Abstract
Oxons are the bioactivated metabolites of organophosphorus insecticides formed via cytochrome P450 monooxygenase-catalyzed desulfuration of the parent compound. Oxons react covalently with the active site serine residue of serine hydrolases, thereby inactivating the enzyme. A number of serine hydrolases other than acetylcholinesterase, the canonical target of oxons, have been reported to react with and be inhibited by oxons. These off-target serine hydrolases include carboxylesterase 1 (CES1), CES2, and monoacylglycerol lipase. Carboxylesterases (CES, EC 3.1.1.1) metabolize a number of xenobiotic and endobiotic compounds containing ester, amide, and thioester bonds and are important in the metabolism of many pharmaceuticals. Monoglyceride lipase (MGL, EC 3.1.1.23) hydrolyzes monoglycerides including the endocannabinoid, 2-arachidonoylglycerol (2-AG). The physiological consequences and toxicity related to the inhibition of off-target serine hydrolases by oxons due to chronic, low level environmental exposures are poorly understood. Here, we determined the potency of inhibition (IC50 values; 15 min preincubation, enzyme and inhibitor) of recombinant CES1, CES2, and MGL by chlorpyrifos oxon, paraoxon and methyl paraoxon. The order of potency for these three oxons with CES1, CES2, and MGL was chlorpyrifos oxon > paraoxon > methyl paraoxon, although the difference in potency for chlorpyrifos oxon with CES1 and CES2 did not reach statistical significance. We also determined the bimolecular rate constants (kinact/KI) for the covalent reaction of chlorpyrifos oxon, paraoxon and methyl paraoxon with CES1 and CES2. Consistent with the results for the IC50 values, the order of reactivity for each of the three oxons with CES1 and CES2 was chlorpyrifos oxon > paraoxon > methyl paraoxon. The bimolecular rate constant for the reaction of chlorpyrifos oxon with MGL was also determined and was less than the values determined for chlorpyrifos oxon with CES1 and CES2 respectively. Together, the results define the kinetics of inhibition of three important hydrolytic enzymes by activated metabolites of widely used agrochemicals.
Keywords: Carboxylesterase, Monoglyceride lipase, Monoacylglycerol lipase, Organophosphate, Oxon, Bimolecular rate constant
Introduction
Organophosphorus (OP) pesticides have been used extensively since the 1960s for agricultural and domestic purposes. Although their use has declined since the development of the synthetic pyrethroids, they are still used in significant amounts. As recently as 2001, the EPA estimated 73 million pounds of OP insecticides were used in the United States (Kiely et al., 2004). At least one of the dialkyl phosphate metabolites (DAP) of OP insecticides was found in 50% of the United States general population in urine samples collected between 2003–2004 (http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf ). The presence of DAP in urine indicates recent exposure to an OP insecticide. Children between 6–11 years of age had significantly higher levels of DAP than did adults and adolescents (Barr et al., 2004). Young children are more likely to be exposed to OP insecticides because they eat, drink and breathe more per unit of body weight than do adults and adolescents (National Research Council (U.S.) Committee on Pesticides in the Diets of Infants and Children., 1993). In addition, prenatal exposure to OP insecticides has been associated with abnormal primitive reflexes (Engel et al., 2007) and possibly with mental development (Eskenazi et al., 2007). These findings emphasize the importance of accurately modeling OP insecticide metabolism post exposure in order to aid in risk assessment.
Organophosphorus oxons, the active metabolites of many OP insecticides, exert their acute toxicity by inhibiting acetylcholinesterase via phosphorylation of the serine residue in the catalytic site (reviewed by Aldridge, 1996; Ebichon, 1996; Mileson et al., 1998). These oxons also react with other off-target proteins, many of which are members of the serine hydrolase superfamily (Aldridge, 1953; Maxwell, 1992). In fact, the covalent reaction of oxons with CES1, a serine hydrolase found in large quantities in the human liver, is one mechanism by which these compounds are detoxified and removed (Maxwell, 1992). Because CES1 is predominantly expressed in human liver, it metabolizes a range of xenobiotics containing ester, amide, and thioester bonds, e.g. ester prodrugs. CES1 is also one of several enzymes that is proposed to be responsible for the neutral cholesteryl ester hydrolase activity in macrophages (reviwed by Ghosh et al., 2010), which regulates the liberation of free cholesterol from cholesteryl esters stored in cytoplasmic lipid droplets for eventual transport to the liver and subsequent excretion (reverse cholesterol transport). Furthermore, we recently showed that CES1 regulates the amounts of the endocannabinoid 2-arachidonoylglycerol and prostaglandin glyceryl esters produced by THP1 cells (Xie et al., 2010).
