Abstract
Oplopanax horridus (OH) or Devil's club is an ethnobotanical used by the indigenous people native to the Pacific Northwest. There are three species in the genus Oplopanax, and OH is the only species that is distributed in North America. Compared with the extensive research on OH's “cousin,” American ginseng, there is comparatively little about the chemical makeup and pharmacological effects of OH. Nevertheless, there has been some research over the past few years that shows promise for future usage perspectives of OH. To date, seventeen compounds were isolated and elucidated including polyynes, glycosides, lignans, and polyenes, with most of the attention being paid to the polyynes. GC and HPLC were used to determine the contents of volatile compounds and polyynes in the essential oil and extracts of OH. For the pharmacological studies, antibacterial and anti-diabetes effects of polyynes were reported. Our recent study has focused more on the anticancer effects of OH and the involved mechanisms of action. In this review, we will summarize the research status in the botany, phytochemistry and pharmacology of OH.
Keywords: Oplopanax horridus, Devil's club, Polyynes, Phytochemistry, Pharmacology, Anticancer
Introduction
Oplopanax horridus (OH) or Devil's club is a botanical found in the Pacific Northwest of North America. This understory shrub is found in dense, old growth forests and clonally reproduces, forming larger groups of plants through layering [1]. The plant is a member of the family Araliaceae, the same family as more familiar plants such as American ginseng and is therefore occasionally referred to as “Alaskan ginseng” or “Pacific ginseng,” although they are in different genera [2-4]. There are three different species within Oplopanax, which include O. horridus, O. japonicus and O. elatus [5]. O. japonicus and O. elatus are distributed throughout Japan and eastern Asia, respectively [5], and there are beyond the scope of this article.
OH has a reported history of use by the Pacific indigenous peoples. In fact, the plant is used by people from over 38 linguistic groups for the treatment of upwards of 34 different medical conditions [2]. OH is also used during the spiritual practices of the Pacific indigenous tribes. The plant is used to cleanse and protect against supernatural entities; fight witchcraft; and is used by shaman in a variety of ceremonial practices. Table 1 summarizes some of the uses of OH as well which part of the plant that is used. It seems that OH has numerous applications and is important to these tribes [2].
Table 1.
Some indigenous and current uses of Oplopanax horridus (OH) in various ailments.
| Use | Part used | Preparation | Reference |
|---|---|---|---|
| Respiratory (cough pneumonia) | Inner bark, stems and roots | Decoction, chewing | [2, 57] |
| Cardiovascular (hemorrhaging heart disease) | Berries, inner bark | Paste taken internally, infusion | [2, 57] |
| Gastrointestinal (diarrhea, laxative, emetic) | Inner bark, berries | Infusion or decoction, paste taken internally | [2, 57] |
| Cancer | Inner bark | Infusion | [2, 5, 51-56] |
| Cold or infection (flu, fever) | Inner bark | Infusion or decoction | [2, 57] |
| Diabetes | Inner bark or roots | Infusion or decoction | [2, 49, 50] |
| Arthritis | Inner bark, leaves, or roots | Infusion or decoction, pounded for bath | [2, 57] |
Although pharmacological study on OH is sparse, as mentioned above, the indigenous of the Pacific Northwest have used the plant to heal a variety of ailments [2]; therefore, recent pharmacological studies that have been done focus on investigating the plant's effects on similar conditions. Up to now, OH has been identified to potentially possess antidiabetic, antiviral, antibacterial, as well as cancer chemopreventive potentials.
Compared to extensive observations on ginseng (genus Panax) [6-8], chemical studies including phytochemical isolation and analytical evaluation on OH is quite limited. In this review, in addition to introducing the botanical research information, we discuss chemical studies, including identified novel compounds and quantitative determination of isolated compounds in OH extracts. Pharmacological studies in different organ systems, including potential cancer chemopreventive effects are also presented. The up-to-date research observations will provide considerations for future study directions of OH.
Botanical studies
Distribution
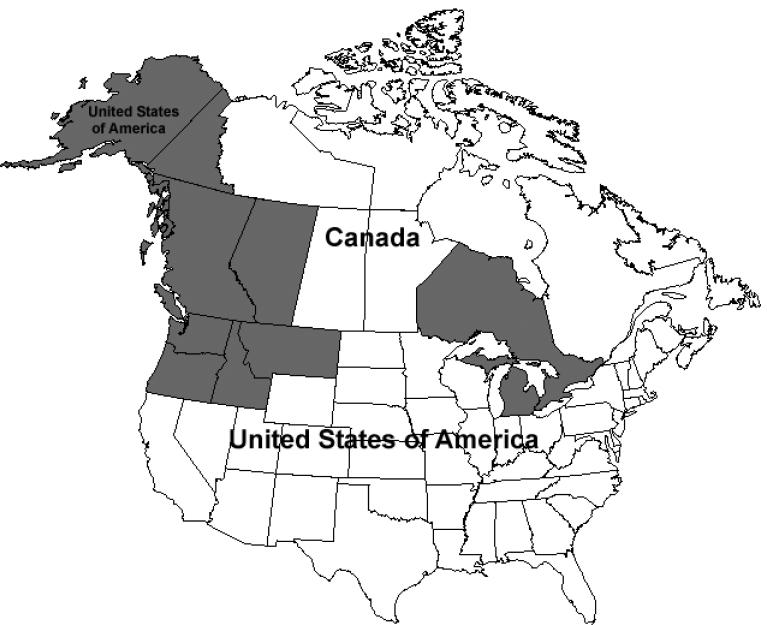
Oplopanax horridus (Sm.) Miq. is distributed throughout the Pacific Northwest of North America, which includes the area from Alaska along the Pacific Coast down to Oregon, Idaho, and Montana in the south and east to the southwestern Yukon Territory. There are also disjunct populations around Michigan and the islands in Lake Superior (Figure 1). The plant was originally extended into eastern Asia but this plant has since been identified as a closely related species O. elatus [9]. O. elatus is distributed throughout eastern Asia, including northeast China, Korea and the far east of Russia. Another species from this genus, O. japonicus, is distributed throughout Japan [5].
