Abstract
Background
Neurocognitive deficits are a recognized late effect of curative brain tumor therapy. We evaluated the feasibility, tolerance, and impact of a pilot pharmacologic intervention with the acetylcholinesterase (AChe) inhibitor, donepezil, in pediatric brain tumor (BT) survivors at risk for neurocognitive dysfunction.
Procedure
A single institution open-label pilot study was conducted in childhood BT survivors: ≥ 1 year from cancer treatment; and who received > 23.5 Gy cranial radiation therapy (RT). Toxicity, adherence and neurocognitive outcomes were evaluated at baseline and serially during 24 weeks of donepezil, and following a 12-week washout period off drug.
Results
From a pool of subjects, 13 were successfully contacted and screened, and 11 met all eligibility criteria to initiate donepezil at a median of 4.7 (1.9–11.9) years from RT. Seventy-two percent of patients completed the 24-week drug study visit. Despite transient gastrointestinal toxicity (vomiting and diarrhea) in 30% of patients there was no weight loss on donepezil. Significant improvement in performance was noted at 24 weeks on the Dellis-Kaplan Executive Function (D-KEF) Tower test (p<=0.001), the Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML-2) Visual memory (p= 0.007), and the Number/Letter task (p= 0.018).
Conclusions
Donepezil was well tolerated among childhood BT survivors who had received substantial prior therapy. Based on improved executive function and memory performance in this pilot trial, a randomized placebo controlled trial of this pharmacologic agent is warranted to fully evaluate its efficacy in remediating neurocognitive dysfunction.
Keywords: childhood brain tumor, survivors, AChe inhibitor, donepezil, executive function
INTRODUCTION
With diagnostic and therapeutic advances, childhood brain tumor (BT) survivors represent 16% of the estimated 300,000 childhood cancer survivors in the US (1). With follow-up, neurocognitive deficits are prevalent in 50–80% of these survivors (2). Deficits include a spectrum of dysfunction in attention, processing speed, memory, and executive function (3). In BT survivors treated during childhood, these deficits are compounded over time with loss of skill acquisition, neuro-developmental and neurobehavioral problems, and risk of social maladjustment, and unemployment. The culmination is poor health-related quality of life (HRQL) for survivors and their families (4–6).
Interventions to alleviate neurocognitive dysfunction in pediatric BT survivors have included trials of cognitive remediation (7), and stimulant pharmacotherapy targeting the cognitive domain of attention (8, 9). Although deficits in the core domain of attention are one manifestation of neurocognitive dysfunction, up to 40% of survivors who received cranial radiation have not met the profile for attentional dysfunction in prior studies (10). Additionally, trials of the stimulant methylphenidate noted a substantial rate of study termination due to medication side effects among BT survivors, suggesting their vulnerability with this agent (11). Thus, treatment strategies targeting alternative neural pathways or neuropsychologic domains are needed (3). No studies of pharmacologic agents targeting executive function have been done to date.
Executive function is a multi-dimensional construct of supervisory regulation of cognition and behavior, influenced by the core domains of attention and memory, and germane to everyday real-world functioning. Acetylcholinesterase (AChe) inhibitors have demonstrated efficacy in stabilizing executive functioning, cognitive abilities, and behavioral symptoms in some adults with Alzheimer’s or vascular dementia (12, 13). More recently, AChe inhibitors have shown promise in improving executive function, attention and memory in adults and children with Down syndrome or autism (14–16). Donepezil (Aricept™, Eisai Pharmaceuticals, New London, CT), is the most widely used AChe inhibitor. The rationale for trials of AChe inhibitors in adult dementia is based on the putative role of cholinergic deficits associated with ischemia affecting basal forebrain nuclei (17). In younger children the role is hypothesized to be related to the role of the cholinergic system in neuronal differentiation and synapse formation (18).
Neurocognitive sequelae following whole brain or cranio-spinal radiation therapy (RT) in childhood are associated with decreased white matter volume and vasculopathy (19). Given that cranial radiation during childhood results in decreased cerebral perfusion and white matter loss at a time when neuronal differentiation is still occurring, we theorized that cholinergic deficits may contribute to impaired executive function in childhood BT survivors. Specific to the cancer population, donepezil was associated with significant improvement in multiple domains of neurocognitive function and HRQL in a 24-week Phase II study in adult survivors of partial or whole brain irradiation (20). These results led to the ongoing phase III study of Donepezil in adult BT survivors (clinicaltrials.gov ID: NCT00369785) and informed the development of our single institution, open label pilot trial of donepezil in pediatric BT survivors (clinicaltrials.gov ID: NCT00452868). The purpose of our study was to explore the hypothesized impact of donepezil on executive function in this population. We now report on the feasibility, tolerance and impact of donepezil on executive function and other neurocognitive domains.
METHODS
Potential study participants were identified from institutional pediatric or radiation oncology databases, based on the diagnosis of a primary tumor of the neuraxis and receipt of radiation. Recruitment was through an introductory letter to parents, or during scheduled visits to the pediatric or radiation oncology clinics, Phone follow up of the study invitation was done whenever contact information was available. Eligibility was established during a screening visit: 8–17 years of age; receipt of partial or whole brain radiation at dose > 23.4 Gy at ≥12 months prior to enrollment; no imaging evidence of tumor progression in the previous 3 months; no weight loss over the prior 6 month period; stable or decreasing steroid dose; Lansky or Karnofsky Performance Status score ≥70; no treatment for BT planned during course of study; estimated IQ > 70 based on the Peabody Picture Vocabulary Test (third edition; PPVT-III), and English speaking status.