Besides CES1, other serine hydrolases, including carboxylesterase 2 (CES2) and monoglyceride lipase (MGL), are also potential off targets for organophosphorus oxons. CES1, CES2, and MGL were recently dubbed “metabolic serine hydrolases” (reviewed in Simon and Cravatt, 2010). Metabolic serine hydrolases indicate enzymes that hydrolyze signaling molecules (e.g., endocannabinoids), energy storage molecules (e.g., triacylglycerols), and precursors of membrane structural components (e.g., cholesteryl esters). Products of these hydrolytic reactions include fatty acids, which are a rich fuel source. CES2 is predominantly found in the small intestine, liver, and kidney (reviewed by Satoh and Hosokawa, 2006), and has 46.8% amino acid sequence identity with CES1 (Schwer et al., 1997). CES2 can activate the anticancer drug CPT-11 (Khanna et al., 2000), but its endogenous substrates are presently unknown. MGL is primarily expressed in adipose tissue where it hydrolyzes monoglycerides, thereby freeing fatty acids for use as a source of energy, and in brain where it hydrolyzes and inactivates 2-arachidonoylglycerol (reviewed in Saario and Laitinen, 2007). The physiological consequences and toxicity, if any, related to the inhibition of these off-target serine hydrolases due to low level environmental exposures is not well understood (Carr et al., 2011). A first step toward investigating this possibility is a thorough study of the kinetics of organophosphorus oxon reactions with purified serine hydrolases. In addition, defining the reaction kinetics of organophosphorus oxons with off-target serine hydrolases could increase the accuracy of physiologically based pharmacokinetic/dynamic models that are used to predict the toxic effects of these compounds in humans.
Here, we determined the potency of inhibition (IC50 values, 15 min preincubation) for chlorpyrifos oxon, paraoxon, and methyl paraoxon, the oxons derived from chlorpyrifos, parathion, and methyl parathion, three commonly used pesticides, with pure CES1, CES2, and MGL proteins in vitro. In addition, we determined the bimolecular rate constants for the reactions of chlorpyrifos oxon, paraoxon, and methyl paraoxon with CES1 and CES2, and the bimolecular rate constant for the reaction of chlorpyrifos oxon with MGL.
Materials and Methods
Chemicals and Reagents
Human recombinant CES1 and CES2 proteins were obtained by expression in baculovirus-infected Spodoptera frugiperda cells and purified as previously described (Morton and Potter, 2000). Human recombinant MGL was purchased from Cayman Chemical (Ann Arbor, MI). Rat hydrolase A was purified from adult male Sprague-Dawley rat liver as described previously (Ross et al., 2006). Chlorpyrifos oxon, paraoxon, and methyl paraoxon were all kind gifts from Dr. Howard Chambers, Department of Entomology, Mississippi State University. The oxons were of greater than 99% purity when assessed by thin-layer chromatography (Chambers et al., 1990). para-Nitrophenyl valerate (pNPV) and all other reagents and buffers were purchased from Sigma (St. Louis, MO).
Enzyme Assays
Hydrolysis reactions were performed at 37°C in a 96-well plate format in a total volume of 300 µL in 50 mM Tris-HCl (which had been adjusted to pH 7.4 at room temperature). CES1 and CES2 were diluted to final concentrations between 0.5–0.75 nM in the reaction mixtures. MGL was diluted to a final concentration of 5.5 nM in the reaction mixtures. The oxons were diluted in ethanol and added to the reaction mixture to give the desired concentrations. The final volume of ethanol in the wells was 1.5% (v/v) with CES1 and MGL, and 0.6% (v/v) with CES2. This amount of ethanol had no effect on enzymatic activity for each of the three enzymes. All reactions were corrected for nonenzymatic hydrolysis of pNPV. Nonenzymatic hydrolysis of pNPV was typically < 5% of enzymatic activity. For the IC50 measurements, the enzyme and inhibitor were incubated at 37°C for 15 minutes, followed by addition of pNPV to a final concentration of 500 µM. The reaction progress was monitored by measuring the absorbance at 405 nm for 5 minutes to estimate the rate of formation of para-nitrophenol. The slopes were determined and used to calculate the enzymatic activity. The curves were linear during the 5 minute reaction period. IC50 values were determined by plotting the fractional inhibition versus the concentration of oxon. Fractional inhibition was defined as: (the rate of the reaction with no oxon – the rate of the reaction with oxon) / the rate of the reaction with no oxon. IC50 values were interpolated from the curve.
