Abstract
Marijuana (MJ) remains the most widely abused illicit substance in the United States, and in recent years, a decline in perceived risk of MJ use has been accompanied by a simultaneous increase in rates of use among adolescents. In the current study, we hypothesized that chronic MJ smokers would perform cognitive tasks, specifically those that require executive function more poorly than control subjects, and that individuals who started smoking MJ regularly prior to age 16 (early onset) would have more difficulty than those who started after age 16 (late onset). Thirty-four chronic, heavy MJ smokers separated into early and late onset groups and 28 non-MJ smoking controls completed a battery of neurocognitive measures. As hypothesized, MJ smokers performed more poorly than controls on several measures of executive function. Age of onset analyses revealed that these between-group differences were largely attributed to the early onset group, who were also shown to smoke twice as often and nearly three times as much MJ per week relative to the late onset smokers. Age of onset, frequency and magnitude of MJ use were all shown to impact cognitive performance. Findings suggest that earlier MJ onset is related to poorer cognitive function and increased frequency and magnitude of MJ use relative to later MJ onset. Exposure to MJ during a period of neurodevelopmental vulnerability, such as adolescence, may result in altered brain development and enduring neuropsychological changes.
Keywords: Cognitive function, marijuana, adolescence, early onset
Introduction
Within the United States, marijuana (MJ) remains the most widely used illicit substance. In 2009, 16.7 million Americans aged 12 and older reported at least one instance of use in the past month, and MJ use within youths aged 12–17 rose to 7.3%, a significant increase from 2008 (SAMHSA, 2010). Relatively few studies have examined the direct relationship between age of onset of MJ use and cognitive performance, despite the alarming number of adolescent consumers. National survey data suggest that attentional problems exist in young smokers, with 71.7% of adolescents who used MJ at least once a week reporting trouble concentrating compared to 50.8% of non-smokers (SAMHSA, 1998). Additionally, surveys have shown that perceived risk and perceived disapproval are linked to increased rates of MJ use among adolescents, with current MJ use much less prevalent among youths who perceived strong parental disapproval for trying MJ than for those who did not (4.8 vs. 31.3 %) (SAMHSA, 2010). In recent years, a decline in perceived risk of MJ has been accompanied by a simultaneous increase in rates of use among adolescents (Johnston, O’Malley, Bachman, & Schulenberg, 2011). As rates of perceived risk decline and MJ use among adolescents increases, age of onset of first regular MJ use has also dropped significantly (Copeland & Swift, 2009; Degenhardt et al., 2008; SAMHSA 2010). Adolescence is a time of neuromaturation, with increasing evidence that the adolescent brain may be more vulnerable to the effects of drugs and alcohol than the adult brain (Monti et al., 2005), and those who are at the greatest risk for adverse consequences appear to represent a growing population of consumers of MJ (Schneider, 2008).
Previous studies focused on neurocognitive function have reported significantly altered frontal/executive function in MJ smokers. Pope and Yurgelun-Todd (1996) reported lower performance scores on tests designed to measure frontal/executive function in MJ smokers relative to control subjects, and Solowij and colleagues (2002) reported significantly worse performance on a battery of neurocognitive measures that included attention, memory and executive function in heavy MJ smokers relative to both lighter smokers and non-smoking controls. Studies of the cognitive effects of MJ following a brief abstinence period have also reported that heavy MJ use is associated with deficits in cognitive tasks mediated by the frontal system (Fletcher et al. 1996; Harvey, Sellman, Porter, & Frampton, 2007; Pope & Yurgelun-Todd, 1996). Bolla, Brown, Eldreth, Tate, & Cadet (2002) reported that heavy MJ smokers had persistent, dose-related cognitive decrements on a battery of neurocognitive tasks, including frontal/executive measures despite a full 28-day abstinence period. Harvey et al. (2007) administered a battery of cognitive tests to adolescent MJ users after 12 hours of abstinence, also finding significant cognitive deficits in smokers, specifically on tasks of verbal learning and spatial working memory. In a study of chronic MJ smokers abstinent for 24 hours, McHale and Hunt (2008) reported deficits in executive function in the smoking cohort as compared to both a non-smoking and a former smoking cohort. A recent review concluded that MJ use impairs memory, attention, inhibitory control, executive function, and decision making and that these effects can persist beyond acute intoxication, lasting days, weeks, or more, with long-term heavy MJ use resulting in longer-lasting cognitive abnormalities (Solowij & Pesa, 2010). Further, the authors found that greater adverse effects are associated with MJ use commencing in early adolescence.
