Abstract
Influenza remains the single most important cause of excess disability and mortality during the winter months. In spite of widespread influenza vaccination programs leading to demonstrated cost-savings in the over 65 population, hospitalization and death rates for acute respiratory illnesses continue to rise. As a person ages, increased serum levels of inflammatory cytokines are commonly recorded (TNF-α, IL-1, IL-6). Termed “inflammaging”, this has been linked to persistent cytomegalovirus (CMV) infection and immune senescence, while increased anti-inflammatory cytokines (IL-10, TGF-β) are possibly associated with more healthy aging. Paradoxically, a shift with aging toward an anti-inflammatory (IL-10) response and decline in the IFN-γ:IL-10 ratio in influenza-challenged peripheral blood mononuclear cells is associated with a decline in the cytolytic capacity of CD8+ T cells responsible for clearing influenza virus from infected lung tissue. Thus, it is seemingly counter intuitive that the immune phenotype of healthy aging predicts a poor cell-mediated immune response and more serious outcomes of influenza. Herein we postulate a mechanistic link between the accumulation of late-stage, potentially terminally-differentiated T cells, many or most of which result from CMV infection, and the immunopathogenesis of influenza infection, mediated by granzyme B in older adults. Further, adjuvanted influenza vaccines that stimulate inflammatory cytokines and suppress the IL-10 response to influenza challenge, would be expected to enhance protection in the 65+ population.
Keywords: older adults, elderly, influenza, influenza vaccine, granzyme B, inflammation, CMV
1. Impact of Influenza in Older Adults
The decline of immune function in older people is a hallmark of aging and affects the ability of this vulnerable population to resist influenza and respond to vaccination. Influenza is foremost among all infectious diseases in the increase of age-related risk for serious complications. During the winter months, influenza is the probable cause of most of the excess mortality from cardiovascular diseases, strokes, diabetes, and pneumonia in the population aged 70 years and older [1, 2]. Of the current estimated 36,000 influenza deaths in the United States each year, 90% occur in older adults and most are related to cardiovascular and pneumonia complications of influenza A/H3N2 infections [3]. Furthermore, influenza-related hospitalizations (~200,000/year) are associated with a significant loss of independence in activities of daily living [4]. Specifically, influenza, pneumonia and the cardiovascular complications thereof are among the six leading causes of catastrophic disability in older adults [5]. The potential for losing as much as 50% of lower limb muscle strength (5% per day) during hospitalization [6–8], is an often unrecognized sequela associated with significant and long-lasting diminished quality of life. A rise in hospitalization rates and increased lengths of hospital stay for acute respiratory illnesses and cardiovascular diseases during the influenza season has also been found [9–11]. Given the burden of cardiovascular diseases, particularly heart failure in older adults, which peaks at a prevalence of 8% in the 70 years and older age group [12], the number of older persons who are permanently disabled from influenza-related illnesses will continue to increase.
2. Influenza Subtype and Risk for Influenza Illness
In older adults (compared to children and younger adults), A/H3N2 strains are by far the most common cause of hospitalization and death, followed by the B strains, and then A/H1N1 strains including the pandemic and previous seasonal strains [3, 11, 13–16]. This distinction becomes important in the clinical trials of new influenza vaccines; attack rates will be highest during the years in which A/H3N2 strains predominate as the circulating strain, and thus are more likely to detect a difference between the vaccines being compared. Further, increased disability from the complications of influenza illness will contribute to frailty in the population during the years when A/H3N2 strains circulate.
Older people have recently experienced somewhat of a reprieve from the impact of influenza when the usual seasonal strains were replaced by pandemic H1N1, which can be explained by the immunologic recall of the protection offered by their childhood exposure to influenza strains similar to pandemic A/H1N1. As we return to influenza seasons with an anticipated more prevalent circulation of A/H3N2 strains, the steadily increasing population aged 65 years and older, will again be vulnerable to seasonal influenza. Preventive measures taken, of which widespread influenza vaccination programs are the most prominent, often result in vaccine failures in this population [9]. This, along with the rising prevalence of conditions that lead to a high-risk for complicated influenza illness (vide infra), is a significant factor in the increased rates of influenza-related hospitalization and death in older adults.
3. Co-morbidity Burden and Influenza Risk
The Clinical Prediction Rule predicts risk for hospitalization or death due to pneumonia or influenza in older people. Notably, the most vulnerable older adults experience more than 60 times the risk of hospitalization and death compared to that of healthy adults aged 65 to 75 years. Not surprisingly, very advanced age, a prior hospital admission for influenza or pneumonia, and chronic conditions particularly those requiring monthly medical follow-up (specifically, chronic heart and lung conditions, renal diseases or transplant, dementia or stroke, and haematological and non-haematological cancer), were associated with the highest risk. Influenza vaccination was shown to reduce this risk by one third [17, 18]. However, rising hospitalization rates for influenza-like illness may be attributed to the increasing prevalence of chronic diseases and increased risk for complicated respiratory illness due to influenza and other respiratory viruses [4, 9].
4. Frailty and Influenza Risk
Frailty is a complex, dynamic and multifactorial syndrome in older adults that represents a reduction in physiological reserve, limited ability to resist environmental stressors, and increased risk of functional decline and mortality [19–22]. Physical frailty can be classified based on the presence of three or more of: slowed walking speed, self-reported exhaustion, grip strength, weight loss, and low physical activity [23], and has been associated with a loss of vaccine-mediated protection against influenza illness [24]. The Frailty Index, which measures the degree to which a person is frail [25], relates to the accumulation of deficits in 30 or more aspects of self-reported health including co-morbidities and functional status [26], and predicts mortality risk [27]. The Frailty Index has been shown to predict outcomes of chronic diseases such as heart failure [28], and the response to pneumococcal vaccination [29]. The Frailty Index can be used to evaluate the relationship between overall health status, biomarkers of immunologic age, and T cell correlates of protection, as predictors of clinical outcomes of vaccination in older adults.
5. Inflammaging, Immune Senescence, and Influenza Risk
An emerging theory is that “inflammaging”, the chronic elevation of inflammatory mediators during aging (See Figure 1), drives a number of adverse changes including those related to immune senescence [30, 31]. These inflammatory changes including high serum levels of IL-6 and increased frailty have been associated with persistent cytomegalovirus (CMV) infection [32]. Detected as the presence of antibody to CMV with a ca. 90% prevalence in ≥ 80 year olds [33]), CMV is postulated to drive T cells to late and terminally differentiated states reflected in the accumulation of potentially senescent T cells [34]. The serious complications of influenza have been attributed to age-related changes in T cell-mediated immunity, which have also been linked to persistent CMV infection and functional decline [35].
Figure 1.