Figure 1.
Distribution of Oplopanax horridus in the United States and Canada.
Cultivation
OH is not widely cultivated and is mostly harvested from the wild for use in supplements or in traditional medicine systems. Harvesting of the clonal plants may have an impact on its population and range, especially since the plant grows quite slowly. Additionally, ecological disruption due to excessive logging may further affect OH populations [2]. However, OH is sometimes cultivated as an ornamental landscaping shrub and is occasionally propagated to block undesired off-trail use in sites sensitive to trampling like those found in state or national parks. It has also been sporadically cultivated on farms to protect natural stands from collecting pressure [10].
Identification
Morphological identification is a traditional authentication method, and such description of herbal material is commonly used for crude drug discrimination of OH. In the genus Oplopanax, OH is native to North America but is extremely closely related with other species, especially O. elatus [11]. It is hypothesized that they may even be subspecies or extremely young species. This is evidenced by the fact that the internal transcribed spacer (ITS) data of the nuclear ribosomal DNA sequence show O. elatus differing at only two or three positions when compared to the same sequence in OH [11]. This similarity perhaps explains why many of the chemical components of O. elatus and OH are similar with only slight variation in the sugar moieties of identified compounds [12, 13]. Zhao et al. studied the HPLC fingerprints of both O. elatus and OH and found that they had a similarity of about 90% [14]. Some important differences among the three plants within Oplopanax are summarized in Table 2.
Table 2.
Phytochemical and pharmacological studies on botanicals in genus Oplopanax.
| Oplopanax species | Major distribution | Phytochemistry | Pharmacology | ||
|---|---|---|---|---|---|
| Identified constituent | Study depth | Bioactivity | Study depth | ||
| O. horridus (OH) | West Canada Northwest USA |
7 polyynes 5 glycosides 2 sesquiterpenes 3 others |
++ | Antibacteria Antidiabetes Anticancer |
+ |
| O. japonicus | Japan | 4 triterpene glycosides 2 sesquiterpenes |
+ | N/A | N/A |
| O. elatus | Far East Russia Northeast China Korea |
28 triterpene glycosides Phenolic glycosides Polyynes Other types |
+++ | Antipsoriasis Antiarthritis Antifungus Anticonvulsant |
++ |
Study depth, from + to +++, is based on the quality and relevance of the published articles; N/A, not available.
Chemical studies
Numerous studies have focused on characterizing the complicated chemical makeup of O. horridus. Due to OH's relation to ginseng, it is often called Alaskan ginseng; yet, studies indicate that the two species are quite different in terms of chemical constituents. In ginseng, the major bioactive constituents are triterpene glycosides called dammarane saponins [7, 15, 16]. However, these dammarane saponins are not found in any of the Oplopanax species. Nevertheless, other types of triterpene glycosides have been isolated from O. japonicus, O. elatus, and O. horridus [5]. Even though the composition of chemical constituents varies depending on what part of the plant is examined, the main components of OH tend to be polyynes, glycosides, polyenes and lignans.
Isolation and compound elucidation
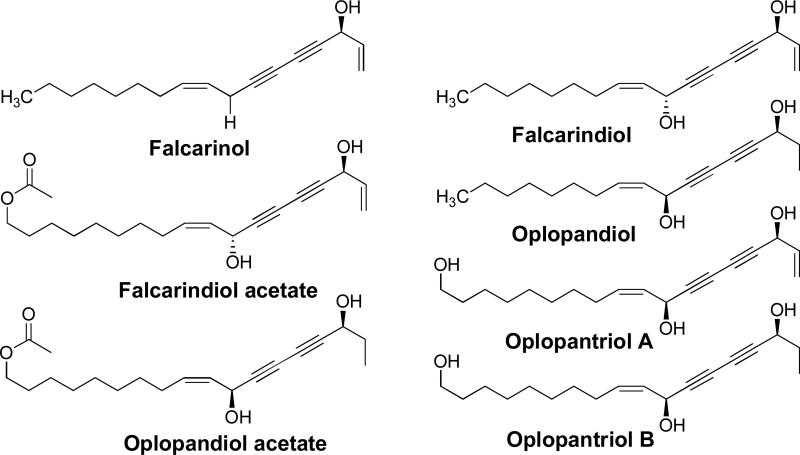
Studies about phytochemical isolation of OH have mainly focused on the root bark from which several polyynes were isolated and identified. In 1997, five polyynes were isolated from O. horridus: falcarinol; falcarindiol; oplopandiol; oplopandiol acetate; 9,17-octadecadiene-12,14-diyne-1,11,16-triol 1-acetate [17]. These polyynes have been linked to the plant's effective antimycobacterial properties [2, 17-21]. Recently, two more polyynes, oplopantriol A and B were isolated. The identified polyynes from OH are shown in Figure 2 [20].