This finding is consistent with those from studies of brain structure which utilized growth mapping techniques and indicate that the prefrontal cortex matures more slowly than other regions of the brain, and that the development of this region parallels the improvements in both cognitive control and behavioral inhibition that emerge during the transition into adulthood (Casey et al., 2005; Blakemore & Choudhury 2006). Sowell and colleagues reported that the largest maturational changes observed between twelve to sixteen and twenty-three to thirty years occurred in dorsal, medial, and lateral regions of the frontal lobes, as compared to parietal and occipital lobes (Sowell et al., 1999). One structural imaging study reported that the dorsolateral prefrontal cortex does not reach its full volume until the early twenties (Giedd et al., 2004), a finding of particular importance given that many complex executive processes continue to develop well into the early adult years (Hogan et al., 2005). Functional imaging studies have confirmed this finding and suggest a pattern of age-related activity within prefrontal areas during inhibitory tasks; heavier dependence on this region is noted in children as compared to adults, and during adolescence, the network recruited for this task is modified until adulthood when a more focal region is used to perform the same task (Blakemore & Choudhury 2006; Barker et al. 2010). Given this dynamic process, it is likely that the neuromaturational changes that occur during adolescence, which result in cognitive and emotional changes, may be adversely affected by early exposure to marijuana.
Perhaps not surprisingly, studies that have examined the relationship between age of onset of MJ use and cognitive function in adults have reported alterations on a range of tasks. Ehrenreich and colleagues (1999) reported that early onset MJ use (prior to age 16) predicted significantly longer reaction times on a task of visual scanning and attention, while those who began use at age 16 or later performed similarly to controls. Pope and colleagues (2003) analyzed cognitive data from long-term heavy MJ users following a 28-day period of abstinence, and compared early onset users (prior to age 17) with late onset users (use at age 17 or later). After adjusting for age, gender, ethnicity and family background, late onset MJ users were no different from control subjects; however, early onset users demonstrated reduced performance relative to control subjects on measures of verbal learning, fluency and overall verbal intelligence quotient (VIQ). The authors concluded that this difference may reflect an innate difference between groups in cognitive ability preceding first MJ use, an actual neurotoxic effect of MJ on the developing brain, or poorer learning of conventional cognitive skills by young MJ users who avoid academics. More recently, Novaes and colleagues (2008) reported that MJ smokers who started smoking before age 15 performed significantly worse on a battery of neurocognitive tasks requiring executive function and sustained attention relative to late onset MJ smokers and non-smoking controls. In an electrophysiology study of auditory selective attention in MJ smokers and controls, Kempel, Lampe, Parnefjord, Henning, and Kunert (2003) reported that MJ smokers had shorter latency negative components relative to controls. Within the MJ smokers, the early onset users (prior to age 16) exhibited greater impairment on the task than late onset MJ users. A more recent neurophysiology study using a modified Stroop task reported that chronic MJ smokers demonstrated increased errors on the interference condition relative to control subjects, and that poorer performance was predicted by earlier age of onset of MJ use (Battisti et al., 2010).
Given these findings and our own reports of altered brain function in MJ smokers relative to non-smoking control subjects (Gruber, Rogowska, & Yurgelun-Todd, 2009; Gruber & Yurgelun-Todd, 2005), we explored the relationship between age of onset of MJ use and cognitive performance, with a focus on executive function, in chronic, heavy MJ smokers and non-MJ smoking controls. Further, in order to determine the specific impact of early onset MJ use, we directly compared the performance of early onset MJ smokers, defined as those with regular, heavy MJ use prior to age 16 to late onset MJ smokers, who began regular MJ use after age 16 and control subjects, as few studies thus far have made this comparison. We hypothesized that overall, MJ smokers would perform more poorly on tasks of cognitive function than non-MJ smoking control subjects and that individuals who started smoking MJ prior to age 16 would have more difficulty on the tasks relative to those who started later.
Methods
Participants
Selected from ongoing neuroimaging studies, thirty-four (29 male, 5 female) well-characterized, chronic, heavy, MJ smokers (22.8 ± 6.57 years) and twenty-eight (19 male, 9 female) non-MJ smoking healthy control participants (24.3 ± 6.64 years) were included in this investigation (see Table 1). Subjects were recruited from the greater Boston area, with participants from both downtown and suburban locations included. Recruitment sites included local colleges and universities, sports clubs and athletic centers, supermarkets, community centers and other public locations. All subjects received the Structured Clinical Interview for DSM-IV, Patient Edition (SCID-P) to ensure that no Axis I pathology was present and that they did not meet criteria for current or previous drug or alcohol abuse or dependence (excluding MJ abuse for the smokers), binge drinking, or routinely had more than 15 drinks per week. Given that diagnostic criteria for both alcohol abuse and dependence are exclusive of the total number of drinks per week, and our desire to enroll subjects without excessive alcohol use, we used criteria that is consistent with several of our previous investigations (Gruber et al., 2009; Gruber, Silveri, Dahlgren & Yurgelun-Todd, 2011; Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001). In addition, subjects were excluded if they reported more than 15 lifetime uses of any category of illicit drugs or had a positive urine screen for any illicit drug (excluding MJ for the smokers), were non-native English speakers, if they had ever experienced a head injury with loss of consciousness or associated symptoms or sequelae or reported a neurological disorder, or if they had ever used psychotropic medications.