Kinetic Studies
The competitive kinetic scheme describing the covalent inhibition of serine hydrolases (E) by organophosphorus oxons (I) in the presence of ester substrate (S) is shown in Figure 1A. To determine the bimolecular rate constants of enzyme inactivation, an oxon (various concentrations) and pNPV (500µM) were added to the reaction buffer and brought to 37°C (5 min). The enzyme was then added to initiate the reaction. The progress of the reaction was followed by measuring the absorbance at 405 nm for either 15 minutes or 45 minutes, depending on the combination of the enzyme and oxon used. The reaction curves were fit to the equation:
| (1) |
using SigmaPlot 8.0, and a value for the apparent first-order rate constant of enzyme inactivation (kobs) was determined for each oxon concentration. A0 is absorbance at time 0, At is absorbance at time t, A∞ is absorbance at time infinity, t is time in s, and kobs is the observed rate constant in s−1. kobs was then plotted against the oxon concentration and fitted to the equation:
| (2) |
where kinact is the rate constant for the inactivation (phosphorylation) of the enzyme by the oxon, KI is the dissociation constant for EI (enzyme·inhibitor complex; i.e., the enzyme·oxon complex), [I] is the inhibitor (oxon) concentration, [S] is the pNPV concentration, and Km is the Michaelis constant for pNPV. If one substitutes KI’ for KI(1 + [S]/Km) and assumes that KI’ >> [I], Equation (2) simplifies to:
| (3) |
which is a linear function, where the slope is the apparent bimolecular rate constant ki’ = kinact / KI’ and KI’ is the apparent dissociation constant for the EI complex. The plots of kobs corrected vs [I] for each reaction was fitted to both the equation for a hyperbola and the equation for a line. For each enzyme and oxon pair studied, the data typically fit the equation of a line better than that of a hyperbola as judged by r2 values. The apparent bimolecular rate constants were determined from the slopes of the lines and then the true bimolecular rate constant was calculated as follows:
| (4) |
where ki represents the true bimolecular rate constant, as defined by (Main and Dauterman, 1963). Km values were experimentally obtained for each enzyme by determining the reaction rates with varying concentrations of pNPV substrate in the absence of inhibitor. The reaction rate vs. the concentration of pNPV was plotted and the substrate concentration giving half the maximum reaction rate was determined by non-linear regression using the Michaelis-Menten equation. The values obtained for pNPV were as follows: CES1 Km = 136 ± 40 µM, CES2 Km = 90 ± 12 µM, MGL Km = 158 ± 37 µM, and rat hydrolase A Km = 32.6 µM The Km values for CES1 and CES2 are in reasonable agreement with those previously published (Hatfield et al., 2010).
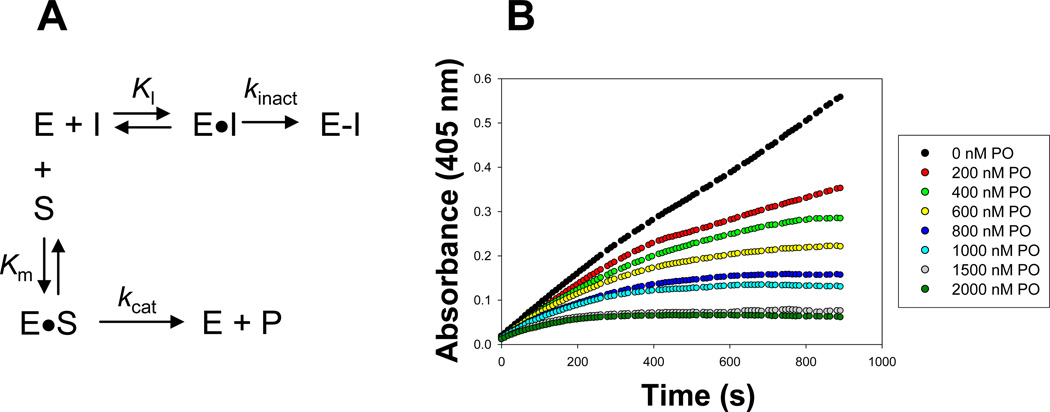
Figure 1. Effect of substrate on the progressive inhibition of a serine hydrolase by oxon.
(A) Kinetic scheme describing the inhibition of serine hydrolases (E) by oxons (I) in the presence of an ester substrate (S). The turnover number kcat for the ester substrate (S) is a function of the rates of acylation (k2) and deacylation (k3) [i.e., kcat = k2*k3/(k2+ k3)] (Streit et al., 2008); however, these steps are not explicitly shown for simplicity. (B) Inhibition of CES2 hydrolytic activity by varying concentrations of paraoxon. The progress of the pNPV hydrolysis reaction was followed by measuring the absorbance of liberated p-nitrophenol at 405 nm every 9 s for 15 minutes.
Statistical Analysis
IC50 values and bimolecular rate constants were log transformed. Values in each row and each column were compared using a one way analysis of variance with post hoc Tukey analysis for three groups of data and a Student’s t-test for two groups of data. Results are noted in the footnotes for Tables 1 and 2.
Table 1.