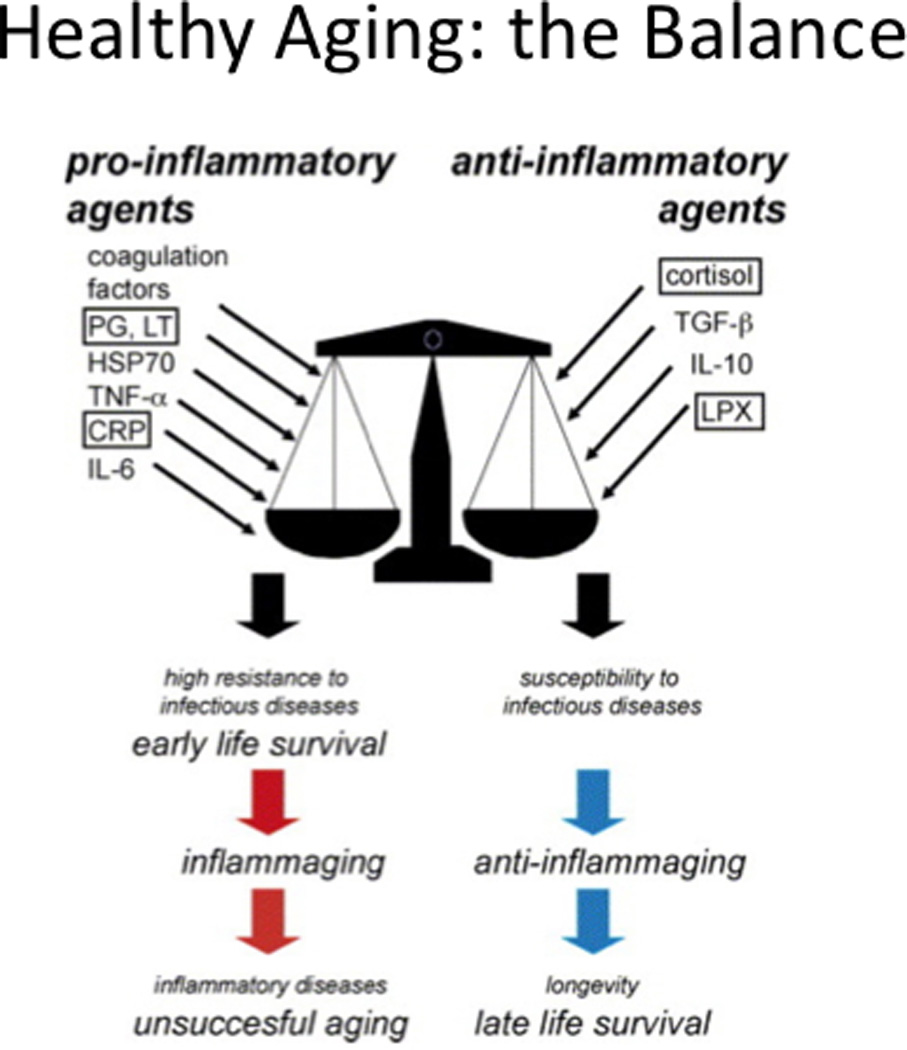
With permission reproduced from Franceschi C et al. [33]. Health aging: the Balance.
Thymic involution and a decline in naïve T cell output with increasing age, together with a lifetime of exposure to a variety of pathogens, leads to a dramatic reduction in the naïve T cell pool and a relative increase in the proportion of memory T cells. Within the total memory pool, the most notable functional changes occur in the CD8+ T cell (CTL) subset, where increased proportions of CD8+ T cells, which no longer express the co-stimulatory molecule, CD28, have been associated with poor antibody responses to influenza vaccination [36, 37], a decline in influenza-specific memory T cells [38], and seropositivity for CMV [39]. Indeed, it has been shown that most of these CD8+CD28− memory T cells are part of large clonal expansions that are specific for persistent viruses, mainly CMV [40]. However, a direct link between changes in CD8+ T cells related to CMV seropositivity, and the dramatic increase with age in the risk for vaccine failure and complicated influenza illness has yet to be made.
Terminally-differentiated (including potentially senescent) T cells upregulate IL-10 production [34], a cytokine that has been to suppress CTL responses [41]. Indeed, CMV itself has also been shown to encode a viral homolog of human IL-10, which may further contribute to dysregulated immune responses in old age [42]. In addition to the suppression of IFN-γ production, IL-10 has been shown to downregulate the expression of costimulatory molecules on antigen-presenting dendritic cells (DC), which limits the stimulation of T cell memory and has been correlated with poorer responses to influenza vaccination in the elderly [43]. Thus, downregulating IL-10 production and increasing viral antigen presentation could improve the CTL response to vaccination.
6. Effects of Late and Terminally Differentiated T cells and Extracellular Granzyme B
We have now developed a biomarker, based on an ex vivo assay indicating the baseline level of granzyme B (GrzB, a proapoptotic serine protease) activity in unstimulated T cells, called “bGrzB”. The abnormal expression of active GrzB in this context is highly correlated with the frequency of T cells with a terminally differentiated or senescent phenotype (unpublished observations). The levels of bGrzB acitivity in resting T cells are significantly increased in older adults who are seropositive for persistent CMV infection compared to seronegative age-matched controls (See Figure 2). In contrast, young adults have low levels of bGrzB activity independent of CMV serologic status (unpublished observations.
Figure 2.
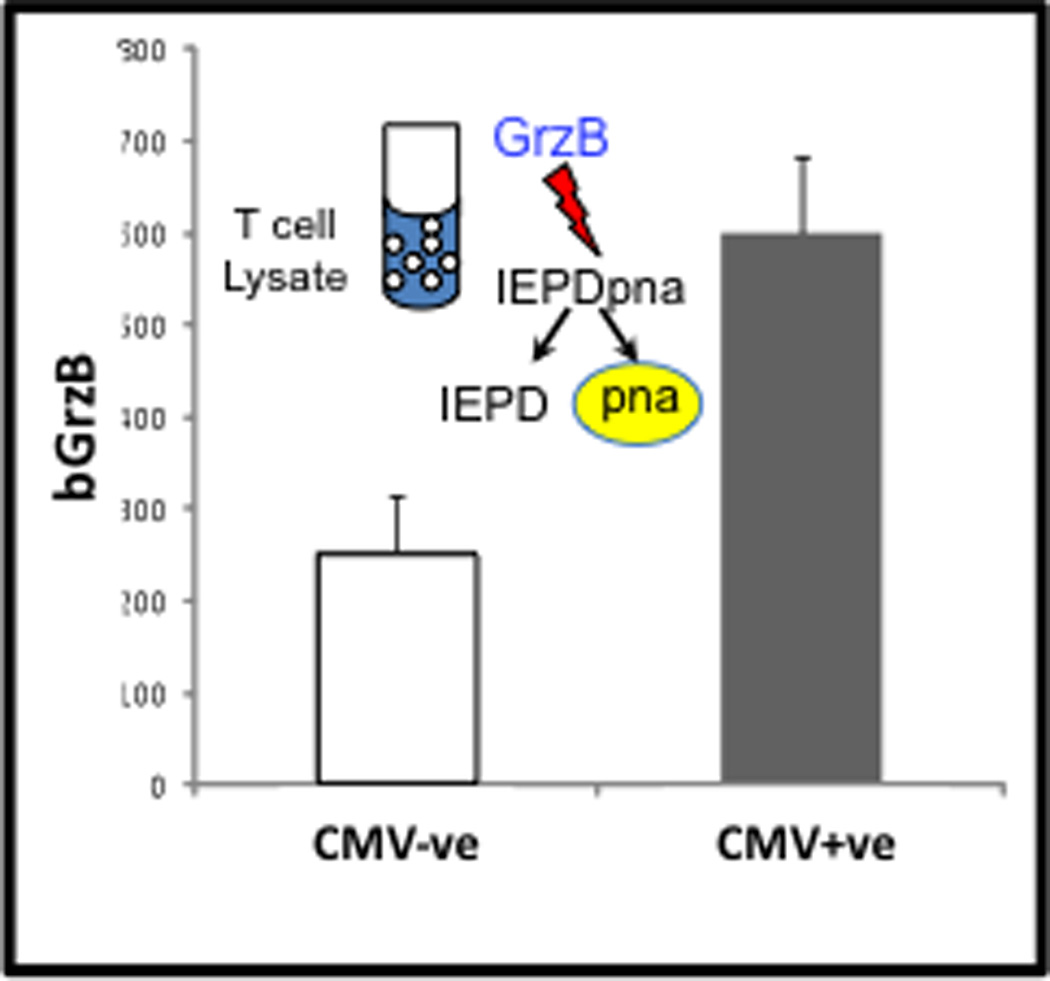
The GrzB assay is based on the enzyme’s unique substrate specificity to cleave the four amino acid sequence (IEPD) at the aspartate (D) residue releasing the paranitroanalide, which undergoes a colorimetric change detected on a plate reader. In this case, “bGrzB” is measured in lysates of unstimulated CD3+ T cells purified by magnetic bead selection. bGrzB activity is significantly higher in CMV+ vs. CMV− older adults (p=0.029) and corresponds to the high proportion of GrzB+ CD8+ T cells found in unstimulated PBMC shown in Figure 3.