Specific exclusion criteria were: stereotactic radiosurgery as sole treatment; steroid dose greater than physiologic replacement (18–30 mg/m2 hydrocortisone equivalent); concurrent use of a cognitive function-enhancing drug including, any AChe inhibitor, ginkgo bilboa, methylphenidate, or other stimulants within 6 wk of study enrollment; pregnancy; Type 2 neurofibromatosis; diagnosis of attention deficit hyperactivity disorder before cancer diagnosis; or uncontrolled seizures or endocrinopathies. A two-tiered informed consent and child assent was obtained for: (1) screening with the PPVT-III; and then (2) for trial of donepezil. Participants received gift cards to a retail store upon completion of each visit to incentivize adherence. An Investigational New Drug Application (IND) for donepezil was filed with the FDA, and received a status of “exempt.”
The intervention trial was an open-label study design with escalation to target dose of the pharmacologic agent at week 6, and a washout period off drug from weeks 24–36. Consenting participants meeting screening criteria were evaluated with a complete neurocognitive assessment (Table I), and neurological exam at baseline prior to start of the drug, week 12, week 24, and following drug washout at week 36. A clinic-based assessment and physical exam were repeated prior to dose escalation at week 6.
Table I.
Study assessments and median testing time (minutes) at baseline (week 0) in pilot study of donepezil in childhood BT survivors
| Neuropsychologic Domain | Measure | Week | Expected testing time# | Actual testing time (min) at wk 0 (range) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 12 | 24 | 36 | ||||
| Global Intelligence | Wechsler Abbreviated Scale of Intelligence (WASI)(44) | X | X | 30 | 40 (35–57) | ||
| Executive Function | Delis-Kaplan Executive Function System (D-KEFS)(21): subtests (Tower Test; Letter Fluency; Color-Word Interference-Inhibition; Sorting) | X | X | X | X | 65 | 63 (41–90) |
| Memory | Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML-2)(22) | X | X | X | X | 45 | 25 (17–60) 12 (2–15)) |
| Sustained Attention and Concentration | Conners Continuous Performance Test (CPT) II (23) | X | X | X | X | 14 | 22 (19–30) |
| Processing Speed | Wechsler Intelligence Scale for Children – IV (WISC-IV) Symbol Search subtest (24) | X | X | X | X | 5 | 4 (2–11) |
| Achievement | Woodcock Reading Mastery Test(25) | X | X | 25 | 28 (9–45) | ||
| Woodcock Johnson III Calculations (26) | X | X | 10 | 10 (5–13) | |||
| HRQL | Pediatric Quality of Life Inventory, version 4.0 (Peds QL™ 4.0)(30) | X | X | X | X | 6 (4–9) | |
| Quality of Life Assessment (QUOLA)(31) | X | X | X | X | 13 (8–25) | ||
| Total testing time for Child | 224 (167–278) | ||||||
| Parent Report of Cognition and Behavior | |||||||
| HRQL of child | Peds QL™ 4.0 | X | X | X | X | ||
| Executive Function of Child | Behavior Rating Inventory of Executive Functioning (BRIEF)(27): The Parent form | X | X | X | X | ||
| Behavioral Adjustment and Social Competency of Child | Behavior Assessment System for Children, Second Edition (BASC-2) (28) | X | X | X | X | ||
| Life Events Checklist: A 5 item assessment of major life events in the past year | X | X | |||||
| Family Impact | Pediatric Inventory for Parents (PIP): A 42 item evaluation of parenting stress related to the child’s chronic illness. | X | X | ||||
Estimates for completion of specified battery, based on test manufacturer indicated times: 195 min. for full battery; 130 min. for abbreviated battery
Neurocognitive assessments were broadly chosen to evaluate: executive function (Dellis-Kaplan Executive Function Test; D-KEFS)(21), memory (Wide Range Assessment of Memory and Learning, 2nd Edition; WRAML-2)(22), attention (Conners Continuous Performance Test (CPT) II)(23), processing speed (Wechsler Intelligence Scale for Children – IV (WISC-IV) Symbol Search subtest)(24) and achievement (Woodcock Reading Mastery Test; Woodcock Johnson III Calculations) (25, 26) (Table I, Supplemental Table I). The battery of tests was administered and timed by a licensed psychologist in the pediatric neuropsychology clinic. In addition, parents completed measures on executive function (Behavior Rating Inventory of Executive Function; BRIEF) (27). Parent report on behavior (Behavior Assessment System for Children, Second Edition (BASC-2) (28) and family function, (29) child and parent proxy report on HRQL (30, 31) will be reported separately. The Wake Forest School of Medicine Institutional Review Board approved the study invitation, consenting, screening, and intervention phases and associated forms.
Donepezil was bottled by the institutional pharmacy, and dispensed at scheduled study visits. Based on prior pediatric studies (14, 16), donepezil was dosed based on the child’s weight: 5 mg orally once a day, with escalation to 10 mg daily at week 6 in participants ≥ 35 kg; 5 mg on an alternate day schedule, with escalation to 5 mg daily at week 6 in subjects < 35 kg. Parents were provided a list of common side effects, and instructed to contact the study team if any symptoms developed upon initiation of the drug.