IC50 values for Reactions of Serine Hydrolases with Oxons*
| Serine Hydrolase, IC50 (nM)** | |||
|---|---|---|---|
| Oxon | Carboxylesterase 1 | Carboxylesterase 2 | Monoacylglycerol lipase |
| Chlorpyrifos Oxon | 0.15 ± 0.05a | 0.33 ± 0.05a | 5.1 ± 3.7b |
| Paraoxon | 0.38 ± 0.10a | 6.2 ± 0.3b | 120 ± 40c |
| Methyl Paraoxon | 4.8 ± 0.2b | 220 ± 50c | 1600 ± 960d |
All reactions were carried out at 37°C with enzyme and inhibitor preincubated for 15 min prior to the addition of substrate.
Values are means ± standard deviation of at least 3 independent experiments. All values in the same row or column with different superscripts are significantly different (p< 0.001, ANOVA and Tukey’s post-hoc test).
Table 2.
Bimolecular Rate Constants for Reactions of Serine Hydrolases with Oxons
| Serine Hydrolase, kinact/KI(M−1·s−1)* | |||
|---|---|---|---|
| Oxon | Carboxylesterase 1 | Carboxylesterase 2 | Monoacylglycerol lipase |
| Chlorpyrifos Oxon | 2.0 (±0.50) × 107 | 4.4 (±2.7) × 105 | 1.4 (±0.37) × 104 |
| Paraoxon | 1.9 (±0.48) × 106 | 3.4 (±2.3) × 104 | N.D. |
| Methyl Paraoxon | 1.2 (±0.57) × 105 | 8.1 (±2.8) × 102 | N.D. |
Values are means ± standard deviation of at least 6 independent experiments. All values have p< 0.001 when compared with values in the same row or column. N.D., not determined (insufficient inhibition to accurately measure).
Results
Inhibition Potency: IC50 Measurements
IC50 values were determined for chlorpyrifos oxon, paraoxon and methyl paraoxon with CES1, CES2, and MGL by preincubating each enzyme with inhibitor for 15 min at 37°C (Table 1). In general, the potency of inhibition was chlorpyrifos oxon > paraoxon > methyl paraoxon for CES1, CES2, and MGL, and IC50 values were significantly different (p< 0.001) for all pair wise comparisons for each oxon with the respective enzyme (i.e., comparing values within a column of Table 1). One exception was found when comparing the inhibition of CES1 by chlorpyrifos oxon and paraoxon (p=0.209). Likewise, for each of the three oxons studied, the rank order of inhibition of the three enzymes was CES1 > CES2 > MGL, and all pair wise comparisons for each enzyme with the respective oxon (i.e., comparing values in each row of Table 1) was significantly different except when comparing the inhibition of CES1 and CES2 by chlorpyrifos oxon (p=0.161).
Determination of the Bimolecular Rate Constants (kinact/KI)
The bimolecular rate constant was determined for the reaction of the three oxons with the three enzymes, as described in detail in the Materials and Methods. As a representative example, the determination of the bimolecular rate constant for CES2 and paraoxon is shown in Figure 1B, supplemental Table 1, and supplemental Figure 1. Various concentrations of paraoxon were mixed with pNPV (final concentration 500 µM) in buffer and warmed to 37°C. Recombinant CES2 was added to initiate the reaction. The progress of the reaction at each concentration of paraoxon was followed by determining the absorbance at 405 nm at 9 s intervals for 15 minutes (Figure 1B). Each progress curve was then fit to an equation that describes an exponential rise to maximum (Equation 1) to obtain the apparent first-order rate constant kobs. The value for kobs at each paraoxon concentration was corrected by subtracting the kobs determined in the absence of paraoxon (which represents inactivation of the enzyme not caused by the oxon inhibitor), thus generating the kobs (corrected) values shown in supplemental Table 1. The values for kobs (corrected) were then plotted versus each concentration of paraoxon and fit to the equation of a straight line (supplemental Figure 1, r2= 0.991), where the slope is the apparent bimolecular rate constant (ki’= kinact/KI’). For this particular experiment, the apparent bimolecular rate constant was 4.4 × 103 M−1·s−1. Using Equation (4), the true bimolecular rate constant was then calculated to be 2.9 × 104 M−1·s−1. The true bimolecular rate constants for each enzyme and oxon are reported in Table 2. MGL reacts too slowly with paraoxon and methyl paraoxon to accurately determine the bimolecular rate constants. Each value in a column was significantly different than the other two values in the same column. Likewise, each value in a row was significantly different than the other values in the same row. In general, for all the enzyme-oxon pairs, the plots of kobs (corrected) against oxon concentrations fit the equation of a straight line better than that of a hyperbola. The bimolecular rate constants for each enzyme-oxon pair were then compared to their corresponding IC50 values. The two parameters exhibited a linear relationship with r2=0.8960 (plot not shown).
The bimolecular rate constant for rat hydrolase A, purified from rat liver, and chlorpyrifos oxon was also determined to be 3.3 × 106 M−1·s−1, which lies in the middle of the range of the values found for CES1 and chlorpyrifos oxon and CES2 and chlorpyrifos oxon (Table 2).