Figure 2.
Seven polyynes isolated from Oplopanax horridus.
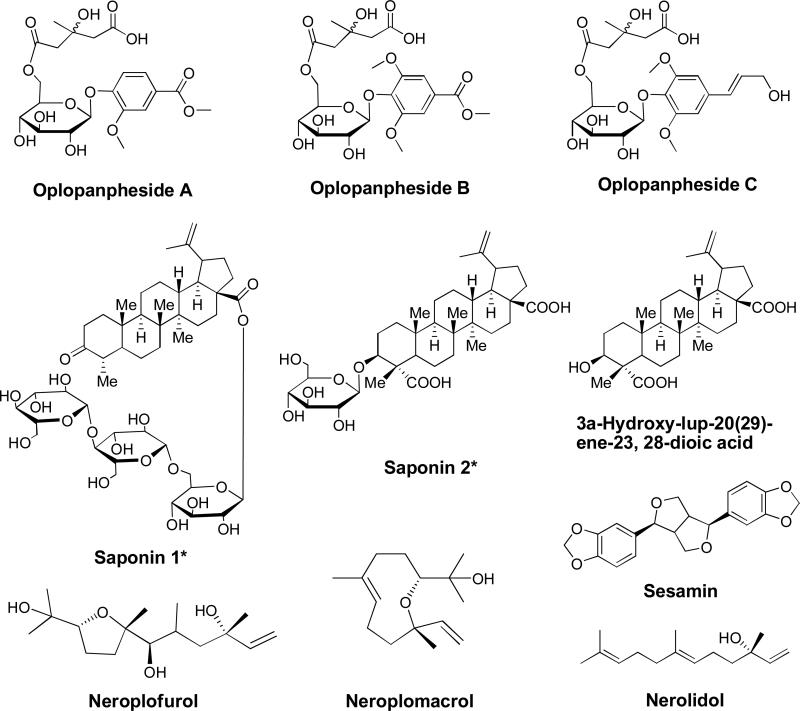
Hydrophilic components of OH have rarely been elucidated. Several glycosides were isolated from OH. Two lupane-type saponins, 3α-hydroxy-lup-20(29)-ene-23, 28-dioic acid-3-O-β-D-glucopyranoside, and 24-nor-3-oxo-lup-20(29)-en-28-oic acid-28-O-α-L-rhamnopyranosyl(1'”→4”)-β-D-glucopyranosyl(1”→6')-β-D-glucopyranoside, as well as a lupane aglycon 3α-hydroxy-lup-20(29)-ene-23,28-dioic acid were isolated from the leaves of OH [13]. Three phenolic glycosides, oplopanphesides A - C were isolated from the root bark of OH [22]. Other isolated and identified compounds include two sesquiterpenes, neroplomacrol and neroplofurol, together with a lignan compound, sesamin from the stem bark of OH [18], and a polyene compound nerolidol from the root bark of OH [19]. Besides polyynes (Figure 2), other isolated and elucidated compounds from OH are summarized in Figure 3. Further investigation into different plant parts of OH may uncover more novel compounds.
Figure 3.
Glycosides and other compounds isolated from Oplopanax horridus. Saponin 1*: 24-nor-3-oxo-lup-20(29)-en-28-oic acid-28-O-α-L-rhamnopyranosyl(1'”→4”)-β-D-glucopyranosyl(1”→6')-β-D-glucopyranoside; Saponin 2*: 3α-hydroxy-lup-20(29)-ene-23, 28-dioic acid-3-O-β-D-glucopyranoside [13].
Analytical evaluations
The chemical composition and proportions in the essential oil from OH were assayed using GC-MS. It was observed that the main constituent in both the stem and root essential oils was nerolidol with over 50% of the oil being composed of this compound, a finding corroborated by a number of studies [19, 23, 24]. Another study examined the volatile oil from the leaves of OH, and the results showed that 2-methyl-6-p-methylbenzene-2-heptene (34.4%) and phytol (8.13%) are the main constituents among a chromatogram with forty-five peaks [25].
In comparison with the GC-MS technique, which was applied to separate volatile compounds, high performance liquid chromatography (HPLC) and thin layer chromatography (TLC) were suitable for the characterization and identification of OH and detected some sterols in a root bark extract [23].