A baseline review of systems was obtained and scored using the NCI Common Toxicity Criteria for Adverse Events (CTC - version 3.0) to establish prevalent symptoms (32). Symptoms were re-assessed at weeks 6, 12, 24 and 36, with additional evaluation for side effects by phone contact at weeks 1 and 8. Based on the adult experience with donepezil, pre-defined toxicities specifically assessed included fatigue, anorexia, insomnia, nausea, vomiting, diarrhea and muscle cramps. Additional symptoms were coded and tracked based on parent report and medical record review for the duration of study or 36 weeks post enrollment, whichever came last. Stopping rules were set such that the study would be halted if the incidence of grade 4 or 5 events exceeded 35% at any point over 24 weeks.
Demographics were collected on the patient, parent, and family unit using the 18-item Child Health Ratings Inventories (CHRIs) demographics form (33), supplemented with items from the National Survey on Children with Special Health Care Needs (http://cshcndata.org/content/Guide2005). Cancer treatment history and co-morbidities were abstracted from the treating institution’s medical records. Adherence to prescribed donepezil was evaluated both by PI review of diaries returned by parent(s), and pill counts done by the study nurse on return visit (s). We defined “good adherence” as return of a bottle with less than five pills.
Statistical considerations
Descriptive statistics were used to summarize patient demographics, clinical characteristics, testing time and toxicities. Characteristics of completers and non-completers were compared using t-tests. Changes over time in the neurocognitive battery measures and the BRIEF parent report were estimated using a longitudinal mixed model adjusted for age at radiation and baseline IQ. Changes in neurocognitive performance at 24 weeks are expressed as effect size (ES) based on the adjusted change from baseline to 24 weeks divided by the population standard deviation. Effect sizes are interpreted as: small = 0.20 to 0.49; medium = 0.50 to 0.79; and 0.80 and above = large (34). Negative ES indicates improvements in measures that are initially reverse scored (CPT-II; BRIEF), such that higher scores suggest a higher level of dysfunction. All statistical tests were performed in SAS (v. 9.1, SAS Institute, Cary, NC), and were two tailed, with the threshold for significance set at p<0.05.
RESULTS
Screening and participant selection
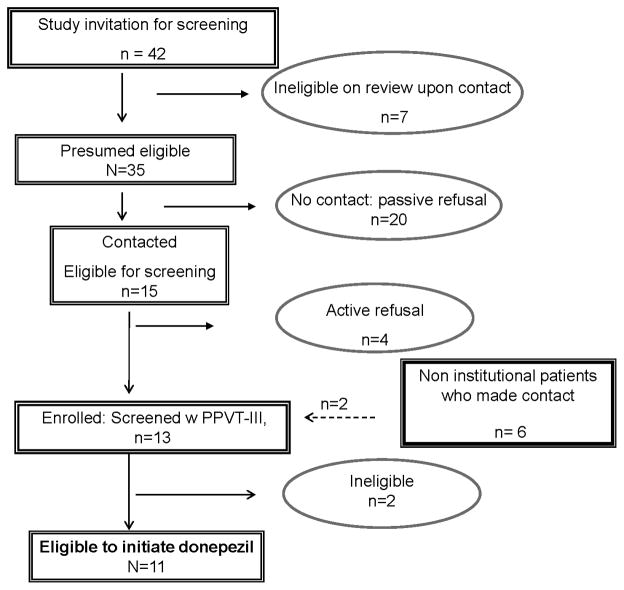
Among 35 childhood BT survivors who were sent study invitations there were 20 passive refusals and 4 active refusals (Figure 1). Parent articulated reasons for active refusals included: time constraint; no perceived cognitive dysfunction in the survivor; reluctance to add another medication; reluctance to invest time in with no planned feedback of neuropsychological testing results for clinical or academic use.
Figure 1.
Pediatric brain tumor patients identified, screened and enrolled in pilot trial of donepezil.
Between September 2006 and September 2009, 11 (31%) institutional and 2 BT patients treated elsewhere were enrolled and screened. The median standardized PPVT-III score among 13 screened subjects was 89 (range 61–117), as previously reported (35). One patient was ineligible for donepezil due to uncorrected hypothyroidism on baseline evaluation. A second patient was taken off study at week 13 after being found ineligible by re-review of PPVT-III scoring. We report on the efficacy of donepezil on all eligible participants (n=11), and toxicity among all who initiated drug in this pilot setting (n=12).
Patient Characteristics
Baseline demographics, treatment characteristics and baseline co-morbidities of eligible participants who received donepezil are listed in Table II. Most subjects received combined modality therapy (n=8). Median age at cranial RT was 5.6 years (range 4.2–13.2 years), and the median time from RT to study enrollment was 4.7 years (range 1.9–11.9 yrs). The median cumulative whole or partial brain dose of radiation was 55.8 Gy (50.4–60.3 Gy). Among the nine patients who received craniospinal field with a posterior fossa boost, the median whole brain dose was 36 Gy (23.4–45 Gy) with a boost of 24.3 Gy (10.8–32.4 Gy).
