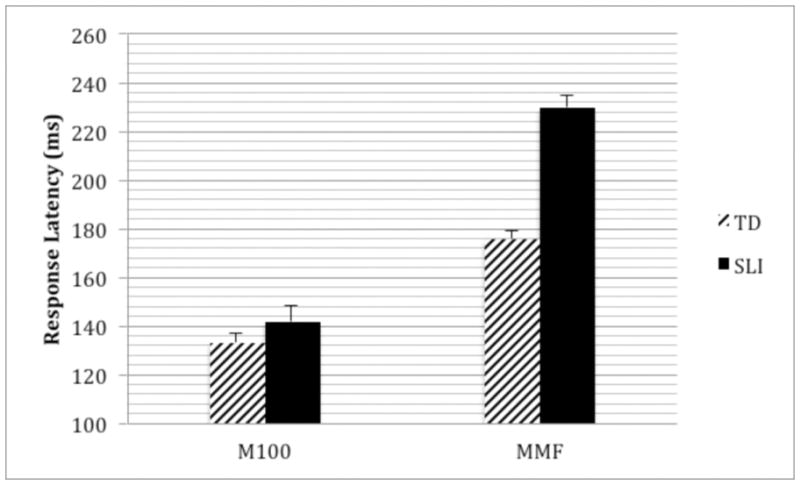
M100 and mismatch field (MMF) latencies for typically developing (TD) controls vs children with specific language impairment (SLI). Whereas there are no group differences in M100 peak latency, mismatch field peak latencies are significantly prolonged in specific language impairment versus controls (~50ms). The y axis shows age-corrected marginal means (represented at the mean age of 9.8yrs).
The 5-fold cross-validated linear discriminant function analysis showed between group classification accuracy of 89.1%, with sensitivity for specific language impairment of 84% and specificity of 92% (Fig. 3). The weighting of the factors in the optimum linear discriminant solution was heavily biased towards mismatch field peak latency (92%) and very little influenced by M100 peak latency (8%).
Figure 3.
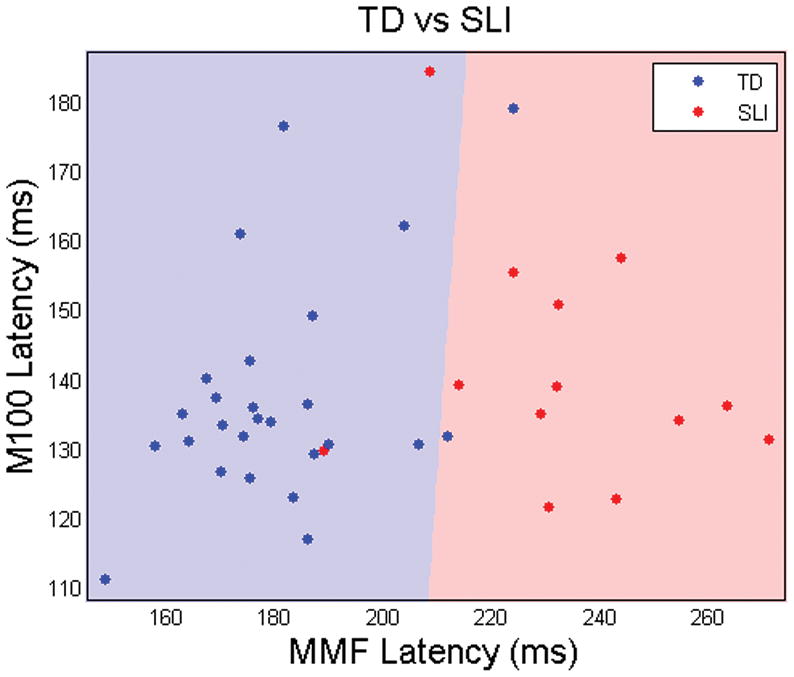
Age-corrected M100 and mismatch field peak latencies (blue dots=TD controls, red=SLI). Zones are shaded blue or red according to the response to that gridpoint from the 5-fold cross-validated linear discriminant function trained on the control and specific language impairment groups. The near vertical nature of the red/blue border reflects the major role of mismatch field peak latency and the minor role of M100 peak latency in predicting group membership.
Discussion
The main results of this study were:
M100 peak latency – a late latency auditory evoked neuromagnetic response - did not distinguish children with specific language impairment from controls. This is consistent with the notion of intact early auditory system detection and feature extraction in specific language impairment.
The later mismatch field peak response, elicited by oddball paradigms of tones or vowels, was significantly prolonged in specific language impairment than controls, supporting the interpretation that this response indexes auditory processes associated with language.
Considering previous studies, present findings add support to the hypothesis that a delayed M100 peak latency is associated with non-language features of autism spectrum disorder pathology. Note that there was a small and non-significant prolongation of the M100 peak latency in the specific language impairment group, suggesting that there may, however, be some overlap of early auditory cortex dysfunction in autism spectrum disorders and specific language impairment.