An HPLC fingerprint method was developed and used on OH stem and berry extracts, and the UV spectra of representative peaks in chromatograms were compared. Although chemical structures of each chromatographic peak were not characterized, results from this study suggest that polyynes are not major constituents in OH stem and berries [5]. For quantification, an online SPE-HPLC was developed to assay polyyne compounds, and observed that all the OH root bark samples contained six polyynes (range 0.25-6.68 mg/g) [19].
Comparing Oplopanax species
There has been some study of the other species within Oplopanax and some insight has been gained as to their similarities within the genus in terms of chemical makeup. Two sesquiterpenes, oplopanone and oplodiol, and four triterpene glycosides, oplopanoxoside A, B, C, D were isolated and identified from O. japonicus. There have been no documented pharmacological studies performed on O. japonicus [26]. Several types of compounds have been isolated from parts of O. elatus, with the representative compounds being saponins, which are also believed to be bioactive constituents. To date, 28 triterpene glycosides have been isolated and elucidated from O. elatus, of which 27 were identified from the leaves. From O. elatus leaves, cirensenoside E, F, G, H [27]; cirensenoside I, J, K, L [28]; cirensenoside M, N [29]; cirensenoside O, P [30]; cirensenoside Q, R [31]; cirensenoside S, T, U, V [12, 32]; 1H-cyclopenta[α] chrysene [33]; nipponoside B, saponins [161400-70-0] (CAS registration number), [161400-72-2], [161400-73-3] [34]; kalopanaxsaponin G, cussonoside A, saponins [136182-02-0], [136182-02-0] [35] were isolated and identified. In addition, daucosterol was isolated from the roots [36] and stems [37] and oploxynes A and B [38], polyacetylenes, were isolated from the stems of O. elatus. For the bioactivity observation, the effect of O. elatus on antiarthritis [39], antifungal [40], antipsoriasis [41], and anticonvulsant [42] have been reported.
Using on-line SPE-HPLC, seven compounds were determined in different lots of root bark of both OH and O. elatus. Both species contain high amounts of falcarindiol, oplopandiol and nerolidol, but falcarindiol was mainly contained in O. elatus, while (S,E)-nerolidol was the main component in OH. The contents of (11S,16S,9Z)-9,17-octadecadiene-12,14-diyne-1,11,16-triol,1-acetate, oplopantriol A, oplopantriol B and oplopandiol acetate in OH were low, but these four compounds were not detected in the sample of O. elatus, which warrants further investigation [19].
Pharmacological studies
Supplements of OH are marketed claiming to help cope with type II diabetes to respiratory problems, as well as a host of other ailments that the indigenous people treated with OH. Interestingly, supplements often contain root bark as the main ingredient while most indigenous use centered on the stem bark [2]. This may be an attempt to mimic the use of ginseng in which the root is considered to the more desirable part of the plant [43-45]. While some studies have been performed investigating the pharmacological effects of OH (Table 2), more study is needed to shed light on popular claims as well as highlight the differences between the chemical makeups of the various parts of the plant.
Antibacterial
The most important applications of OH come from its apparent antibacterial properties. Several studies have found OH to possess antibacterial and specifically antimycobacterial properties, which has a particular efficacy against Mycobacterium, the genus of bacteria that includes those that cause leprosy and tuberculosis in humans [17, 46-48]. Therefore, OH is most widely used to treat internal infections, particularly tuberculosis, among the indigenous.
OH extract has shown to be effective in treating tuberculosis [2, 17, 46-48]. It was identified that two polyynes, namely oplopandiol and falcarindiol are the main effective antimycobacterial constituents of the extract [18]. Other polyynes, falcarinol, oplopandiol acetate, and (Z)-9,17-octadecadiene-12,14-diyne-1,11,16-triol 1-acetate, were also shown to be effective against both Mycobacterium tuberculosis and M. avium [17].
In addition, the polyynes of OH extracts have shown to be effective against two Gram-positive bacteria S. aureus and B. subtilis, two Gram-negative bacteria E. coli DC2 and P. aeruginosa Z61, and the yeast Candida albicans. Falcarinol proved to be the most effective among the tested polyynes [17].
Antidiabetes
One of the commonly listed health benefits of OH extracts comes in its reported antidiabetic effects. However, there has been almost no study investigating these claims other than reporting that the indigenous people used it to treat diabetes. A pilot study performed by Thommasen, et al. found that there was no significant hypoglycemic effect of an OH tea [49]. However, they only used a tea extract of OH and had a very small dose. Nevertheless, most support for the antidiabetic effects of OH is anecdotal [49].
The findings by the aforementioned study dispute the only other study on the hypoglycemic effects of OH extract, performed in 1938, which found that there was a substance that lowered blood sugar in OH [50]. More study is needed to determine the effects of OH on blood sugar.