Table II.
Baseline characteristics of childhood brain tumor survivors eligible for donepezil (n=11)
| Sex | |
| Male | 6 (55%) |
|
| |
| Race | |
| White | 10 (91%) |
| Asian | 1 (9%) |
|
| |
| Age at study enrollment (years)* | 11.1 (9.3–17.3) |
|
| |
| Parent education# | |
| High School or less | 3 (27%) |
| High school + some college | 8 (73%) |
|
| |
| Annual household Income# | |
| <20,000 | 1(8%) |
| 20–34,999 | 3 (25%) |
| 35,000–49,999 | 2 (17%) |
| 75,0000+ | 5(45%) |
|
| |
| Parent marital status (married)# | 7 (63%) |
|
| |
| Diagnosis | |
| Medulloblastoma | 7 |
| Craniopharyngioma | 1 |
| Pilocytic astrocytoma | 1 |
| Ependymoma | 1 |
| Pineoblastoma | 1 |
|
| |
| Age at cranial RT* | 5.55 (3.4–13.2) |
|
| |
| Cranial RT Dose (Gy)* | 55.8 (50.4–60.3) |
|
| |
| Median time since radiation (years) | 4.7 (1.9–11.9) |
|
| |
| Chemotherapy treatment | |
| Yes | 8 (73%) |
|
| |
| Lansky/Karnofsky performance status | 90 (70–100) |
|
| |
| Endocrinopathy## | |
|
| |
| GH deficiency | 7 (64%) |
|
| |
| Cortisol deficiency | 3 (27%) |
|
| |
| Hypothyroidism | 7 (64%) |
|
| |
| VP shunt | 2 (18%) |
|
| |
| Cranial nerve deficit | 7 (64%) |
|
| |
| Gross motor deficit | 6 (55%) |
Median and range;
based on CHRIS demographics;
RT= radiation therapy;
Mean(sd) Intelligence quotient (IQ) measured by Weschler Abbreviated Scale of Intelligence(WASI)(44);
Patients on hormone replacement therapy at time of study
Feasibility and adherence
Seventy-two percent (8/11) of participants completed the week 24 assessment. Dropouts occurred at median of 16 weeks (range 11 to 29.6 weeks). Reasons for non-completion included: perceived lack of efficacy by patient or parent (n=2; beyond week 12); nonspecific report of deviant behavior on study drug (n=1; after dose escalation but prior to week 12); misunderstanding of end of study drug period (n=1; following week 12 visit); and loss of contact during drug washout period (n=1). Baseline characteristics of non-completers were not statistically different from completers with regard to age or time since RT, or full scale IQ.
Medication adherence diaries were returned by 64% (7/11), 45% (5/11) and 25% (2/8) of participants at weeks 6, 12 and 24 respectively. Good adherence, was noted in 63% (5/8), based on pill count at week 24. At the earlier time points, good adherence was detected in 75 % (9/12) at week 6 and 64% (7/11) at week 12.
The baseline and week 24 neurocognitive assessment was completed in 224 minutes (3.7 hrs; range 167–278 min), and the abbreviated (weeks 12 and 36) version in 141 minutes (2.4 hrs; range 106–169 min). Testing times in this group, which exceed test manufacturer estimates (Table I), do not include additional break times needed to accommodate a high level of participant testing fatigue noted by the neuropsychologist.
Toxicity
Table III delineates new symptoms reported during donepezil therapy, compared to symptoms at baseline. All subjects reported at least one symptom (grade 1 or 2, CTC v.3) prior to beginning donepezil. Prevalent complaints at baseline included: fatigue (92%), anorexia (33%), intermittent nausea (25%), insomnia (25%), muscle pain (42%) and headaches (8%). New symptoms of transient diarrhea and vomiting were reported by 30% of participants within two weeks of initiation or dose escalation of the study drug. One CTC grade 3 event of emesis and fever required hospitalization for IV hydration and administration of of stress dose hydrocortisone in a child with stable pan-hypopituitarism at baseline. There was a 17% (2/12) incidence of non-specific mood alteration (defiant behavior, n=1) and confusion (n=1), which led to study dropout.
Table III.