Discussion
Accurate modeling of OP insecticide metabolism in vivo requires an understanding of the interaction of the OPs and their metabolites with the enzymes responsible for their metabolism and/or detoxication. Here, we determined the potency of inhibition (IC50 values) of the liver carboxylesterases CES1 and CES2, as well as MGL, by chlorpyrifos oxon, paraoxon, and methyl paraoxon. IC50 values are defined functionally and vary amongst different laboratories. We routinely preincubate the enzyme and oxon for 15 minutes at 37°C before measuring the enzyme activity. We were able to measure an IC50 for each enzyme-oxon pair. In general, we found that chlorpyrifos oxon was the most effective inhibitor of the enzymes tested and that CES1 was the most reactive enzyme (Table 1). Paraoxon was noted to be a more potent inhibitor than was methyl paraoxon for each of the three enzymes (Table 1). This difference in potency is likely due to the greater reactivity of paraoxon with the enzymes as the fold difference in IC50 values was almost identical to the reciprocal of the fold difference in the bimolecular rate constants for the enzyme-oxon pairs for which bimolecular rate constants could be determined (Table 2). Previous reported values for the IC50 of chlorpyrifos oxon and paraoxon for the inhibition of porcine liver carboxylesterase were 2 and 3 nM, respectively (Quistad and Casida, 2000). These values are approximately one order of magnitude larger than the corresponding values we determined for CES1, which is the carboxylesterase present in the largest amount in human liver. This suggests that CES1 is more sensitive to inhibition by chlorpyrifos oxon and paraoxon than are porcine carboxylesterases. However, we do note that our incubations of enzyme and oxon were done at 37°C versus the 25°C used by Quistad and Casida (2000), which may account for some of the differences observed.
Using an approach similar to that described by Main and Dauterman (1963), we also determined the bimolecular rate constants for the reaction of chlorpyrifos oxon, paraoxon, and methyl paraoxon with CES1 and CES2 and for chlorpyrifos oxon with MGL. For both CES1 and CES2, the rank order of oxon reactivity was chlorpyrifos oxon > paraoxon > methyl paraoxon (Table 2). For chlorpyrifos oxon, the rank order of enzyme reactivity was CES1 > CES2 > MGL. The rate of reaction of MGL with paraoxon and methyl paraoxon in the presence of the substrate pNPV could not be accurately measured because the reactions were too slow to yield useful data. We also attempted to determine the two components of the bimolecular rate constant (actually referred to as the bimolecular reaction constant by Main (1964)), namely kinact and KI, but were unable to do so because the plots of kobs(corrected) versus [I] were linear, implying that KI’ was >> than [I]. Attempts to increase the concentration of the oxons so that KI’ was not >> than [I] (see Materials and Methods) resulted in enzyme inhibition that occurred so rapidly that we were unable to capture the curvilinear portion of the exponential rise to maximum function. In general, we found good correlation between the bimolecular rate constants we were able to measure and the corresponding IC50 values for the enzyme-oxon pairs.
The carboxylesterases are localized in the endoplasmic reticulum (ER) of cells (Robbi and Beaufay, 1991). They are not integral membrane proteins, rather they are found within the ER lumen tethered to the inner leaflet of the ER membrane via an integral KDEL receptor that interacts with the HIEL tetrapeptide on the C-terminus of CES proteins. It is currently unclear whether the lipid membrane affects CES kinetics in any significant manner since the active site of the enzyme is not buried within the lipid bilayer, although data from our laboratory showed that free arachidonic acid and 27-hydroxycholesterol, which are likely embedded in the membrane environment, can inhibit CES1 activity of recombinant protein and CES1 activity within intact living cells (Crow et al., 2010). Moreover, we also recently showed that the lipid peroxidation product, 4-hydroxynonenal, can inhibit CES1 activity in vitro (Borazjani et al., 2011). Therefore, in cells, it is possible that components of the ER membrane (e.g., fatty acids, oxysterols, and reactive aldehydes) might modulate the activity of the CES1 active site toward inhibitors and substrates.