Cancer chemoprevention
The most extensive pharmacological research that has been performed on OH has involved its anticancer abilities. Nerolidol, a constituent of OH extracts, was shown to inhibit azoxymethane-induced neoplasia of the large bowel in male F344 rats [51]. Tai et al. found that the root bark extract is effective in the inhibition of the growth of several ovarian cancer cell lines [52]. Additionally, a study by the same group found that a similar extract inhibited the proliferation of different cancer cell lines, although the mechanisms of action in these studies were not explored in depth [53]. These observations suggested that extracts or constituents from OH showed anticancer potential. To date, cancer chemopreventive studies are more focused on colorectal cancer. The effects of OH on other types of cancers, such as breast cancer and lung cancer, have also been evaluated.
Breast cancer
Effects of water and ethanol fractions of OH root bark on human breast cancer MCF-7 cells have been studied. It was shown that the 70% and 100% ethanol fractions possessed more potent antiproliferative effects than the total extract. However, the water and 30% ethanol fractions significantly promoted cell proliferation on MCF-7 cells at concentrations > 100 μg/mL, which suggested that the hydrophilic fractions should be removed from the extract when used for cancer chemoprevention in order to achieve desirable activities. The total extract and the two active fractions obviously induced cell apoptosis. The 70% ethanol fraction, which possessed the most potent antiproliferative activity, showed the strongest apoptotic induction activity [54].
Additionally, stem and berry extracts were assayed and compared for their effectiveness on MCF-7 cells. Compared to the berry extract, the stem extract showed significant potent antiproliferative effects [5]. The effects of an OH root bark extract containing only hydrophobic constituents was also examined on MCF-7 cells. All of the isolated compounds presented inhibitive effects with falcarindiol being identified as the most potent antiproliferative agent [55].
Lung cancer
The antiproliferative effects of OH extracts on lung cancer cell lines has been investigated. In one study, non-small cell lung cancer (NSCLC) cells were treated with various root bark fractions. The study found that the 70% and 100% ethanol fractions demonstrated more potent antiproliferative effects than the total extract [54].
In another study, the effectiveness of stem and berry extract on NSCLC cells were evaluated. Compared to the berry extract, the stem extract showed significant potent antiproliferative effects for as low a concentration as 0.1 mg/mL stem extract versus 1 mg/mL for the berry extract, suggesting that the stem extract contains anti-lung cancer bioactive natural compounds [5].
Colorectal cancer
Within the antiproliferative evaluations, extracts from different plant parts were screened, including root, stem, and berry extracts. Compared to the berry extract, the stem extract showed significant potent antiproliferative effects on different colorectal cancer cell lines. The stem extract induced cell apoptosis, arrested cancer cells in S- and G2/M-phases, and significantly induced expression of cyclin A, suggesting the cell cycle arrest and induction of apoptosis may play a critical role in cancer chemoprevention of an OH stem extract [5].
Because the root bark is the most commonly used plant part of OH, systemic fractionation and active component isolation were performed on the root bark extract. Various fractions of root bark extract were tested on colorectal cancer cell lines, and only the hydrophobic fractions showed potent antiproliferative effects. It was found that the percentage of apoptotic cells, including early and late apoptosis, was much higher in the hydrophobic fractions. Analysis of the cell cycle distribution in the study demonstrated that G2/M-phase was arrested in cancer cells in response to the OH extract, which suggests that G2/M arrest is the target cell cycle checkpoint. Further investigation on the expression of cyclin A and cyclin B1 supported the cell cycle results [56].
To explore the active compounds in hydrophobic fractions of OH, several polyynes were isolated and their bioactivities were evaluated [55]. All of the polyynes presented inhibitive effects on colon cancer cells. Therefore, it was concluded that the observed anticancer activities of OH root bark are likely related to those hydrophobic compounds. While falcarindiol showed the most potent effects, primary structure–activity analysis suggested that the observed bioactivities were influenced by the ethylenic bonds and acylations of polyacetylenes [54, 55].
Summary and future perspective
This potentially important ethnobotanical has provided a starting point for a variety of research disciplines. While several important natural products have been isolated and characterized from root, stem and leaf, most studies have focused on the root bark of OH. For the biological study, the next step could include testing the effects of single compounds or the possible synergistic effects of several compounds isolated from the plant, and assaying the functional-bioactivity relationship. Mechanistic evaluation of the OH extract or active compounds on different diseases could help discover effective uses for this herbal medicine. Additionally, testing the metabolites of OH compounds for potential anticancer modulation may be an interesting direction as the plant is often taken orally and the major compounds would be converted by gut microbiota. Overall, there are likely more avenues to be explored by the coming studies on this largely unexplored botanical.
Acknowledgement
This work was supported in part by the NIH/NCCAM grants (AT004418, AT005362), the University of Chicago Digestive Disease Research Core Center (5P30DK042086), and University of Macau grant (UL015/09-Y1).