In contrast to M100 findings, mismatch field peak latencies were longer in specific language impairment than controls (by >50ms on average), results comparable to language impaired autism spectrum disorder versus control group differences reported in Roberts et al. [1]. This is further supported by the LDA analysis which suggests that the bulk (92%) of the discriminatory influence between groups stems from mismatch field peak latency differences with only a minor (8%) contribution from M100 peak latency. Of note, with reference to [1] and [6], this latency prolongation was still significant after covarying non-verbal IQ (Perceptual Reasoning Index), suggesting that the effect is not accounted for by general cognitive differences.
Taken together, present findings support a line of reasoning that considers M100 peak latency as a marker for autism spectrum disorders, perhaps secondary to abnormal white matter development of the acoustic radiations [14] or other local cortical circuitry abnormalities. Along this line of reasoning, Verhoeven et al. [15] recently reported reduced fractional anisotropy in the superior longitudinal fasciculus of patients with specific language impairment but not those with autism spectrum disorder and language impairment when compared to healthy controls. Mean superior longitudinal fasciculus fractional anisotropy was also correlated with language performance in specific language impairment, but similar relationships could not be established for either language impaired children with autism spectrum disorder or healthy controls. Similarly, despite the overlap in mismatch field peak latency between children with specific language impairment and prior studies of language impaired children with autism spectrum disorders [1], the lack of distinction on the basis of M100 might be considered consistent with Bishop and Frazier-Norbury [16], noting also that all children in this study considered in the specific language impairment group tested negative for autism spectrum disorder on the basis of tests including ADOS.
Conversely, mismatch field peak latency prolongation may index a dysfunction of auditory processing necessary for language, thus representing neural dysfunction common to language impairment in the setting of autism spectrum disorder and specific language impairment. It remains to be explored whether similar mismatch field differences are observed in other disorders characterized by language impairment. Whereas measures that provide diagnostic specificity are appealing, it is also useful to identify electrophysiological dysfunction common to multiple diagnoses, measures that perhaps reflect co-morbidity or neural circuit abnormalities common to multiple disorders. Such abnormalities might suggest similar targeted therapeutic intervention across disorders.
Conclusion
Whereas M100 peak latency did not distinguish children with specific language impairment from controls (in contrast to the M100 group differences reported between children with autism spectrum disorders and controls [2]), the mismatch field latency was delayed in the specific language impairment group, a delay also observed in children with autism spectrum disorders with concomitant language impairment [1]. These observations reinforce the specificity of M100 latency prolongation in autism spectrum disorders, and suggest that mismatch field latency is sensitive to a common neural system anomaly in language impaired children with autism spectrum disorders as well as in children with specific language impairment.
Acknowledgments
This work was supported by NIH R01-DC008871 (TR), and in part by the Nancy Lurie Marks Family Foundation and the Jeffery and Christina Lurie Family Foundation. Dr Roberts would like to thank the Oberkircher family for the Oberkircher Family Chair in Pediatric Radiology. Ragini Verma and Drew Parker were supported by R01-MH092862 (RV). This work is dedicated posthumously to Prof Judy Gravel who was instrumental in establishing this endeavor.
References
- 1.Roberts TP, et al. Auditory magnetic mismatch field latency: a biomarker for language impairment in autism. Biol Psychiatry. 2011;70:263–9. doi: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts TP, et al. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 2010;3:8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oram Cardy JE, et al. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 2005;16:521–5. doi: 10.1097/00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- 4.Oram Cardy JE, et al. Auditory evoked fields predict language ability and impairment in children. Int J Psychophysiol. 2008;68:170–5. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Bishop DV. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychol Bull. 2007;133:651–72. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- 6.Naatanen R, Kujala T. The Mismatch Negativity and Its Magnetic Equivalent: An Index of Language Impairment or More General Cognitive Decline in Autism? Biol Psychiatry. 2011;70:212–3. doi: 10.1016/j.biopsych.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Semel EM, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals (CELF-4) San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 8.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 9.Wechsler D. Wechsler individual achievement test. 2. San Antonio, Texas: The Psychological Corporation; 2001. [Google Scholar]
- 10.Wagner RK, et al. Comprehensive Test of Phonological Processing. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- 11.Lord C, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- 12.Krug D, Arick JR. Krug Asperger’s Disorder Index. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 13.Rutter M, Bailey A, Lloyd C. SCQ: Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 14.Roberts TP, et al. Developmental correlation of diffusion anisotropy with auditory-evoked response. Neuroreport. 2009;20:1586–91. doi: 10.1097/WNR.0b013e3283306854. [DOI] [PubMed] [Google Scholar]
- 15.Verhoeven JS, Rommel N, Prodi E, Leeman A, Zink I, Vandewalle E, et al. Is There a Common Neuroanatomical Substrate of Language Deficit between Autism Spectrum Disorder and Specific Language Impairment? Cereb Cortex. 2011 doi: 10.1093/cercor/bhr292. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Bishop DV, Frazier-Norbury C. Exploring the borderlands of autistic disorder and specific language impairment: a study using standardised diagnostic instruments. J Child Psychol Psychiatry. 2002;43:917–29. doi: 10.1111/1469-7610.00114. [DOI] [PubMed] [Google Scholar]


