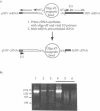
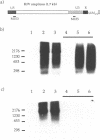
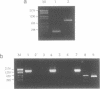
Abstract
To study host gene activation by retroviral promotor insertion, a polymerase chain reaction (PCR) assay was developed. This method allows a sensitive and selective detection of chimeric provirus-host gene transcripts, hallmarks of insertional activation events, which does not rely on an induction of tumor cell growth. We analysed HIV-1 infected cells of a CD4+ T-cell line (H9), infected peripheral blood mononuclear cells and cells in broncho-alveolar washes of AIDS patients. In each case, a variety of chimeric mRNA molecules were detected using a PCR amplification reaction and 5' primers specific to the HIV-1 LTR and 3' primers specific to poly A of mRNA. In infected H9 lymphocytes, a mRNA was identified encoding a putative protein of 145 amino-acids that was not expressed in uninfected H9 cells. This shows for the first time that HIV-1 can activate transcription of host cellular genes by promotor insertion in a fashion similar to slow-transforming avian and murine retroviruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boerkoel C. F., Kung H. J. Transcriptional interaction between retroviral long terminal repeats (LTRs): mechanism of 5' LTR suppression and 3' LTR promoter activation of c-myc in avian B-cell lymphomas. J Virol. 1992 Aug;66(8):4814–4823. doi: 10.1128/jvi.66.8.4814-4823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington J., Ganem D. Regulation of polyadenylation in human immunodeficiency virus (HIV): contributions of promoter proximity and upstream sequences. EMBO J. 1992 Apr;11(4):1513–1524. doi: 10.1002/j.1460-2075.1992.tb05196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Honoré B., Madsen P., Basse B., Andersen A., Walbum E., Celis J. E., Leffers H. Nucleotide sequence of cDNA covering the complete coding part of the human vimentin gene. Nucleic Acids Res. 1990 Nov 25;18(22):6692–6692. doi: 10.1093/nar/18.22.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W., Patel M. D., Lobel L. I., Goff S. P., Nguyen-Huu M. C. Insertion mutagenesis of embryonal carcinoma cells by retroviruses. Science. 1985 May 3;228(4699):554–558. doi: 10.1126/science.3838595. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkosky J., Katz R. A., Skalka A. M. Terminal nucleotides of the preintegrative linear form of HIV-1 DNA deduced from the sequence of circular DNA junctions. J Acquir Immune Defic Syndr. 1990;3(9):852–858. [PubMed] [Google Scholar]
- Kulkosky J., Skalka A. M. HIV DNA integration: observations and interferences. J Acquir Immune Defic Syndr. 1990;3(9):839–851. [PubMed] [Google Scholar]
- Kung H. J., Boerkoel C., Carter T. H. Retroviral mutagenesis of cellular oncogenes: a review with insights into the mechanisms of insertional activation. Curr Top Microbiol Immunol. 1991;171:1–25. doi: 10.1007/978-3-642-76524-7_1. [DOI] [PubMed] [Google Scholar]
- Laurence J., Kulkosky J., Dong B., Early E., Snyderman R., Cianciolo G. J. A soluble inhibitor of T lymphocyte function induced by HIV-1 infection of CD4+ T cells: characterization of a cellular protein and its relationship to p15E. Cell Immunol. 1990 Jul;128(2):337–352. doi: 10.1016/0008-8749(90)90031-l. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Crowe S., Sadick M. D., Heinzel F. P., Gardner K. D., Jr, McGrath M. S., Mills J. Release of interleukin 1 inhibitory activity (contra-IL-1) by human monocyte-derived macrophages infected with human immunodeficiency virus in vitro and in vivo. J Clin Invest. 1988 Dec;82(6):2097–2105. doi: 10.1172/JCI113831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Hoover D. L., Hanson B. D., Turpin J. A., Kalter D. C., Gendelman H. E. Macrophages and the human immunodeficiency virus. Immunol Today. 1990 Jun;11(6):217–223. doi: 10.1016/0167-5699(90)90086-o. [DOI] [PubMed] [Google Scholar]
- Mooslehner K., Karls U., Harbers K. Retroviral integration sites in transgenic Mov mice frequently map in the vicinity of transcribed DNA regions. J Virol. 1990 Jun;64(6):3056–3058. doi: 10.1128/jvi.64.6.3056-3058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B. The polymerase chain reaction in an anemic mode: how to avoid cold oligodeoxyribonuclear fusion. PCR Methods Appl. 1991 Aug;1(1):1–4. doi: 10.1101/gr.1.1.1. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Salahuddin S. Z., Biberfeld P., Ensoli B., Markham P. D., Wong-Staal F., Gallo R. C. Kaposi's sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science. 1988 Oct 21;242(4877):426–430. doi: 10.1126/science.3262925. [DOI] [PubMed] [Google Scholar]
- Pauza C. D., Galindo J. Persistent human immunodeficiency virus type 1 infection of monoblastoid cells leads to accumulation of self-integrated viral DNA and to production of defective virions. J Virol. 1989 Sep;63(9):3700–3707. doi: 10.1128/jvi.63.9.3700-3707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. Oncogenes at viral integration sites. Cell Growth Differ. 1990 Oct;1(10):503–510. [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Pulliam L., Herndier B. G., Tang N. M., McGrath M. S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991 Feb;87(2):503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri I., Moroni C., Senn H. P. Improved efficiency for single-sided PCR by creating a reusable pool of first-strand cDNA coupled to a solid phase. Nucleic Acids Res. 1991 Jul 25;19(14):4010–4010. doi: 10.1093/nar/19.14.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Benko D. M., Fenyö E. M., Pavlakis G. N. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J Virol. 1990 Jun;64(6):2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. C., Stoye J. P., Coffin J. M. Highly preferred targets for retrovirus integration. Cell. 1988 May 20;53(4):531–537. doi: 10.1016/0092-8674(88)90569-7. [DOI] [PubMed] [Google Scholar]
- Stricker R. B., Abrams D. I., Corash L., Shuman M. A. Target platelet antigen in homosexual men with immune thrombocytopenia. N Engl J Med. 1985 Nov 28;313(22):1375–1380. doi: 10.1056/NEJM198511283132202. [DOI] [PubMed] [Google Scholar]
- Swain A., Coffin J. M. Mechanism of transduction by retroviruses. Science. 1992 Feb 14;255(5046):841–845. doi: 10.1126/science.1371365. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Lazo P. A. Virus-host interactions and the pathogenesis of murine and human oncogenic retroviruses. Curr Top Microbiol Immunol. 1991;171:95–171. doi: 10.1007/978-3-642-76524-7_5. [DOI] [PubMed] [Google Scholar]
- Via C. S., Shearer G. M. Autoimmunity and the acquired immune deficiency syndrome. Curr Opin Immunol. 1989 Apr;1(4):753–756. doi: 10.1016/0952-7915(89)90053-8. [DOI] [PubMed] [Google Scholar]
- Weichs an der Glon C., Monks J., Proudfoot N. J. Occlusion of the HIV poly(A) site. Genes Dev. 1991 Feb;5(2):244–253. doi: 10.1101/gad.5.2.244. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M., Berns A. Tumorigenesis by slow-transforming retroviruses--an update. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):213–235. doi: 10.1016/0304-419x(90)90005-l. [DOI] [PubMed] [Google Scholar]