To our knowledge, the bimolecular rate constants for the reaction of human CES1 and CES2 with chlorpyrifos oxon, paraoxon, and methyl paraoxon, and MGL with chlorpyrifos oxon, have not been reported. Thus, we present here for the first time the determination of the bimolecular rate constants for the reaction of pure recombinant human CES1, CES2, and MGL with these oxons. The bimolecular rates of inhibition for CES1 and pesticide oxons reported here are between one and four orders of magnitude higher than those seen for CES1 and nerve agent analogs (sarin, soman, and cyclosarin) (Hemmert et al., 2010). Therefore, CES1 appears to be very efficient at quenching pesticide oxons and is likely a critical enzyme that protects against OP poisoning in humans. A previous study (Timchalk et al., 2002) used a physiologically based pharmacokinetic/ pharmacodynamic model (PBPK/PD) to estimate the value of the bimolecular rate constant for the reaction of chlorpyrifos oxon and CES1 by fitting their model to data obtained from human studies. The value that they estimated was 5.56 × 103 M−1·s−1, which is vastly lower than the value we determined, 2.0 × 107 M−1·s−1. However, the human data used in the modeling did not include explicit chlorpyrifos oxon amounts raising some question about the accuracy of the bimolecular rate constants that were predicted by the PBPK/PD model. In fact, the authors noted that the bimolecular rate constants estimated by their model for the reaction of chlorpyrifos oxon with butyrlcholinesterase and acetylcholinesterase in humans (5.56 × 105 M−1·s−1and 5.56 × 103 M−1·s−1, respectively) were significantly lower than experimental values determined using recombinant human enzymes (2.78 × 107 M−1·s−1and 1.67 × 105 M−1·s−1) (Amitai et al., 1998). Other studies using purified enzymes (Shenouda et al., 2009) also determined much higher bimolecular rate constants for chlorpyrifos oxon with butrylcholinesterase and acetylcholinesterase (8.47 × 108 M−1·s−1 and 3.33 × 105 M−1·s−1 to 5.36 × 106 M−1·s−1, respectively) than was estimated by (Timchalk et al., 2002). Presumably, the reason for these discrepancies is that the PBPK/PD models for OPs are examining the kinetics of OP metabolism by whole tissues and organs and not the kinetics of the interaction of OPs with isolated enzymes.
Recently, CES1 expression has been shown to be age dependent. Prenatal individuals and children less than 70 days old were found to have significantly lower levels of CES1 mRNA and protein than were adults (Yang et al., 2009) (Shi et al., 2011). Juvenile animals are more sensitive to the effects of acute exposure to OP insecticides than are adults ((Atterberry et al., 1997) (Benke and Murphy, 1975) (Pope et al., 1991)). The changes in sensitivity to OP insecticides in animals with age correlates with the expression of carboxylesterases (Benke and Murphy, 1975) (Karanth and Pope, 2000) (Moser et al., 1998). Age-dependent PBPK/PD models for rodents that incorporate the age dependent expression of carboxylesterases have been constructed (Timchalk et al., 2007). However, human PBPK/PD models in general have not taken the age dependent expression of carboxylesterases into account (Timchalk et al., 2002). Given the high level of reactivity of the oxons examined here with CES1, incorporating the age dependent hepatic expression of CES1 into the human PBPK/PD models for acute OP exposure would be expected to increase the accuracy of these models for predicting toxicity in prenatal individuals and young children.
A previous study (Mortensen et al., 1998) noted a significant difference in the IC50 values of acetylcholinesterase in crude tissue preparations with chlorpyrifos oxon. When the acetylcholinesterase from these tissue fractions was isolated by immunoprecipitation, the purified enzyme had the same IC50 value regardless of the tissue it was purified from. Mortensen et al. (1998) concluded that the differences observed in the IC50 values using the crude preparations were due to the binding or hydrolysis of chlorpyrifos oxon by components of the tissue fraction other than acetylcholinesterase. Thus, it seems likely that some of the discrepancies in values determined by the PBPK/PD model versus those measured for pure enzymes may be the result of not including other B esterases such as CES2 and MGL in the model. It was also noted by Timchalk et al. (2002) that their model does not incorporate intestinal metabolism of chlorpyrifos, which is a possible pathway of its biotransformation since isoforms of cytochrome P450 are present in enterocytes. In addition, CES2 is abundantly expressed in the intestine and would likely react with oxons generated in situ and be inhibited. For PBPK/PD models to improve and OP metabolism in individual organs and tissues at the molecular level to be simulated, the rate constants for the interaction of OPs with individual enzymes will be needed. Knowledge of species differences in rate constants for these interactions should allow more accurate extrapolation of models from one species to another. The bimolecular rate constants we have determined for chlorpyrifos oxon, paraoxon, and methyl paraoxon with human CES1 and CES2 and for chlorpyrifos oxon with human MGL should prove useful in this modeling.
Highlights.
IC50 values and bimolecular rate constants (kinact/KI) of human recombinant CES1, CES2, and MGL proteins and chlorpyrifos oxon, paraoxon and methyl paraoxon were determined.
The IC50 values for the oxons with CES1, CES2, and MGL followed the rank order: chlorpyrifos oxon > paraoxon > methyl paraoxon.
The order of reactivity for the oxons with CES1 and CES2 was chlorpyrifos oxon > paraoxon > methyl paraoxon
Chlorpyrifos oxon was less reactive with MGL than with either CES1 or CES2
Supplementary Material
Acknowledgements
Research support was provided by NIH 1R15ES015348-01A1, 3R15ES015348-01A1S1, and 3R15ES015348-01A1S2. Work in Dr. Potter’s laboratory is supported in part by the American Lebanese Syrian Associated Charities and St Jude Children's Research Hospital (SJCRH).