NCI grade 1/2/3 toxicities in childhood brain tumor survivors at study entry and during donepezil therapy
| Baseline (n = 12) (%) | Week 1 (n=11) (%) | Week 6 (n=11) (%) | Week 8 (n=11) (%) | Week 12 (n = 11) (%) | Week 24 (n = 7) (%) | Week 36a (n = 7) (%) | |
|---|---|---|---|---|---|---|---|
| Anorexia | |||||||
| Total | 4 (33.3) | 3 (27.3) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1(14.3) |
| Newb | NA | 2 (18.2) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||||||
| Nausea | |||||||
| Total | 3 (25.0) | 1 (9.1) | 3 (27.3) | 0 (0.0) | 3 (27.3) | 1 (14.3) | 0 (0.0) |
| New | NA | 1 (9.1) | 1 (9.1) | 0 (0.0) | 2 (18.2) | 1 (14.3) | 0 (0.0) |
|
| |||||||
| Fatigue | |||||||
| Total | 11 (91.7) | 2 (18.2) | 4 (36.4) | 3 (27.3) | 7 (63.6) | 4 (57.1) | 4 (57.1) |
| New | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
|
| |||||||
| Insomnia | |||||||
| Total | 3 (25.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 2 (28.6) | 1 (14.3) |
| New | NA | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 1 (14.3) | 1 (14.3) |
|
| |||||||
| Muscle Pain | |||||||
| Total | 5 (41.7) | 3 (27.3) | 2 (18.2) | 1 (9.1) | 4 (36.4) | 0 (0.0) | 1 (14.3) |
| New | NA | 1 (9.1) | 1 (9.1) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) |
|
| |||||||
| Dizziness | |||||||
| Total | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) |
| New | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||||||
| Headache | |||||||
| Total | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 1 (9.1) | 0 (0.0) | 0 (0.0) |
| New | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||||||
| Vomiting | 0 (0.0) | 1 (9.1) | 2 (18.2) | 0 (0.0) | 3 (27.3) | 1 (14.3) | 0 (0.0) |
|
| |||||||
| Diarrhea | 0 (0.0) | 1 (9.1) | 1 (9.1) | 0 (0.0) | 3 (27.3) | 1 (14.3) | 0 (0.0) |
|
| |||||||
| Fever and Dehydration | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 1 (14.3) | 0 (0.0) |
|
| |||||||
| Confusion | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) |
|
| |||||||
| Muscle Weakness | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 1 (14.3) | 1 (14.3) |
|
| |||||||
| Infection | 0 (0.0) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) |
|
| |||||||
| Urinary Incontinence | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
|
| |||||||
| Abdominal Pain | 0 (0.0) | 1 (9.1) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
Week 36 represents the evaluation following 12 weeks off donepezil;
Indicates not present at baseline
There was no decrease in weight among participants, and a resolution of anorexia in affected children (baseline report) by week 8. Patient weight, tracked at each visit, indicated a median increase of 1.5 kg (range −0.4 to 3 kg) from baseline to the last visit on drug. There was no adverse change in neurologic exam from baseline. The only subtle finding was improved dystonia in two patients while on donepezil, which recurred when off the drug.
Based on perceived benefit, caregivers requested re-initiation of the drug following the washout window in 75% (6/8) that completed 24 weeks of therapy. This represented 54% of all those who initiated donepezil.
Neurocognitive Outcomes on donepezil
Performance accuracy on the executive function D-KEFS Tower task increased significantly on donepezil, evidenced by a large effect on the Tower Total score, which changed from a mean baseline scaled score (adjusted for age at radiation and baseline IQ) of 8.3 to 11.7 at week 24 (ES 1.14; p<0.001) (Table IV). Improvement was also seen for the Time-per-Move Ratio score (ES 0.74; p=0.013) which assesses the ratio of time to accurate moves to reproduce the tower disc pattern. A medium effect was seen on the Color/Word Interference Inhibition (ES 0.62; p=0.04), a subtest which assesses verbal inhibition, simultaneous processing and cognitive flexibility. However, there were no significant changes in Letter Fluency and Sorting tasks. After the drug washout period, the mean Tower Total score dropped to 10.1, which was not significantly lower than the 24 week mean (ES compared to 24 weeks 0.53; p=0.0703). While parent report by the BRIEF indicated a small, non-significant improvement in global executive function (ES −0.33, p=0.47), there was a medium effect on the plan/organize (ES −0.66; p=0.06), and working memory (ES −0.50, p=0.26) clinical scales. Notably there was also a medium impact on the emotional control scale by parent report (ES −0.74, p=0.08).
Table IV.
Neurocognitive functioning at baseline and 24 week change on Donepezil
| Neurocognitive Test | Baseline Least Squares Mean^ | Effect Size (SE)† Baseline to Week 24 | p-value |
|---|---|---|---|
| Global Intelligence* | |||
| WASI Verbal IQ | 89.8 | −0.06 (0.18) | 0.7365 |
| WASI Performance IQ | 83.4 | 0.05 (0.12) | 0.6563 |
| WASI Full scale IQ | 85.6 | −0.01 (0.09) | 0.9016 |
| Executive Functioning** | |||
| D-KEFS Tower Total | 8.3 | 1.14 (0.27) | 0.0003 |
| D-KEFS Towers Time Ratio | 7.7 | 0.74 (0.27) | 0.0131 |
| D-KEFS Letter Fluency | 6.6 | −0.02 (0.21) | 0.9250 |
| D-KEFS Color/Word Inhibition | 8.6 | 0.62 (0.28) | 0.0408 |
| D-KEFS Sorting | 4.9 | 0.24 (0.19) | 0.2330 |
| Memory | |||
| WRAML-2 Visual Memory* | 81.5 | 0.67 (0.22) | 0.0070 |
| WRAML-2 Verbal Learning*** | 7.7 | 0.32 (0.21) | 0.1495 |
| WRAML-2 Number/Letter*** | 7.8 | 0.39 (0.15) | 0.0180 |
| Sustained Attention and Concentration*** | |||
| CPT II Omissions@ | 62.1 | −0.59 (0.49) | 0.2366 |
| CPT II Commissions@ | 49.1 | −0.15 (0.25) | 0.5523 |
| CPT II Response Style@ | 57.0 | −0.67 (0.46) | 0.1531 |
| CPT II Detectability(d′)@ | 56.6 | −0.65 (0.35) | 0.0731 |
| Processing Speed** | |||
| WISC-IV Symbol Search | 4.8 | 0.45 (0.27) | 0.1048 |
Model is adjusted for baseline IQ and age at radiation therapy;
Effect size calculated as difference score (adjusted difference between 24 weeks and baseline) divided by test standard deviation;
mean=100, s.d.=15;
mean=10, s.d.=3;
mean=50, s.d.=10;
CPT is reverse scored such that negative effect sizes represent improvement;
WASI=Weschler Abbreviated Scale of Intelligence; IQ= intelligence quotient; D-KEFS= Dellis-Kaplan Executive Function Scale; WRAML-2= Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML-2); CPT II= Connor’s Performance Test II; WISC-IV=Wechsler Intelligence Scale for Children, 4th Edition
Memory was positively impacted by donepezil, measured by a medium effect in the mean Visual Memory composite score on the WRAML-2 (ES 0.67; p=0.007). There was a small effect in Number/Letter Memory, repeating number/letter strings (ES 0.39; p=0.018).