The higher level of bGrzB activity in older compared to young adults is associated with an increase in the proportion of CD8+ T cells that express GrzB. Further, a greater proportion of these GrzB+ T cells from older compared to young adults degranulate in response to influenza challenge, suggesting some of them are non-specifically stimulated as bystanders in the process (See Figure 3 and [44]). In older adults with heart failure, these cells are degranulating and releasing GrzB in the absence of ex vivo stimulation (Figure 3). Such cytotoxic T lymphocytes (CTL) show no response to influenza vaccination; degranulation in the absence of virus stimulation may be related to in vivo exposure to the inflammatory environment associated with heart failure and the damaging effects of extracellular GrzB [45].
Figure 3.
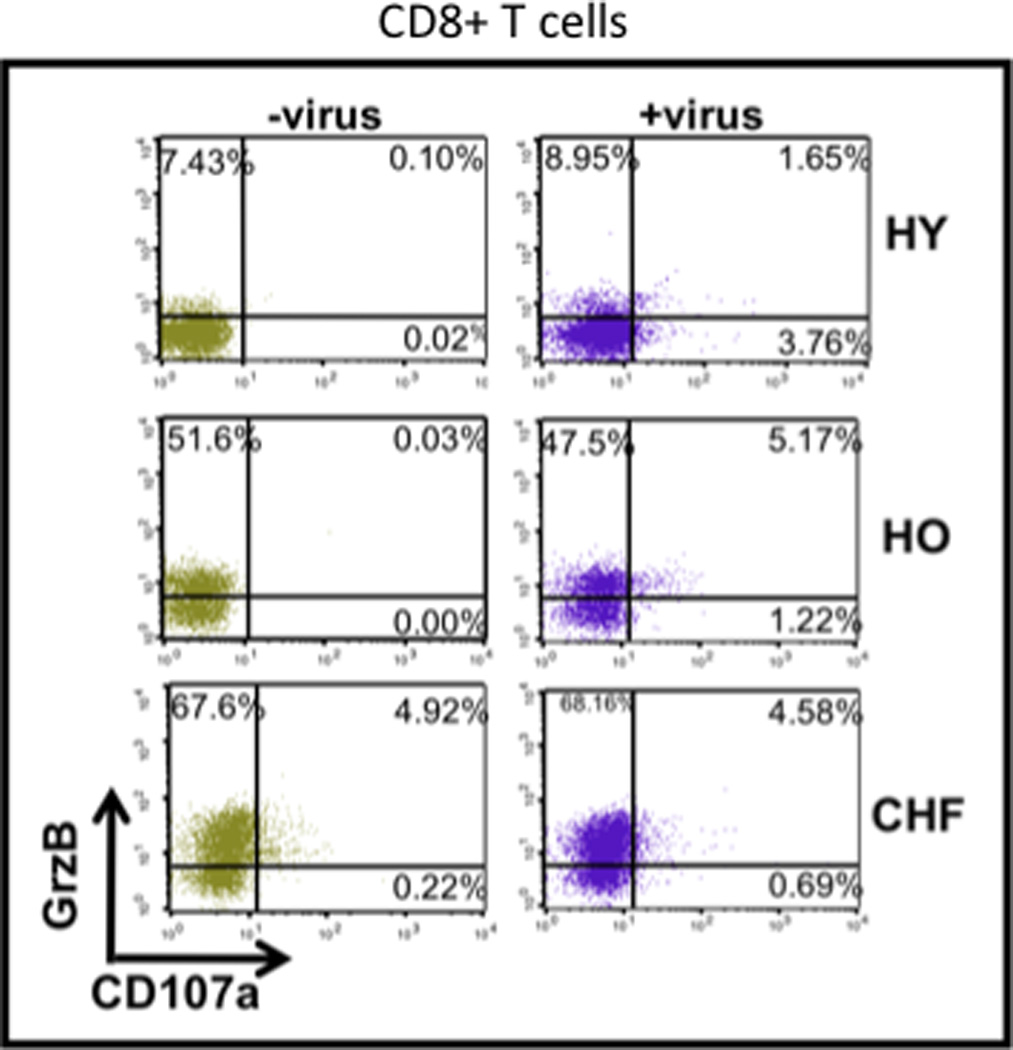
With permission, modified from McElhaney et al. [44]. Intracellular GrzB and the degranulation marker CD107a are shown for effector CD45RA+CD8+ T cells. Healthy young (HY) adults show low levels of GrzB+ cells (−virus) and degranulate (CD107a+) only in response to influenza stimulation (+virus). Healthy older adults (HO) vs. HY show a significantly increased proportion of GrzB+ cells (−virus) and higher proportions degranulate in response to influenza (+virus). Older adults with CHF (CHF) show a further increase in the proportion of GrzB+ cells, degranulate (CD107a+) in the resting state (in the absence of virus) and show no response to virus stimulation.
A high proportion of CD8+ T cells, express GrzB at baseline and do not co-express perforin (Perf) in response to influenza challenge [46]. In the absence of Perf, degranulation of GrzB and release into the extracellular space results in degradation of the extracellular matrix (ECM) and inflammation (See Figure 4). GrzB co-localizes with CD8+ T cells in atherosclerotic lesions leading to plaque instability by inducing apoptosis and/or anoikis of vascular smooth muscle cells and potentially inducing cleavage of extracellular matrix [47–50]. Extracellular GrzB has also been implicated in animal and human models of age-related dermatological, cardiovascular and pulmonary diseases [51–56]. Similarly in animal models, GrzB has been shown to be a key enzyme in tissue degradation that leads to aortic aneurysm rupture; rupture is delayed and survival increases in GrzB knock-out mice and in the presence of a biologic inhibitor of GrzB [54, 55]. Inflammatory responses stimulated by extracellular GrzB have recently been linked to its proteolytic cleavage of IL-1α, which enhances the biological activity of this cytokine and results in a 2-3-fold increase in IL-6, IL-8 and GM-CSF levels [56]. These results suggest that increased levels of bGrzB may be a common thread between immune dysfunction and the increased risk for serious illness including the cardiovascular complications of influenza infection in older adults.
Figure 4.