References
- 1.Lantz TC, Antos JA. Clonal expansion in the deciduous understory shrub, devil's club (Oplopanax horridus; Araliaceae). Can J Bot. 2002;80:1052–1062. [Google Scholar]
- 2.Lantz TC, Swerhun K, Turner NJ. Devil's club (Oplopanax horridus): An ethnobotanical review. HerbalGram. 2004;62:33–48. [Google Scholar]
- 3.Lin WN, Lu HY, Lee MS, Yang SY, Chen HJ, Chang YS, Chang WT. Evaluation of the cultivation age of dried ginseng radix and its commercial products by using (1)H-NMR fingerprint analysis. Am J Chin Med. 2010;38:205–218. doi: 10.1142/S0192415X10007762. [DOI] [PubMed] [Google Scholar]
- 4.Chan PC, Peckham JC, Malarkey DE, Kissling GE, Travlos GS, Fu PP. Two-year toxicity and carcinogenicity studies of Panax ginseng in Fischer 344 rats and B6C3F1 mice. Am J Chin Med. 2011;39:779–788. doi: 10.1142/S0192415X11009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CZ, Aung HH, Mehendale SR, Shoyama Y, Yuan CS. High performance liquid chromatographic analysis and anticancer potential of Oplopanax horridus: comparison of stem and berry extracts. Fitoterapia. 2010;81:132–139. doi: 10.1016/j.fitote.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taira S, Ikeda R, Yokota N, Osaka I, Sakamoto M, Kato M, Sahashi Y. Mass spectrometric imaging of ginsenosides localization in Panax ginseng root. Am J Chin Med. 2010;38:485–493. doi: 10.1142/S0192415X10008007. [DOI] [PubMed] [Google Scholar]
- 7.Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang HI, Shin HM. Wild Panax ginseng (Panax ginseng C.A. Meyer) protects against methotrexate-induced cell regression by enhancing the immune response in RAW 264.7 macrophages. Am J Chin Med. 2010;38:949–960. doi: 10.1142/S0192415X10008378. [DOI] [PubMed] [Google Scholar]
- 9.Reunova GD, Kats IL, Zhuravlev YN. Genetic variation of Oplopanax elatus (Araliaceae) populations estimated using DNA molecular markers. Dokl Biol Sci. 2010;433:252–256. doi: 10.1134/S0012496610040058. [DOI] [PubMed] [Google Scholar]
- 10.Luna T. Propagation Protocol for Devil's Club (Oplopanax horridus). Native Plants J. 2001;2:106–108. [Google Scholar]
- 11.Artyukova EV, Gontcharov AA, Kozyrenko MM, Reunova GD, Zhuravlev YN. Phylogenetic relationships of the Far Eastern Araliaceae inferred from ITS sequences of nuclear rDNA. Russ J Genet. 2005;41:649–658. [PubMed] [Google Scholar]
- 12.Wang GS, Yang XH, Xu JD. Structures of four new triterpenoid saponins from the leaves of Oplopanax elatus Nakai. Yao Xue Xue Bao. 2004;39:354–358. [PubMed] [Google Scholar]
- 13.Liu P-P, Li M, Kang T-G, Dou D-Q, Smith DC. New lupane-type triterpenoid saponins from leaves of Oplopanax horridus (Devil's Club). Nat Prod Commun. 2010;5:1019–1022. [PubMed] [Google Scholar]
- 14.Zhao Y, Kang T, Dou D, Zhang F, Wang S. HPLC fingerprints of Oplopanax horridus and the comparision of constituents between O. horridus and O. elatus Nakai. Shizhen Guoyi Guoyao. 2008;19:546–548. [Google Scholar]
- 15.Rodriguez M, Du GJ, Wang CZ, Yuan CS. Letter to the editor: Panaxadiol's anticancer activity is enhanced by epicatechin. Am J Chin Med. 2010;38:1233–1235. doi: 10.1142/S0192415X10008597. [DOI] [PubMed] [Google Scholar]
- 16.Si YC, Zhang JP, Xie CE, Zhang LJ, Jiang XN. Effects of Panax notoginseng saponins on proliferation and differentiation of rat hippocampal neural stem cells. Am J Chin Med. 2011;39:999–1013. doi: 10.1142/S0192415X11009366. [DOI] [PubMed] [Google Scholar]
- 17.Kobaisy M, Abramowski Z, Lermer L, Saxena G, Hancock RE, Towers GH, Doxsee D, Stokes RW. Antimycobacterial polyynes of Devil's Club (Oplopanax horridus), a North American native medicinal plant. J Nat Prod. 1997;60:1210–1213. doi: 10.1021/np970182j. [DOI] [PubMed] [Google Scholar]