On measures of attention and concentration, there were non-significant but medium effects in two indicators from the CPT-II at 24 weeks: Detectability score (d prime) (ES −0.65; p=0.073) which measures attentiveness to discrimination between a target and non target; and Overall Response Style (ES −0.67; p=0.15), an indicator of willingness to risk speed for accuracy. Notably, the effects for these two measures were significantly stronger at 12 weeks: d prime (ES −0.91; p=0.0094); Overall Response Style (ES −0.88; p=.048). There was no observed impact on processing speed as measured by the WISC symbol search, or in achievement on the Woodcock Reading or calculations test over the study period.
DISCUSSION
Metrics for evaluating feasibility of interventions in survivorship studies include: acceptability; recruitment and retention timelines; detailed assessment of side effects; and preliminary evidence of efficacy (36). In the first trial of an AChe inhibitor in childhood cancer survivors we demonstrate that donepezil is well tolerated with no weight loss and no adverse neurologic symptoms over a 6-month period in children who received high doses of ionizing cranial RT for a primary BT. Importantly, in an open label pilot setting we report preliminary evidence of efficacy of this AChe inhibitor on executive function, and memory. Notably, this is the longest exposure to donepezil reported in a childhood trial (18, 37). Consistent with manufacturer listed side effects in adults and previous childhood trials (18, 37), we observed transient, non dose-limiting gastrointestinal symptoms within two weeks of drug initiation.
Despite the burden of cognitive dysfunction in BT survivors, our acceptance rate for this pharmacologic intervention trial was 31% (11/35 eligible) in a single institution setting. Enrollment of childhood cancer patients in trials of anti-tumor therapy has been exemplary (38), yet similar success in survivorship intervention studies has been challenging. While parents of BT survivors live with the daily burden of their child’s neurocognitive dysfunction, competing priorities of return to work and school limit acceptability of longitudinal studies in the survivorship period. This is a reminder of changing parent perception of needs after completion of cancer therapy. The barriers to accrual in longitudinal survivorship studies have been reported by others studying behavioral and pharmacologic interventions in this population (39, 40). As neurocognitive late effects are recognized as part of a continuum of the BT experience, future studies need to consider pharmacologic intervention during or juxtaposed to completion of cancer treatment, with assessment batteries and study schedules that minimize days of school and work missed for survivor and parent respectively.
We did not observe weight loss over the treatment period on donepezil, in contrast to the methylphenidate experience (41). Our evaluation of toxicity of an AChe inhibitor builds on prior experience with methylphenidate in childhood cancer survivors, where individual tolerability to methylphenidate was related to the number and severity of baseline behavioral and somatic symptoms (11), many of which are paradoxically considered to be adverse effects of stimulant medicines. Similarly, we observed many baseline symptoms in our study group. Specifically, a required overnight hospitalization (Grade 3 vomiting) in one survivor of craniopharyngioma with panhypopituitarism, chronic nausea and headaches at baseline underscores the vulnerability of BT survivors, and the need for caution and careful titration of pharmacologic interventions into the survivorship period.
While tasks designed to evaluate executive function are sensitive to injury in the frontal lobes, it is increasingly found that executive functioning requires coordination of activity among diffuse anatomical and functional areas of the brain (42). This indicator of a child’s ability to organize, plan, self-regulate, and maintain cognitive flexibility is impaired in BT patients who received cranial RT, and may in fact be more indicative of real-world function than attention or memory measured alone. Consistent with our hypothesis, and with outcomes in adult cancer populations (20), we observed enhancement of executive function in childhood BT survivors, evidenced by significant performance improvement on the D-KEFS Tower Task and by improvement on the plan/organize scale on the BRIEF while on donepezil. The Tower test required planning and higher order organizational skills, as the child followed a set of rules to match a pattern by moving disks of varying size onto three vertical pegs. Furthermore, on the Tower task subjects made a greater number of correct moves in less time, suggesting more efficient processing while on donepezil. Improvement during dose escalation and trend of decline during the 12-week washout further supports drug response. Drug-associated improvement in inhibition on the D-KEFS Color/Word task may indicate increased attention and a change to a more cautious response style. The other indicators supporting this change in response style on drug include better ability to detect the target correctly (d prime), and the trend toward fewer errors of omission on the CPT-II. Improved memory span is a further indication that donepezil improves focus in this population.