List of Abbreviations
- 2-AG
2-arachidonoylglycerol
- CES1
Carboxylesterase 1
- CES2
Carboxylesterase 2
- DAP
dialkyl phosphate metabolites
- MGL
monoglyceride lipase
- pNPV
para-nitrophenyl valerate
- OP
organophosphorus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge WN. Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem. J. 1953;53:110–117. doi: 10.1042/bj0530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge WN. Mechanisms and Concepts in Toxicology. London: Taylor and Francis; 1996. Organophosphorus compounds and carbamates; pp. 86–90. [Google Scholar]
- Amitai G, Moorad D, Adani R, Doctor BP. Inhibition of acetylcholinesterase and butyrylcholinesterase by chlorpyrifos-oxon. Biochem. Pharmacol. 1998;56:293–299. doi: 10.1016/s0006-2952(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Atterberry TT, Burnett WT, Chambers JE. Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol Appl Pharmacol. 1997;147:411–418. doi: 10.1006/taap.1997.8303. [DOI] [PubMed] [Google Scholar]
- Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Jr, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ, Jackson RJ, Needham LL. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ. Health. Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke GM, Murphy SD. The influence of age on the toxicity and metabolism of methyl parathion and parathion in male and female rats. Toxicol Appl Pharmacol. 1975;31:254–269. doi: 10.1016/0041-008x(75)90161-1. [DOI] [PubMed] [Google Scholar]
- Borazjani A, Edelmann MJ, Hardin KL, Herring KL, Allen Crow J, Ross MK. Catabolism of 4-hydroxy-2-trans-nonenal by THP1 monocytes/macrophages and inactivation of carboxylesterases by this lipid electrophile. Chem Biol Interact. 2011;194:1–12. doi: 10.1016/j.cbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Borazjani A, Ross MK. Effect of developmental chlorpyrifos exposure, on endocannabinoid metabolizing enzymes, in the brain of juvenile rats. Toxicol Sci. 2011;122:112–120. doi: 10.1093/toxsci/kfr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H, Brown B, Chambers JE. Noncatalytic Detoxication of Six Organophosphorus Compounds by Rat Liver Homogenates. Pesticide Biochemistry and Physiology. 1990;36:308–315. [Google Scholar]
- Crow JA, Herring KL, Xie S, Borazjani A, Potter PM, Ross MK. Inhibition of carboxylesterase activity of THP1 monocytes/macrophages and recombinant human carboxylesterase 1 by oxysterols and fatty acids. Biochim Biophys Acta. 2010;1801:31–41. doi: 10.1016/j.bbalip.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebichon DJ. Toxic effects of pesticides. In: Klaassen CD, editor. Casarett and Doull's Toxicology. New York: McGraw-Hill; 1996. pp. 643–698. [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. J. Epidemiol. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health. Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Zhao B, Bie J, Song J. Macrophage cholesteryl ester mobilization and atherosclerosis. Vascul. Pharmacol. 2010;52:1–10. doi: 10.1016/j.vph.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield MJ, Tsurkan L, Hyatt JL, Yu X, Edwards CC, Hicks LD, Wadkins RM, Potter PM. Biochemical and molecular analysis of carboxylesterase-mediated hydrolysis of cocaine and heroin. Br. J. Pharmacol. 2010;160:1916–1928. doi: 10.1111/j.1476-5381.2010.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmert AC, Otto TC, Wierdl M, Edwards CC, Fleming CD, MacDonald M, Cashman JR, Potter PM, Cerasoli DM, Redinbo MR. Human carboxylesterase 1 stereoselectively binds the nerve agent cyclosarin and spontaneously hydrolyzes the nerve agent sarin. Mol Pharmacol. 2010;77:508–516. doi: 10.1124/mol.109.062356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanth S, Pope C. Carboxylesterase and A-esterase activities during maturation and aging: relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicol Sci. 2000;58:282–289. doi: 10.1093/toxsci/58.2.282. [DOI] [PubMed] [Google Scholar]
- Khanna R, Morton CL, Danks MK, Potter PM. Proficient metabolism of irinotecan by a human intestinal carboxylesterase. Cancer Res. 2000;60:4725–4728. [PubMed] [Google Scholar]
- Kiely T, Donaldson D, Grube A. Pesticide industry sales and usage - 2000 and 2001 market estimates. Washington D.C.: U.S. Environmental Protection Agency; 2004. [Google Scholar]
- Main AR. Affinity and Phosphorylation Constants for the Inhibition of Esterases by Organophosphates. Science. 1964;144:992–993. doi: 10.1126/science.144.3621.992. [DOI] [PubMed] [Google Scholar]
- Main AR, Dauterman WC. Determination of the bimolecular rate constant for the reaction between organophosphorus inhibitors and esterases in the presence of substrate. Nature. 1963;198:551–553. [Google Scholar]