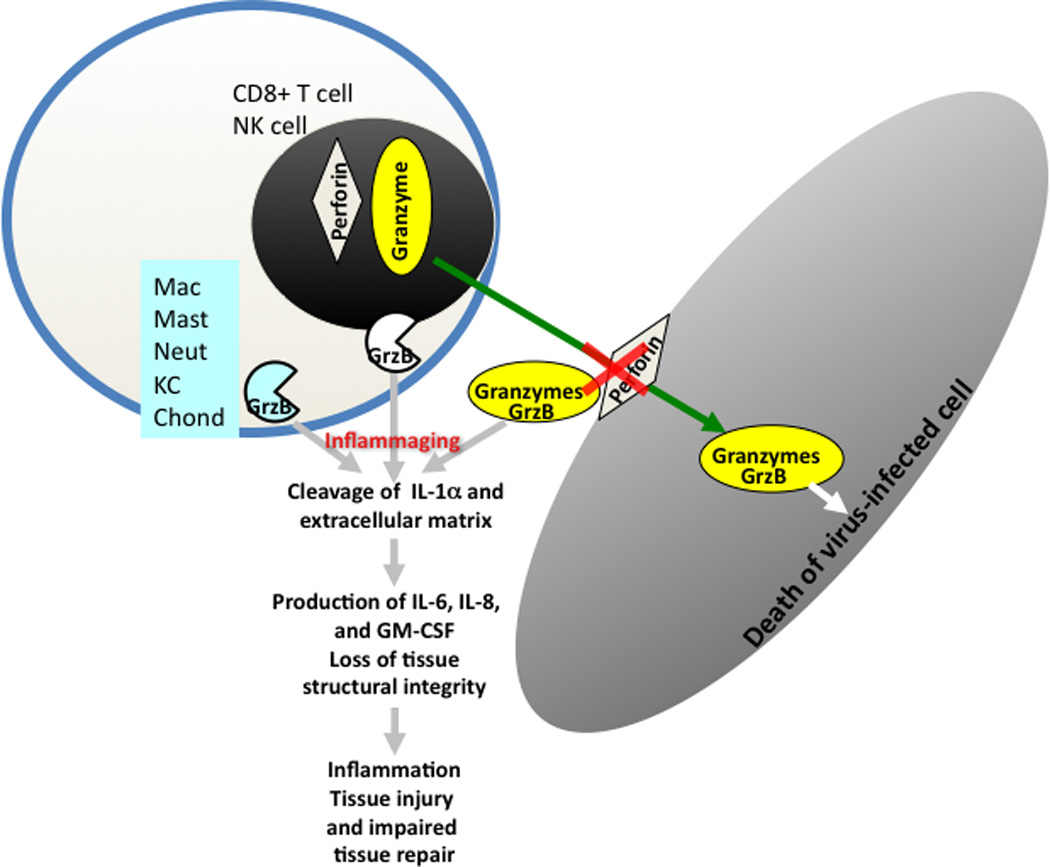
Granzyme B (GrzB) is released by CTL and NK cells to induce apoptosis of virus-infected cells and requires perforin for internalization into cells. Once inside the cell, GrzB induces apoptosis and this is referred to as the classical GrzB/apoptosis pathway. In the absence of perforin, GrzB is released into extracellular space (bGrzB is the biomarker) stimulating GrzB in other cells and causing cleavage of IL-1α and the extracellular matrix causing inflammation and loss of tissue integrity.
7. Immunopathogenesis in Influenza Infection
Inflammatory cytokines including tumour necrosis factor-alpha (TNF-α), and alpha and beta interferons (IFN-α, IFN-β) have been implicated in the pathogenesis of influenza infection. Although critical to the down-regulation of intracellular protein synthesis that limits new virus production, these cytokines along with interleukins (IL-1β, IL-6) produced to stimulate the adaptive immune response, are largely responsible for the systemic symptoms of influenza illness including fever and myalgia. Further, clinical observations of disease mediated by A/H3N2 vs. A/H1N1 or B strains of influenza have been linked to differential stimulation of cytokines in older adults [14]. In particular, higher plasma IL-6 levels correspond to increased respiratory symptom scores and temperature in community-acquired influenza A illness, in association with a number of cytokines which have also been found to be elevated [57]. Paradoxically, older people often do not mount a fever response to influenza infection even though IL-6 levels increase with age and in response to influenza challenge in their PBMC cultures (unpublished observations). This dysregulated response could be mediated by the release of GrzB and extracellular cleavage of IL-1α, resulting in increased levels of inflammatory cytokines, which stimulate an anti-inflammatory response and suppress CTL activity.
8. The Innate Immune Response to Influenza Infection
The innate immune response provides the first line of defence and is stimulated when virus escapes antibody binding and infects the cells of the respiratory tract. The innate immune response is activated within a few hours of infection and lasts for 1 to 2 days before the transition to the adaptive immune response. Type I interferons (IFN-α/β) are among the most important cytokines produced by the innate immune response and have several critical anti-viral functions, stimulating intracellular anti-viral proteins and inhibiting synthesis of cellular proteins, to prevent viral replication. Interferons also recruit monocytes/macrophages. An age-related decline in the IFN-α response to influenza virus has been related to a defect in Toll-like receptor (TLR) signalling, specifically TLR7, which is a receptor for single-stranded RNA derived from influenza virus [58]. The function of TLR on macrophages and dendritic cells is to register the danger signals of invading pathogens, which also contributes to the generation of cytokines involved in innate and adaptive immune mechanisms. Although the expression of TLR declines with aging [59], the functional consequence of this age-related change remains an area of controversy requiring further investigation [60]. Clearly, the role of TLR appears to be important in antigen presentation and the transition to an adaptive immune response to more specifically target clearance of the virus. Defective TLR7 signalling and IFN-α responses to influenza would suggest a critical defect in innate immune function that would facilitate the establishment and spread of influenza to the lungs, contributing to the increased risk for severe influenza infection in older adults.
9. The Adaptive Immune Response during Influenza Infection
Influenza vaccination or natural infection stimulates antibodies to the surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), which bind to the virus to respectively prevent infection of or release from the host cell. Antibodies cannot gain access to the intracellular environment and once infection occurs, the capacity of antibody response to limit the spread of infection may be overwhelmed by the large numbers of virus particles released from infected host cells. In older adults, it appears that the traditional role of vaccines in providing antibody-mediated protection against infection, or “sterilizing immunity”, is replaced by T-cell mediated clearance of the virus once infection occurs, thus providing “clinical protection” against disease.
IFNγ is an important cytokine in the defense against viral infections while IL-10 suppresses these defense mechanisms. Our work has shown a decline with aging in the IFNγ:IL-10 ratio in response to influenza virus challenge [61]. Further, the IFNγ:IL-10 ratio correlates with protection against influenza in older adults [62]. The importance of T cell-mediated clinical protection against influenza in older adults is increasingly recognized and has underscored the importance of including cellular immune measures in the assessment of vaccine efficacy in the over 65 population [63]. Age-related changes in T cell function are associated with a decline in the antibody response to influenza vaccination [36, 37] but mechanistic links with loss of protection against influenza illness yet to be clearly defined.