While we saw moderate effect sizes on several correlative clinical scales reported by parents on the BRIEF (improved planning and organizing, working memory, and emotional control), none of them was statistically significant. This may be due to lack of statistical power in our pilot study. Alternatively, while the BRIEF has been a good indicator of impaired in executive function in many childhood populations, its sensitivity to change over a longitudinal study in this population is unknown (43). Parent request for re-initiation of drug, indicates that at least some parents detected subjective improvements in their child’s function on donepezil.
In conclusion, successful remediation of neurocognitive dysfunction in BT survivors may require a combination of behavioral, educational, and pharmacotherapeutic interventions. Given that donepezil was tolerated well in our pilot experience together with our preliminary data supporting improvements in neurocognitive function, a larger randomized, placebo-controlled clinical trial of safety and efficacy may be justified. Additionally, we were struck by the fact that 54% of those who initiated drug requested re-initiation after the completion of the study. This suggests that patients and their parents are motivated to seek treatment for neurocogntive dysfunction. Our preliminary data add the AChe class of agents to consideration for radiation-induced brain injury in children.
Supplementary Material
Table V.
Executive function, measured by parent report on the Behavior Rating Inventory of Executive Function (BRIEF)
| Least Square Means Baseline T-Scores^ | Effect Sizes† Baseline to Week 24 | p-value | |
|---|---|---|---|
|
| |||
| BRIEF – Behavioral Regulation Index | 56.5 | −0.47 (0.42) | 0.2749 |
| Inhibit | 52.4 | −0.23 (0.35) | 0.5135 |
| Shift | 59.3 | −0.16 (0.47) | 0.7332 |
| Emotional Control | 56.3 | −0.74 (0.40) | 0.0847 |
|
| |||
| BRIEF – Metacognitive Index | 62.3 | −0.38 (0.44) | 0.4039 |
| Initiate | 59.5 | −0.09 (0.42) | 0.8421 |
| Working Memory | 67.2 | −0.50 (0.42) | 0.2585 |
| Plan/Organize | 62.0 | −0.69 (0.35) | 0.0669 |
| Organization of Materials | 55.6 | −0.05 (0.36) | 0.8807 |
| Monitor | 55.6 | −0.15 (0.36) | 0.6778 |
|
| |||
| BRIEF – Global Executive Function | 60.5 | −0.33 (0.44) | 0.4723 |
The BRIEF is reverse scored such that higher scores suggest a higher level of dysfunction (population mean =50, standard deviation=10), and scores of > 65 indicate patients at risk for dysfunction;
Effect size calculated as difference score divided by the test standard deviation of 10; Model is adjusted for baseline IQ and age at radiation therapy.
Acknowledgments
This study was supported by a Lance Armstrong Foundation Young Investigator Award (SMC) and R25 CA122061; S.M.C.
We are grateful to Mr. Graham Keyes for data management, test re-scoring, and data entry; to Ms. Shierina Fareed for support with regulatory issues; to Ms. Terry Gray for manuscript preparation assistance.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to disclose.
References
- 1.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the united states. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 2.Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: A report from the children’s oncology group. Archives of pediatrics & adolescent medicine. 2007;161:798–806. doi: 10.1001/archpedi.161.8.798. [DOI] [PubMed] [Google Scholar]
- 3.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhat SR, Goodwin TL, Burwinkle TM, et al. Profile of daily life in children with brain tumors: An assessment of health-related quality of life. J Clin Oncol. 2005;23:5493–5500. doi: 10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]
- 5.Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2004;22:999–1006. doi: 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- 6.Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10:293–310. doi: 10.1097/01.nrl.0000144287.35993.96. [DOI] [PubMed] [Google Scholar]
- 7.Butler RW, Copeland DR. Attentional processes and their remediation in children treated for cancer: A literature review and the development of a therapeutic approach. J Int Neuropsychol Soc. 2002;8:115–124. [PubMed] [Google Scholar]
- 8.Thompson SJ, Leigh L, Christensen R, et al. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol. 2001;19:1802–1808. doi: 10.1200/JCO.2001.19.6.1802. [DOI] [PubMed] [Google Scholar]
- 9.Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10:180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- 10.Conklin HM, Khan RB, Reddick WE, et al. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: A randomized, double-blind, cross-over trial. J Pediatr Psychol. 2007;32:1127–1139. doi: 10.1093/jpepsy/jsm045. [DOI] [PubMed] [Google Scholar]
- 11.Conklin HM, Lawford J, Jasper BW, et al. Side effects of methylphenidate in childhood cancer survivors: A randomized placebo-controlled trial. Pediatrics. 2009;124:226–233. doi: 10.1542/peds.2008-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowen DM, Francis PT, Pangalos MN, et al. Treatment strategies for alzheimer’s disease. Lancet. 1992;339:132–133. doi: 10.1016/0140-6736(92)91050-i. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 14.Kishnani PS, Sullivan JA, Spiridigliozzi GA, et al. Donepezil use in down syndrome. Arch Neurol. 2004;61:605–606. doi: 10.1001/archneur.61.4.605-b. [DOI] [PubMed] [Google Scholar]