- 18.Inui T, Wang Y, Nikolic D, Smith DC, Franzblau SG, Pauli GF. Sesquiterpenes from Oplopanax horridus. J Nat Prod. 2010;73:563–567. doi: 10.1021/np900674d. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Yang J, Zhao J, Wang CZ, Yuan CS, Li SP. Quantitative analysis of six polyynes and one polyene in Oplopanax horridus and Oplopanax elatus by pressurized liquid extraction and on-line SPE-HPLC. J Pharm Biomed Anal. 2010;53:906–910. doi: 10.1016/j.jpba.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Huang WH, Zhang QW, Wang CZ, Yuan CS, Li SP. Isolation and identification of two new polyynes from a North American ethnic medicinal plant--Oplopanax horridus (Smith) Miq. Molecules. 2010;15:1089–1096. doi: 10.3390/molecules15021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu LA, Wu XH, Zheng GR, Cai JC. First total synthesis of optically active oplopandiol acetate, a potent antimycobacterial polyyne isolated from Oplopanax horridus. Chinese Chem Lett. 2000;11:213–216. [Google Scholar]
- 22.Zhang QW, Huang WH, Meng LZ, Yuan CS, Wang CZ, Li SP. Oplopanphesides A-C, Three New Phenolic Glycosides from the Root Barks of Oplopanax horridus. Chem Pharm Bull (Tokyo) 2011;59:676–679. doi: 10.1248/cpb.59.676. [DOI] [PubMed] [Google Scholar]
- 23.Gruber JW, Kittipongpatana N, Bloxton JD, 2nd, Der Marderosian A, Schaefer FT, Gibbs R. High-performance liquid chromatography and thin-layer chromatography assays for Devil's Club (Oplopanax horridus). J Chromatogr Sci. 2004;42:196–199. doi: 10.1093/chromsci/42.4.196. [DOI] [PubMed] [Google Scholar]
- 24.Garneau FX, Collin G, Gagnon H, Jean FI, Strobl H, Pichette A. The essential oil composition of devil's club, Oplopanax horridus J. E. Smith Miq. Flavour Fragrance J. 2006;21:792–794. [Google Scholar]
- 25.Li M, Dou D, Smith DC. Studies on chemical constituents of volatile oil from the leaves of Oplopanax horridus. Zhongguo Xiandai Zhongyao. 2009;11:24–26. [Google Scholar]
- 26.Hirai Y, Murayama T, Miyakoshi M, Hirono S, Isoda S, Ideura N, Ida Y, Shoji J, Wang G-S, Xu J-D. Three new lupane type glycosyl esters from Oplopanax japonicus leaves. Nat Med. 1995;49:462–467. [Google Scholar]
- 27.Wang GS, Zhao CF, Xu JD, Murayama T, Miyakoshi M, Junzo S. Isolation and structure elucidation of new glycosides from the leaves of Oplopanax elatus Nakai 2. Chem Res Chinese U. 1994;10:285–290. [Google Scholar]
- 28.Wang GS, Xu JD, Murayama T, Shoji J. Isolation and structure elucidation of new glycosides from the leaves of Oplopanax elatus Nakai 3. Chinese Sci Bull. 1994;39:1969–1972. [Google Scholar]
- 29.Wang GS, Xu JD, Ma XL, Sun YX, Liu JZ, Murayama T, Shoji J. Chemical studies on glycosides in the leaves of Oplopanax elatus Nakai. (4). Chem Res Chinese U. 1997;13:34–38. [Google Scholar]
- 30.Wang GS. Isolation and structure elucidation of cirensenosides O and P from the leaves of Oplopanax elatus Nakai. Act Pharmaceut Sinic. 1996;31:940–944. [PubMed] [Google Scholar]
- 31.Wang G, Xu J, Murayama T, Shoji J. Isolation and structure elucidation of cirensenosides Q and R. Zhongguo Zhong Yao Za Zhi. 1997;22:101–103. 128. [PubMed] [Google Scholar]
- 32.Wang G, Xu J. Structure of four new triterpenoids saponins from the leaves of Oplopanax elatus Nakai VII. Stud Plan Sci. 1999;6:52–57. [Google Scholar]
- 33.Wang G, Xu J. Chemical study of glycosides of Oplopanax elatus Nakai. Zhongguo Yaoxue Zazhi. 1993;28:593–594. [Google Scholar]
- 34.Wang GS, Zhao CF, Xu JD, Murayama T, Miyakoshi M, Junzo S. Studies on the glycosides in the leaves of Oplopanax elatus Nakai. Chem Res Chinese U. 1994;10:185–192. [Google Scholar]
- 35.Wang G, Meng Q, Xu J, Chuenshan Z, Zhuanshi S. Clycosides in the leaves of Oplopanax elatus Nakai. Zhongguo Yaoxue Zazhi. 1996;31:522–523. [Google Scholar]
- 36.Liu JZ, Wu G. Chemical constituents of the roots of Oplopanax elatus Nakai. J Chin Pharm Sci. 1993;2:168. [Google Scholar]
- 37.Zhang H, Wu G. Chemical constituents from the stems of Oplopanax elatus Nakai. J Chin Pharm Sci. 1996;5:112. [PubMed] [Google Scholar]
- 38.Yang HO, Yang MC, Kwon HC, Kim YJ, Lee KR. Oploxynes A and B, polyacetylenes from the stems of Oplopanax elatus. J Nat Prod. 2010;73:801–805. doi: 10.1021/np900628j. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Wang K. The effect of Echinopanax elatum Nakai on experimental arthritis and the neuro-hypophyseal-adrenal system. Yao Xue Xue Bao. 1980;15:81–85. [Google Scholar]
- 40.Mi HM, Li CG, Su ZW, Wang NP, Zhao JX, Jiang YG. Studies on the chemical constituents and antifungal activities of essential oil from Oplopanax elatus Nakai. Yao Xue Xue Bao. 1987;22:549–552. [PubMed] [Google Scholar]