Virus-specific killing to clear influenza from the lungs is mediated by granzymes contained in granules within CTL. Granules migrate to the “immune synapse” between the activated CTL and the virus-infected target cell, and through a process facilitated by perforin (Perf), granzymes are transported across the cell membrane into the cytoplasm of the infected host cell (See Figure 4). GrzB is a key element in the enzymatic cascade that leads to apoptotic cell death [64] and clearance of influenza virus from the lungs [65, 66]. In older adults, GrzB activity in virus-stimulated PBMC correlates with protection against influenza [44, 62] but its co-expression with Perf is diminished in the elderly [67]. We have found that there is an age-related decline in the magnitude and duration of the CD8+ T cell response to the current influenza vaccines; by 10 weeks post-vaccination, there is a significant reduction in the proportion of cells that co-express perforin with GrzB and this is associated with a dramatic decline in their cytolytic activity (Summarized in Figure 5 from [46]). These results correspond to low level GrzB activity in influenza-challenged PBMC, which not only predicts increased risk of influenza illness [44, 62], but also illness severity [68] in older adults. However, given that older adults can mount a robust GrzB response to influenza vaccination following a recent influenza infection [44], this suggests that the defect in CD8+ T cell memory is reversible and could be exploited in the design of new influenza vaccines.
Figure 5.
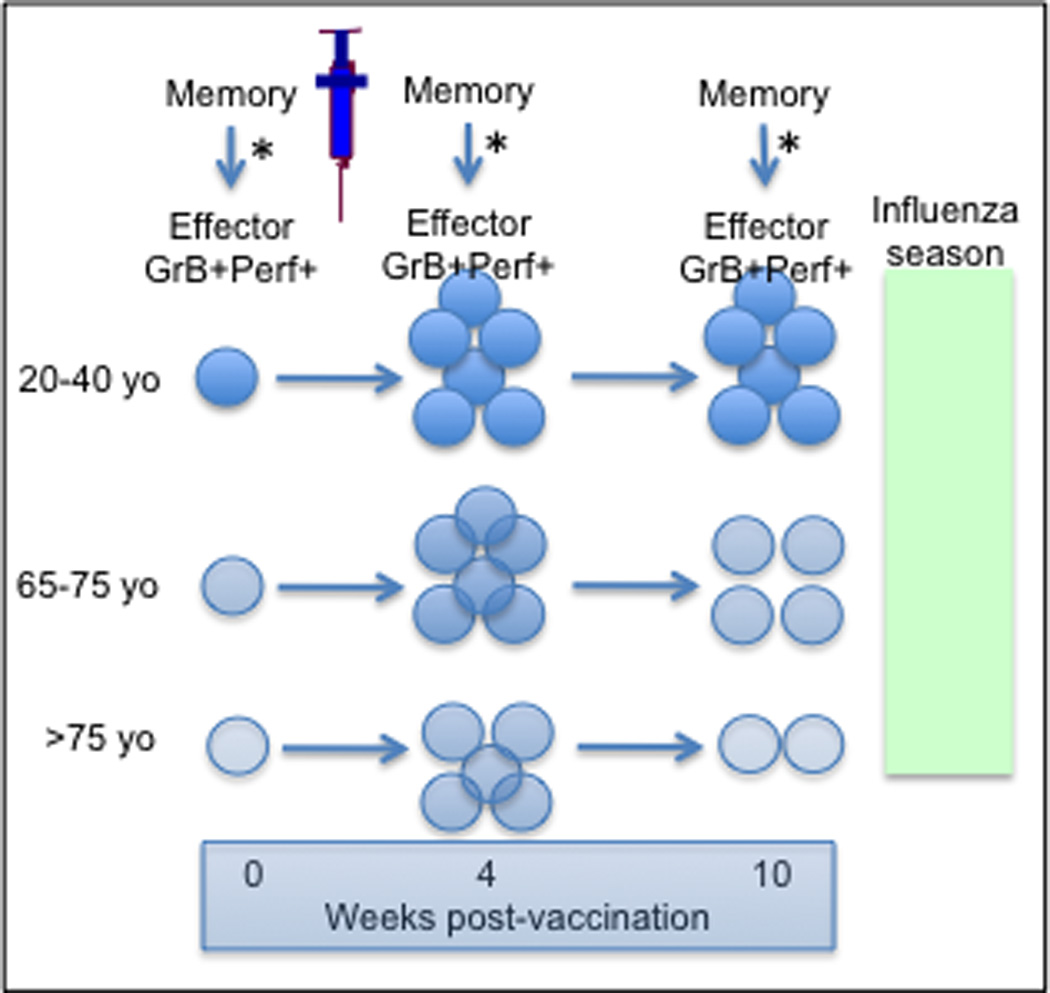
Summary of flow cytometry results from [46]. The response to influenza vaccination is shown as the change in memory T cell frequency and the generation of Effector T cells in response to ex vivo influenza challenge (*). Cytotoxicity of Effectors declines with age and with time after vaccination (lighter colour = less cytotoxicity). The Memory CD8+ T cell frequency is similar at 4 weeks post-vaccination but declines by 10 weeks post-vaccination in older compared to young adults. Thus, CD8+ T cell immunity through vaccination is lost by the time of the influenza season in older adults.
10. Improving Protection Against Influenza
Influenza vaccination programs have been shown to be cost-effective in older people and even cost saving in developed countries. While annual vaccination is the mainstay of influenza management, it is apparent that current influenza vaccines are not keeping pace with the changing risk profile of an aging population [69–81]. A concerted effort to develop more effective influenza vaccines for the 65+ population [82, 83] requires an increased understanding of how these vaccines can be designed to stimulate a cell-mediated response that improves protection especially against serious illness.
In the development of new influenza vaccines, the inclusion of internal proteins of the virus including matrix (M) and nucleoproteins (NP) are necessary to stimulate a CTL (CD8+ T cell) response. In this regard, it is important to realize that the current split-virus vaccines (SVV) differ from subunit influenza vaccines (SuV) with respect to the protein composition: SVV contain all the virus proteins including the internal proteins, whereas SuV contain only the surface proteins, hemaglutinin (HA) and neuraminidase (NA). In vitro evidence suggests that SVV are superior to SuV for activating human T cell responses and that this is dependent on the internal protein content [84]. Studies of CTL epitopes (viral peptides presented on the Major Histocompatibility Complex (MHC I) of the antigen-presenting or virus-infected cell which stimulate influenza-specific CTL) derived from the various influenza A proteins have predicted 167 epitopes within conserved regions on influenza proteins [85, 86]. Of these epitopes only 1 is on HA and 7 on NA. NP contains 17 putative epitopes and an additional 142 epitopes are on the polymerase or matrix proteins, which are not present in SuV. CTL are in general effectively induced by natural infection, which is mimicked by vaccination with live attenuated virus and to some extent by SVV in older adults [43]. However, the often used SuV do not induce any response to internal proteins, and the current killed vaccines are prepared from cloning HA and NA into the PR8 virus that originates from 1934. Thus, when using current vaccines, T cell responses against internal proteins are induced only against cross-specific epitopes if live attenuated or split virus vaccines are used. Optimal re-stimulation of CTL memory may be achieved by including the internal proteins of currently circulating influenza strains in the production of SVV.
Given the potential for TLR agonists as vaccine adjuvants to improve the cell-mediated immune response, we tested several TLR agonists in vitro and found that the TLR4 agonist, Glucopyranosyl Lipid Adjuvant - Stable Emulsion (GLA-SE) could improve DC antigen-presenting capacity when added to the current split-virus influenza vaccine (SVV). It thereby improved the T cell response to influenza challenge in humans similar to what had been found in animal studies [87]. Indeed, we have shown in a pre-clinical model using human PBMC that treatment of PBMC with GLA-SE/SVV compared to SVV alone, suppressed the IL-10 response (10-fold reduction) to live influenza challenge and was associated with a significant increase in GrzB activity [88]. This suggests that the increased IL-10 response to influenza challenge, which is associated with persistent CMV infection, may be a key mediator of a suppressed CTL response to influenza vaccination in older adults. Our future studies are designed to determine how terminally differentiated GrzB+ T cells interact with inflammatory mediators to dysregulate the T cell response to influenza. This may explain the increased risk of serious influenza illness and how defective T cell responses in older adults could be reversed in the design of new vaccines.