- 15.Spiridigliozzi GA, Heller JH, Crissman BG, et al. Preliminary study of the safety and efficacy of donepezil hydrochloride in children with down syndrome: A clinical report series. Am J Med Genet A. 2007;143A:1408–1413. doi: 10.1002/ajmg.a.31790. [DOI] [PubMed] [Google Scholar]
- 16.Hardan AY, Handen BL. A retrospective open trial of adjunctive donepezil in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 2002;12:237–241. doi: 10.1089/104454602760386923. [DOI] [PubMed] [Google Scholar]
- 17.Erkinjuntti T, Roman G, Gauthier S. Treatment of vascular dementia--evidence from clinical trials with cholinesterase inhibitors. J Neurol Sci. 2004;226:63–66. doi: 10.1016/j.jns.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Handen BL, Johnson CR, McAuliffe-Bellin S, et al. Safety and efficacy of donepezil in children and adolescents with autism: Neuropsychological measures. J Child Adolesc Psychopharmacol. 2011;21:43–50. doi: 10.1089/cap.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 20.Shaw EG, Rosdhal R, D’Agostino RB, et al. A phase ii study of donepezil in irradiated brain tumor patients: Effect on health-related quality of life and mood. Neuro-oncol. 2004;6:357. [Google Scholar]
- 21.Delis D, Kaplan E, Kramer J. Delis-kaplan executive function scale. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 22.Sheslow D, Adams W. Wide range assessment of memory and learning. 2. Wilmington, DE: Wide Range, Inc; 2003. [Google Scholar]
- 23.Conners C. Conner’s continuous performance test (CPTII) North Tonawanda, NY: Multi-Health Systems, Inc; 2004. [Google Scholar]
- 24.Weschler D. Weschler intelligence scale for children. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 25.Woodcock R. Woodcock johnson reading mastery-revised, normative update. Pines, MN: American Guidance Service; 1998. [Google Scholar]
- 26.Mather N, Woodcock R. Woodcock-johnson III tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 27.Gioia G, Isquith P, Guy S, et al. Behavior rating inventory of executive function. Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- 28.Reynolds C, Camphaus R. Behavior assessment system for children. 2. Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 29.Stein RE, Jessop DJ. The impact on family scale revisited: Further psychometric data. J Dev Behav Pediatr. 2003;24:9–16. [PubMed] [Google Scholar]
- 30.Varni JW, Seid M, Kurtin PS. Pedsql 4.0: Reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Medical care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Tao ML, Masterman-Smith M, Garvie PA, et al. Quality of life assessment (QUOLA) in pediatric brain tumor population: Feasibility and instrument properties of a new brain-specific instrument. Neuro-oncol. 2004;6:448. [Google Scholar]
- 32.Cancer therapy evaluation program. Common terminology criteria for adverse events, version 3.0. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 33.Parsons SK, Shih MC, Mayer DK, et al. Preliminary psychometric evaluation of the child health ratings inventory (chris) and disease-specific impairment inventory-hematopoietic stem cell transplantation (dsii-hsct) in parents and children. Qual Life Res. 2005;14:1613–1625. doi: 10.1007/s11136-005-1004-2. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 35.Castellino SM, Tooze JA, Flowers L, et al. The peabody picture vocabulary test as a pre-screening tool for global cognitive functioning in childhood brain tumor survivors. J Neurooncol. 2011;104:559–63. doi: 10.1007/s11060-010-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazak AE, Simms S, Alderfer MA, et al. Feasibility and preliminary outcomes from a pilot study of a brief psychological intervention for families of children newly diagnosed with cancer. J Pediatr Psychol. 2005;30:644–655. doi: 10.1093/jpepsy/jsi051. [DOI] [PubMed] [Google Scholar]
- 37.Kishnani PS, Heller JH, Spiridigliozzi GA, et al. Donepezil for treatment of cognitive dysfunction in children with down syndrome aged 10–17. Am J Med Genet A. 2010;152A:3028–3035. doi: 10.1002/ajmg.a.33730. [DOI] [PubMed] [Google Scholar]
- 38.Unguru Y. The successful integration of research and care: How pediatric oncology became the subspecialty in which research defines the standard of care. Pediatr Blood Cancer. 2011;56:1019–1025. doi: 10.1002/pbc.22976. [DOI] [PubMed] [Google Scholar]
- 39.Patel SK, Katz ER, Richardson R, et al. Cognitive and problem solving training in children with cancer: A pilot project. J Pediatr Hematol Oncol. 2009;31:670–677. doi: 10.1097/MPH.0b013e3181b25a1d. [DOI] [PubMed] [Google Scholar]
- 40.Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. Journal of consulting and clinical psychology. 2008;76:367–378. doi: 10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jasper BW, Conklin HM, Lawford J, et al. Growth effects of methylphenidate among childhood cancer survivors: A 12-month case-matched open-label study. Pediatr Blood Cancer. 2009;52:39–43. doi: 10.1002/pbc.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychology review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 43.Gioia GA, Kenworthy LIsquith PK. Executive function in the real world: Brief lessons from mark ylvisaker. The Journal of head trauma rehabilitation. 2010;25:433–439. doi: 10.1097/HTR.0b013e3181fbc272. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D. Wechsler abbreviated scale of intelligence (WASI™) San Antonio, TX: Harcourt Assessment Inc; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.