- Maxwell DM. Detoxication of organophosphorus compounds by carboxylesterase. Organophosphates. 1992:183–199. [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol. Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Mortensen SR, Brimijoin S, Hooper MJ, Padilla S. Comparison of the in vitro sensitivity of rat acetylcholinesterase to chlorpyrifos-oxon: What do tissue IC50 values represent? Toxicol. Appl. Pharmacol. 1998;148:46–49. doi: 10.1006/taap.1997.8287. [DOI] [PubMed] [Google Scholar]
- Morton CL, Potter PM. Comparison of Escherichia coli, Saccharomyces cerevisiae, Pichia pastoris, Spodoptera frugiperda, and COS7 cells for recombinant gene expression: Application to a rabbit liver carboxylesterase. Molecular Biotechnology. 2000;16:193–202. doi: 10.1385/MB:16:3:193. [DOI] [PubMed] [Google Scholar]
- Moser VC, Chanda SM, Mortensen SR, Padilla S. Age- and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol Sci. 1998;46:211–222. doi: 10.1006/toxs.1998.2526. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.) Pesticides in the diets of infants and children. Washington, D.C.: National Academy Press; 1993. Committee on Pesticides in the Diets of Infants and Children. [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD, Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Casida JE. Sensitivity of blood-clotting factors and digestive enzymes to inhibition by organophosphorus pesticides. J. Biochem. Mol. Toxicol. 2000;14:51–56. doi: 10.1002/(sici)1099-0461(2000)14:1<51::aid-jbt7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Robbi M, Beaufay H. The COOH terminus of several liver carboxylesterases targets these enzymes to the lumen of the endoplasmic reticulum. J Biol Chem. 1991;266:20498–20503. [PubMed] [Google Scholar]
- Ross MK, Borazjani A, Edwards CC, Potter PM. Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem. Pharmacol. 2006;71:657–669. doi: 10.1016/j.bcp.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Saario SM, Laitinen JT. Monoglyceride lipase as an enzyme hydrolyzing 2- arachidonoylglycerol. Chem. Biodivers. 2007;4:1903–1913. doi: 10.1002/cbdv.200790158. [DOI] [PubMed] [Google Scholar]
- Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem. Biol. Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Schwer H, Langmann T, Daig R, Becker A, Aslanidis C, Schmitz G. Molecular cloning and characterization of a novel putative carboxylesterase, present in human intestine and liver. Biochem Biophys Res Commun. 1997;233:117–120. doi: 10.1006/bbrc.1997.6413. [DOI] [PubMed] [Google Scholar]
- Shenouda J, Green P, Sultatos L. An evaluation of the inhibition of human butyrylcholinesterase and acetylcholinesterase by the organophosphate chlorpyrifos oxon. Toxicol Appl Pharmacol. 2009;241:135–142. doi: 10.1016/j.taap.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Yang D, Prinssen EP, Davies BE, Yan B. Surge in expression of carboxylesterase 1 during the post-neonatal stage enables a rapid gain of the capacity to activate the anti-influenza prodrug oseltamivir. J Infect Dis. 2011;203:937–942. doi: 10.1093/infdis/jiq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. J. Biol. Chem. 2010;285:11051–11055. doi: 10.1074/jbc.R109.097600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit TM, Borazjani A, Lentz SE, Wierdl M, Potter PM, Gwaltney SR, Ross MK. Evaluation of the 'side door' in carboxylesterase-mediated catalysis and inhibition. Biol Chem. 2008;389:149–162. doi: 10.1515/BC.2008.017. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Kousba AA, Poet TS. An age-dependent physiologically based pharmacokinetic/pharmacodynamic model for the organophosphorus insecticide chlorpyrifos in the preweanling rat. Toxicol Sci. 2007;98:348–365. doi: 10.1093/toxsci/kfm119. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Nolan RJ, Mendrala AL, Dittenber DA, Brzak KA, Mattsson JL. A Physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol Sci. 2002;66:34–53. doi: 10.1093/toxsci/66.1.34. [DOI] [PubMed] [Google Scholar]
- Xie S, Borazjani A, Hatfield MJ, Edwards CC, Potter PM, Ross MK. Inactivation of Lipid Glyceryl Ester Metabolism in Human THP1 Monocytes/Macrophages by Activated Organophosphorus Insecticides: Role of Carboxylesterases 1 and 2. Chem Res Toxicol. 2010;23:1890–1904. doi: 10.1021/tx1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Pearce RE, Wang X, Gaedigk R, Wan YJ, Yan B. Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem Pharmacol. 2009;77:238–247. doi: 10.1016/j.bcp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.