11. Pathways to Developing New Influenza Vaccines
Haemagglutination inhibition (HI) assays are the current industry standard for measuring antibody responses as a correlate of protection to estimate vaccine efficacy. Largely supported by our work showing that antibody titers against influenza may fail to predict protection in older adults [44, 62], there has been a paradigm shift in understanding the limitations of antibody titers as a sole measure of influenza efficacy in older adults [63, 89–93]. Specifically, HI assays may detect an age-related decline in the antibody response that would predict a loss of vaccine efficacy [94, 95] but these results have not been directly linked to vaccine failure in older adults. Even in young adults, post-vaccination titers alone may fail to predict vaccine failures [96]. Virus neutralization assays are gaining acceptance as a more functional assay but have not been established as correlates of protection.
In addition to stimulating an antibody response to HA and NA, current split-virus vaccines effectively stimulate T helper cells, and vaccination of healthy older people increases IL-2 to levels comparable to those in young adults [97–99]. In contrast, IFN-γ [100], IL-10 and the IFN-γ:IL-10 ratio [61] all decline with aging. IFN-γ ELISPOTS, cytotoxicity assays, and intracellular cytokine staining of CD4+ and CD8+ T cells have been used but none have been shown to correlate with protection in older adults. Validated assays of the IFN-γ:IL-10 ratio and the GrzB response to influenza challenge [101] have yet to used as correlates of protection in vaccine trials. Thus, we are left with HI assays of the antibody response to influenza vaccination as a surrogate of vaccine efficacy to advance new influenza vaccines through the different phases of clinical trials. However, the superiority of these novel vaccines over current split virus formulations will need to be proven in trials with PCR-confirmed influenza as an endpoint. Given the pitfalls of using antibody responses to predict protection in older adults, this becomes a major risk in the conduct of Phase III clinical trials requiring in excess of 40,000 older adults to demonstrate superiority over existing SVV. Correlates of protection based on the cell-mediated immune response may complement antibody responses in screening for the best vaccine candidates earlier in the vaccine development pipeline.
12. Summary
Understanding how genetic, environmental and systemic biomarkers of inflammaging interact in their relationship to the onset of frailty and accelerated immune senescence is the subject of ongoing studies [30, 102]. The effects of inflammatory cytokines, IL-6, IL-8, IL-15, and TNF-α contribute to protection from infectious diseases but increased serum levels of IL-6 and CMV antibody have been associated with an increased incidence of frailty [103]. To counterbalance this effect, increased levels of anti-inflammatory cytokines such as IL-10 have been associated with successful aging but diminished resistance to infectious diseases. This paradox may be related to the limitations of serologic biomarkers – are we observing the cause or effect of a dysregulated immune response especially under conditions of an acute infection?
We postulate that late-stage, potentially terminally-differentiated T cells resulting from CMV infection are recruited to the site of infection but have no cytolytic potential. Further, the release of GrzB from these cells into the extracellular environment potentiates the inflammatory response and stimulates anti-inflammatory cytokines that suppress cytolytic T cells and viral clearance. Designing new influenza vaccines to reduce the IL-10 response to influenza infection and more effectively stimulate CTL for early clearance of the virus will be important for improved protection in older adults. Continued reliance on antibody titers as a sole surrogate of vaccine efficacy remains a major barrier to this strategy for vaccine development in the 65+ population.
Highlights.
In older adults:
As part of the “inflammaging” process, innate immune processes may not only be ineffective, they may result in harmful inflammatory responses to infection.
Increased levels of “bGrzB” activity associated with CMV, may be a common thread between immune dysfunction and the risk for cardiovascular complications of influenza.
The IFN-γ:IL-10 ratio and GrzB activity in influenza-challenged PBMC can be used as biomarkers to firmly establish vaccine-mediated protection/failure
Adding specific adjuvants may further enhance vaccine responses even in the face of a suboptimal immune system.
Reliance on antibody titers as a sole predictor of vaccine efficacy is a major barrier to the development of more effective influenza vaccines, which remains an unmet need.
Acknowledgements
The research (JEM) was funded by Operating Grant Number: FRN-89729 from the Canadian Institutes of Health Research (CIHR), and Grant Number: R01 AI68265 and U01 AI074449 from the National Institutes of Health, and the National Institute of Allergy and Infectious Diseases. The Network of Centres of Excellence in Vaccines and Immunotherapeutics (CANVAC) in Canada supported the development of the granzyme B (GrzB) assay, and the CIHR supported the validation of the GrzB activity and cytokine assays according to International Conference on Harmonisation Guidelines. Research (GP) was funded by the Deutsche Forschungsgemeinschaft (DFG PA 361/14-1), the Bundesministerium für Bildung und Forschung (BMBF GerontoSys project GerontoShield, 0315890F) and the European Commission (FP7 project IDEAL, contract no. 259679).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Janet McElhaney has participated on advisory boards for GlaxoSmithKline, Sanofi Pasteur, Novartis, Med-Immune, and Abbott, and on data monitoring boards for Sanofi Pasteur; she has received research grants from the Canadian Institutes of Health Research, the US National Institute of Allergy and Infectious Diseases, BC Lung Association, GlaxoSmithKline, and Merck Frosst; has participated in clinical trials sponsored by Merck, GlaxoSmithKline and Sanofi Pasteur, has received honoraria and travel and accommodation reimbursements for presentations sponsored by Merck, GlaxoSmithKline and Sanofi Pasteur, and travel and accommodation reimbursements for participation on a publication steering committee for GlaxoSmithKline. The bGrzB biomarker has a provisional patent application number, UBC 11-098. H. Keipp Talbot has received research funding from Sanofi Pasteur and Protein Sciences. Graham Pawelec has participated on advisory boards for Sanofi MSD and has received honoraria and travel and accommodation reimbursements for presentations sponsored by Sanofi Pasteur and GlaxoSmithKline; he has received research grants from the Deutsche Forschungsgemeinschaft, the Bundesministerium für Bildung und Forschung and the European Commission,
References
- 1.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160(5):492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 2.Fleming DM, Elliot AJ. The impact of influenza on the health and health care utilisation of elderly people. Vaccine. 2005 Jul 8;23(Suppl 1):S1–S9. doi: 10.1016/j.vaccine.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005 Apr 28;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci L, Guralnik JM, Pahor M, Corti MC, Havlik RJ. Hospital diagnoses, Medicare charges, and nursing home admissions in the year when older persons become severely disabled. Jama. 1997 Mar 5;277(9):728–734. [PubMed] [Google Scholar]
- 6.Muller EA. Influence of training and of activity on muscle strength. Arch Phys Med Rehabil. 1970;51(8):449–462. [PubMed] [Google Scholar]
- 7.Harper CM, Lyles YM. Physiology and complications of bed rest. J Am Geriatr Soc. 1988;36:1047–1054. doi: 10.1111/j.1532-5415.1988.tb04375.x. [DOI] [PubMed] [Google Scholar]
- 8.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. Jama. 2007;297(16):1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 9.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. Jama. 2000;283(4):499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 10.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000;181(3):831–837. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 11.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. Jama. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 12.Chow CM, Donovan L, Manuel D, Johansen H, Tu JV. Regional variation in self-reported heart disease prevalence in Canada. Can J Cardiol. 2005 Dec;21(14):1265–1271. [PubMed] [Google Scholar]