- 41.Kang TG, Dou DQ, Hu XY, Zhao YR, Liu FY, Kuang HX, Smith DC. Studies on the anti-psoriasis constituents of Oplopanax elatus Nakai. Nat Prod Res. 2009;23:334–342. doi: 10.1080/14786410802075806. [DOI] [PubMed] [Google Scholar]
- 42.Qu S, Wu Y, Wang Y, Pan D. Inhibitory effect of tall oplopanax (Oplopanax elatus) oil on the central nervous system. Zhongcaoyao. 1984;15:259–261. [Google Scholar]
- 43.Chan E, Wong CY, Wan CW, Kwok CY, Wu JH, Ng KM, So CH, Au AL, Poon CC, Seto SW, Kwan YW, Yu PH, Chan SW. Evaluation of anti-oxidant capacity of root of Scutellaria baicalensis Georgi, in comparison with roots of Polygonum multiflorum Thunb and Panax ginseng CA Meyer. Am J Chin Med. 2010;38:815–827. doi: 10.1142/S0192415X10008263. [DOI] [PubMed] [Google Scholar]
- 44.Lee JS, Choi HS, Kang SW, Chung JH, Park HK, Ban JY, Kwon OY, Hong HP, Ko YG. Therapeutic effect of Korean red ginseng on inflammatory cytokines in rats with focal cerebral ischemia/reperfusion injury. Am J Chin Med. 2011;39:83–94. doi: 10.1142/S0192415X1100866X. [DOI] [PubMed] [Google Scholar]
- 45.Jung HL, Kwak HE, Kim SS, Kim YC, Lee CD, Byurn HK, Kang HY. Effects of Panax ginseng supplementation on muscle damage and inflammation after uphill treadmill running in humans. Am J Chin Med. 2011;39:441–450. doi: 10.1142/S0192415X11008944. [DOI] [PubMed] [Google Scholar]
- 46.Pauli GF, Inui T, Wang YH, Deng SX, Smith DC, Franzblau SG. Counter-current chromatography based analysis of synergy in an anti-tuberculosis ethnobotanical. J Chromatogr A. 2007;1151:211–215. doi: 10.1016/j.chroma.2007.01.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inui T, Case R, Chou E, Soejarto DD, Fong HHS, Franzblau SG, Smith DC, Pauli GF. CCC in the phytochemical analysis of anti-tuberculosis ethnobotanicals. J Liq Chromatogr R T. 2005;28:2017–2028. [Google Scholar]
- 48.McCutcheon AR, Roberts TE, Gibbons E, Ellis SM, Babiuk LA, Hancock REW, Towers GHN. Antiviral screening of British Columbian medicinal plants. J Ethnopharmacol. 1995;49:101–110. doi: 10.1016/0378-8741(95)90037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thommasen HV, Wilson RA, McIlwain RG. Effect of devil's club tea on blood glucose levels in diabetes mellitus. Can Fam Physician. 1990;36:62–65. [PMC free article] [PubMed] [Google Scholar]
- 50.Large RG, Brocklesby HN. A hypoglycaemic substance from the roots of the devil's club (Fatsia horrida). Can Med Assoc J. 1938;39:32–35. [PMC free article] [PubMed] [Google Scholar]
- 51.Wattenberg LW. Inhibition of azoxymethane-induced neoplasia of the large bowel by 3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene (nerolidol). Carcinogenesis. 1991;12:151–152. doi: 10.1093/carcin/12.1.151. [DOI] [PubMed] [Google Scholar]
- 52.Tai J, Cheung S, Chan E, Hasman D. Inhibition of human ovarian cancer cell lines by devil's club Oplopanax horridus. J Ethnopharmacol. 2010;127:478–485. doi: 10.1016/j.jep.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Tai J, Cheung S, Cheah S, Chan E, Hasman D. In vitro anti-proliferative and antioxidant studies on Devil's Club Oplopanax horridus. J Ethnopharmacol. 2006;108:228–235. doi: 10.1016/j.jep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Sun S, Li XL, Wang CZ, Williams S, Yuan CS. Improving anticancer activities of Oplopanax horridus root bark extract by removing water-soluble components. Phytother Res. 2010;24:1166–1174. doi: 10.1002/ptr.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun S, Du GJ, Qi LW, Williams S, Wang CZ, Yuan CS. Hydrophobic constituents and their potential anticancer activities from Devil's Club (Oplopanax horridus Miq.). J Ethnopharmacol. 2010;132:280–285. doi: 10.1016/j.jep.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li XL, Sun S, Du GJ, Qi LW, Williams S, Wang CZ, Yuan CS. Effects of Oplopanax horridus on human colorectal cancer cells. Anticancer Res. 2010;30:295–302. [PMC free article] [PubMed] [Google Scholar]
- 57.Russel PN. English Bay and Port Graham Alutiiq Plantlore. Homer; Kenai Peninsula: 1991. [Google Scholar]