- 13.Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358(9291):1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 14.Seo SH, Webby R, Webster RG. No apoptotic deaths and different levels of inductions of inflammatory cytokines in alveolar macrophages infected with influenza viruses. Virology. 2004;329(2):270–279. doi: 10.1016/j.virol.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Update: influenza activity - United States, 2009-10 season. Mmwr. Jul 30;59(29):901–908. [PubMed] [Google Scholar]
- 16.Update: influenza activity - United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR. Apr 16;59(14):423–430. [PubMed] [Google Scholar]
- 17.Hak E, Nordin J, Wei F, Mullooly J, Poblete S, Strikas R, et al. Influence of high-risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed-care organizations. Clin Infect Dis. 2002;35(4):370–377. doi: 10.1086/341403. Epub 2002 Jul 19. [DOI] [PubMed] [Google Scholar]
- 18.Hak E, Wei F, Nordin J, Mullooly J, Poblete S, Nichol KL. Development and validation of a clinical prediction rule for hospitalization due to pneumonia or influenza or death during influenza epidemics among community-dwelling elderly persons. J Infect Dis. 2004;189(3):450–458. doi: 10.1086/381165. Epub 2004 Jan 23. [DOI] [PubMed] [Google Scholar]
- 19.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol. 2007 Jul;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strandberg TE, Pitkala KH. Frailty in elderly people. Lancet. 2007 Apr 21;369(9570):1328–1329. doi: 10.1016/S0140-6736(07)60613-8. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol. 2004 Mar;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 22.Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, et al. Aging, frailty and age-related diseases. Biogerontology. 2010 Oct;11(5):547–563. doi: 10.1007/s10522-010-9287-2. [DOI] [PubMed] [Google Scholar]
- 23.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 24.Yao X, Hamilton RG, Weng NP, Xue QL, Bream JH, Li H, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011 Jul 12;29(31):5015–5021. doi: 10.1016/j.vaccine.2011.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stretton CM, Latham NK, Carter KN, Lee AC, Anderson CS. Determinants of physical health in frail older people: the importance of self-efficacy. Clin Rehabil. 2006 Apr;20(4):357–366. doi: 10.1191/0269215506cr946oa. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc. 2010 Feb;58(2):318–323. doi: 10.1111/j.1532-5415.2009.02667.x. [DOI] [PubMed] [Google Scholar]
- 27.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010 Apr;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 28.Muzzarelli S, Leibundgut G, Maeder MT, Rickli H, Handschin R, Gutmann M, et al. Predictors of early readmission or death in elderly patients with heart failure. Am Heart J. 2010 Aug;160(2):308–314. doi: 10.1016/j.ahj.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, et al. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009 Mar 4;27(10):1628–1636. doi: 10.1016/j.vaccine.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007 Jan;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011 Jul;10(3):319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010 May 15;171(10):1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006 Nov 1;43(9):1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 34.Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009 Aug;21(4):440–445. doi: 10.1016/j.coi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Moro-Garcia MA, Alonso-Arias R, Lopez-Vazquez A, Suarez-Garcia FM, Solano-Jaurrieta JJ, Baltar J, et al. Relationship between functional ability in older people, immune system status, and intensity of response to CMV. Age (Dordrecht, Netherlands) Apr 13; doi: 10.1007/s11357-011-9240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75(24):12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 38.Xie D, McElhaney JE. Lower GrB+ CD62Lhigh CD8 TCM effector lymphocyte response to influenza virus in older adults is associated with increased CD28null CD8 T lymphocytes. Mech Ageing Dev. 2007 May-Jun;128(5–6):392–400. doi: 10.1016/j.mad.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005 Mar;79(6):3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006 Feb 15;176(4):2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 41.Elrefaei M, Ventura FL, Baker CA, Clark R, Bangsberg DR, Cao H. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol. 2007 Mar 1;178(5):3265–3271. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins C, Garcia W, Godwin MJ, Spencer JV, Stern JL, Abendroth A, et al. Immunomodulatory properties of a viral homolog of human interleukin-10 expressed by human cytomegalovirus during the latent phase of infection. J Virol. 2008 Apr;82(7):3736–3750. doi: 10.1128/JVI.02173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Duin D, Allore HG, Mohanty S, Ginter S, Newman FK, Belshe RB, et al. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007 Jun 1;195(11):1590–1597. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- 44.McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, et al. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27:2418–2425. doi: 10.1016/j.vaccine.2009.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito Y, Kondo H, Hojo Y. Granzyme B as a novel factor involved in cardiovascular diseases. J Cardiol. 2011 Mar;57(2):141–147. doi: 10.1016/j.jjcc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011 Mar 3;29(11):2169–2177. doi: 10.1016/j.vaccine.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chamberlain CM, Granville DJ. The role of Granzyme B in atheromatous diseases. Can J Physiol Pharmacol. 2007 Jan;85(1):89–95. doi: 10.1139/y06-090. [DOI] [PubMed] [Google Scholar]
- 48.Hendel A, Cooper D, Abraham T, Zhao H, Allard MF, Granville DJ. Proteinase inhibitor 9 is reduced in human atherosclerotic lesion development. Cardiovasc Pathol. 2011 Feb 4; doi: 10.1016/j.carpath.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Choy JC, McDonald PC, Suarez AC, Hung VH, Wilson JE, McManus BM, et al. Granzyme B in atherosclerosis and transplant vascular disease: association with cell death and atherosclerotic disease severity. Mod Pathol. 2003 May;16(5):460–470. doi: 10.1097/01.MP.0000067424.12280.BC. [DOI] [PubMed] [Google Scholar]
- 50.Choy JC, Hung VH, Hunter AL, Cheung PK, Motyka B, Goping IS, et al. Granzyme B induces smooth muscle cell apoptosis in the absence of perforin: involvement of extracellular matrix degradation. Arterioscler Thromb Vasc Biol. 2004 Dec;24(12):2245–2250. doi: 10.1161/01.ATV.0000147162.51930.b7. [DOI] [PubMed] [Google Scholar]
- 51.Hendel A, Hiebert PR, Boivin WA, Williams SJ, Granville DJ. Granzymes in age-related cardiovascular and pulmonary diseases. Cell Death Diff. 2010 Apr;17(4):596–606. doi: 10.1038/cdd.2010.5. [DOI] [PubMed] [Google Scholar]
- 52.Granville DJ. Granzymes in disease: bench to bedside. Cell Death Diff. 2010 Apr;17(4):565–566. doi: 10.1038/cdd.2009.218. [DOI] [PubMed] [Google Scholar]
- 53.Hiebert PR, Boivin WA, Abraham T, Pazooki S, Zhao H, Granville DJ. Granzyme B contributes to extracellular matrix remodeling and skin aging in apolipoprotein E knockout mice. Exp Gerontol. 2011 Jun;46(6):489–499. doi: 10.1016/j.exger.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Chamberlain CM, Ang LS, Boivin WA, Cooper DM, Williams SJ, Zhao H, et al. Perforin-independent extracellular granzyme B activity contributes to abdominal aortic aneurysm. Am J Pathol. 2010 Feb;176(2):1038–1049. doi: 10.2353/ajpath.2010.090700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ang LS, Boivin WA, Williams SJ, Zhao H, Abraham T, Carmine-Simmen K, et al. Serpina3n attenuates granzyme B-mediated decorin cleavage and rupture in a murine model of aortic aneurysm. Cell Death Dis. 2011;2:e209. doi: 10.1038/cddis.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Afonina IS, Tynan GA, Logue SE, Cullen SP, Bots M, Luthi AU, et al. Granzyme B-Dependent Proteolysis Acts as a Switch to Enhance the Proinflammatory Activity of IL-1alpha. Mol Cell. 2011 Oct 21;44(2):265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001;64(3):262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 58.Canaday DH, Amponsah NA, Jones L, Tisch DJ, Hornick TR, Ramachandra L. Influenza-induced production of interferon-alpha is defective in geriatric individuals. J Clin Immunol. 2010 May;30(3):373–383. doi: 10.1007/s10875-010-9374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010 Mar 1;184(5):2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, et al. Murine [corrected] myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006 Dec;5(6):473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 61.Skowronski DM, Hottes TS, McElhaney JE, Janjua NZ, Sabaiduc S, Chan T, et al. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. The Journal of infectious diseases. 2011;203:158–167. doi: 10.1093/infdis/jiq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006 May 15;176(10):6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 63.Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25(4):599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 64.Darmon AJ, Nicholson DW, Bleackley RC. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme. B. Nature. 1995;377(6548):446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 65.Johnson BJ, Costelloe EO, Fitzpatrick DR, Haanen JB, Schumacher TN, Brown LE, et al. Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8+ T cells in influenza virus-infected mice. Proc Natl Acad Sci U S A. 2003;100(5):2657–2662. doi: 10.1073/pnas.0538056100. Epub 003 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005 May 1;174(9):5332–5340. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- 67.Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, et al. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12(8):770–777. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. 2010 Aug 31;28(38):6145–6151. doi: 10.1016/j.vaccine.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006 Apr;35(2):337–344. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- 70.Jackson LA, Nelson JC, Benson P, Neuzil KM, Reid RJ, Psaty BM, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006 Apr;35(2):345–352. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- 71.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007 Oct;7(10):658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 72.Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008 Aug 2;372(9636):398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 73.Nelson JC, Jackson ML, Weiss NS, Jackson LA. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among seniors. J Clin Epidemiol. 2009 Jul;62(7):687–694. doi: 10.1016/j.jclinepi.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Baxter R, Lee J, Fireman B. Evidence of bias in studies of influenza vaccine effectiveness in elderly patients. J Infect Dis. Jan 15;201(2):186–189. doi: 10.1086/649568. [DOI] [PubMed] [Google Scholar]
- 75.Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009 Sep 1;170(5):650–656. doi: 10.1093/aje/kwp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994;331(12):778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 77.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348(14):1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 78.Hak E, Hoes AW, Nordin J, Nichol KL. Benefits of influenza vaccine in US elderly--appreciating issues of confounding bias and precision. Int J Epidemiol. 2006 Jun;35(3):800–802. doi: 10.1093/ije/dyl068. author reply 799–800. [DOI] [PubMed] [Google Scholar]
- 79.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007 Oct 4;357(14):1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 80.Jansen AG, Sanders EA, Nichol KL, van Loon AM, Hoes AW, Hak E. Decline in influenza-associated mortality among Dutch elderly following the introduction of a nationwide vaccination program. Vaccine. 2008 Aug 21; doi: 10.1016/j.vaccine.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. Oct 25; doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 82.Nichol KL. Challenges in evaluating influenza vaccine effectiveness and the mortality benefits controversy. Vaccine. 2009 Oct 23;27(45):6305–6311. doi: 10.1016/j.vaccine.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L. Influenza vaccination and mortality benefits: new insights, new opportunities. Vaccine. 2009 Oct 23;27(45):6300–6304. doi: 10.1016/j.vaccine.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 84.Co MD, Orphin L, Cruz J, Pazoles P, Green KM, Potts J, et al. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine. 2009 Jan 7;27(2):319–327. doi: 10.1016/j.vaccine.2008.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol. 1999;162(12):7578–7583. [PubMed] [Google Scholar]
- 86.Wang M, Lamberth K, Harndahl M, Roder G, Stryhn A, Larsen MV, et al. CTL epitopes for influenza A including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine. 2007 Apr 12;25(15):2823–2831. doi: 10.1016/j.vaccine.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 87.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS ONE. 2010;5(10):e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Behzad H, Huckriede A, Haynes L, Gentleman B, Coyle K, Wilschut JC, et al. GLA-SE, a synthetic TLR4 agonist, enhances T cell responses to influenza vaccine in older adults. J Infect Dis Epub. 2011 Dec 5; doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine. 1999;17(1):82–94. doi: 10.1016/s0264-410x(98)00117-0. [DOI] [PubMed] [Google Scholar]
- 90.Gardner EM, Bernstein ED, Dran S, Munk G, Gross P, Abrutyn E, et al. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine. 2001;19(32):4610–4617. doi: 10.1016/s0264-410x(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 91.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37(2–3):427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 92.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Op Immunol. 2009 Aug;21(4):418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McElhaney JE. Prevention of infectious diseases in older adults through immunization: the challenge of the senescent immune response. Exp Rev Vaccines. 2009;8(5):593–606. doi: 10.1586/erv.09.12. [DOI] [PubMed] [Google Scholar]
- 94.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011 Jul;10(3):330–335. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011 Aug 1;121(8):3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohmit SE, Petrie JG, Cross RT, Johnson E, Monto AS. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis. 2011 Dec;204(12):1879–1885. doi: 10.1093/infdis/jir661. [DOI] [PubMed] [Google Scholar]
- 97.McElhaney JE, Meneilly GS, Beattie BL, Helgason CD, Lee SF, Devine RD, et al. The effect of influenza vaccination on IL2 production in healthy elderly: implications for current vaccination practices. J Gerontol. 1992;47(1):M3–M8. doi: 10.1093/geronj/47.1.m3. [DOI] [PubMed] [Google Scholar]
- 98.McElhaney JE, Meneilly GS, Lechelt KE, Bleackley RC. Split-virus influenza vaccines: do they provide adequate immunity in the elderly? J Gerontol. 1994;49(2):M37–M43. doi: 10.1093/geronj/49.2.m37. [DOI] [PubMed] [Google Scholar]
- 99.McElhaney JE, Meneilly GS, Pinkoski MJ, Lechelt KE, Bleackley RC. Vaccine-related determinants of the interleukin-2 response to influenza vaccination in healthy young and elderly adults. Vaccine. 1995;13(1):6–10. doi: 10.1016/0264-410x(95)80003-v. [DOI] [PubMed] [Google Scholar]
- 100.Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine. 1999;17(1):82–94. doi: 10.1016/s0264-410x(98)00117-0. [DOI] [PubMed] [Google Scholar]
- 101.Gijzen K, Liu WM, Visontai I, Oftung F, van der Werf S, Korsvold GE, et al. Standardization and validation of assays determining cellular immune responses against influenza. Vaccine. 2010 Mar 4;28(19):3416–3422. doi: 10.1016/j.vaccine.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 102.Franceschi C, Olivieri F, Marchegiani F, Cardelli M, Cavallone L, Capri M, et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Ageing Dev. 2005 Feb;126(2):351–361. doi: 10.1016/j.mad.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 103.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005 May;53(5):747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]