Table 1.
Controls vs MJ Smokers Results
| Demographic Data | Controls | MJ Smokers | 1-way ANOVA Significance |
|---|---|---|---|
| N | 28(19M, 9F) | 34(29M, 5F) | - |
| Handedness | 26R, 2L | 31R, 3L | - |
| Age | 24.32±6.65 | 22.76±6.57 | NS |
| WASI (four-factor) | |||
| VIQ | 122.00±11.45 | 119.69±14.43 | NS |
| PIQ | 118.93±15.08 | 118.66±14.52 | NS |
| MJ-Related Variables | |||
| Urinary THC/creatinine level (ng/ml) | - | 414.13±556.11 | - |
| Age of Onset | - | 15.53±2.16 | - |
| Smokes/Week | - | 19.24±19.58 | - |
| Grams/Week | - | 10.86±14.95 | - |
| Duration of Use (yrs) | - | 7.24±7.30 | - |
| Clinical Data | |||
| BDI | 2.19±2.86 | 1.91±3.22 | NS |
| PANAS | |||
| Positive | 32.59±5.79 | 32.26±5.58 | NS |
| Negative | 11.37±2.12 | 12.62±3.46 | NS |
| POMS | |||
| Vigor | 20.56±3.95 | 19.33±4.40 | NS |
| Anger | 3.12±3.91 | 4.70±4.33 | NS |
| Confusion | 4.77±3.94 | 5.18±2.59 | NS |
| Tension | 5.79±5.17 | 5.48±3.67 | NS |
| Fatigue | 4.21±3.40 | 4.12±2.64 | NS |
| Depression | 3.75±3.87 | 3.21±4.00 | NS |
| Total Mood Disturbance | 1.08±17.72 | 3.36±15.04 | NS |
| HAM-A | 1.81±2.48 | 2.12±2.16 | NS |
| BIS | |||
| Attention | 15.32±3.85 | 16.93±2.36 | 0.062 |
| Motor | 19.60±4.89 | 22.57±4.04 | 0.017 |
| Non-Planning | 24.28±4.73 | 26.77±5.15 | 0.070 |
| Total | 59.20±10.28 | 66.27±8.80 | 0.008 |
| Neurocognitive Data | |||
| WCST | |||
| Deck 1 Categories | 4.45±1.14 | 3.87±1.34 | NS |
| Deck 2 Categories | 4.64±0.58 | 4.23±1.02 | 0.096 |
| Total Categories | 9.09±1.38 | 8.19±2.10 | 0.086 |
| Deck 1 Perseverations | 3.41±2.84 | 7.61±6.98 | 0.010 |
| Deck 2 Perseverations | 2.27±2.21 | 3.42±3.66 | NS |
| Total Perseverations | 5.68±3.55 | 11.03±9.28 | 0.013 |
| Loss of Set Deck 1 | 0.09±0.29 | 0.19±0.40 | NS |
| Loss of Set Deck 2 | 0.09±0.29 | 0.39±0.50 | 0.015 |
| Total Losses of Set | 0.18±0.40 | 0.58±0.72 | 0.022 |
| Stroop Color Word Test | |||
| Color Naming % Accuracy | 93.89±6.04 | 93.89±6.53 | NS |
| Color Naming Commissions | 2.00±2.42 | 2.24±2.24 | NS |
| Word Reading % Accuracy | 99.08±1.81 | 98.01±3.11 | NS |
| Word Reading Commissions | 0.29±0.54 | 1.45±1.99 | 0.004 |
| Interference % Accuracy | 96.63±5.61 | 92.96±11.41 | NS |
| Interference Commissions | 1.46±2.87 | 2.64±3.64 | NS |
| Trail Making Test | |||
| A Time (sec) | 21.25±5.96 | 22.50±5.69 | NS |
| A Errors | 0.11±0.32 | 0.25±0.51 | NS |
| B Time (sec) | 42.61±14.37 | 49.66±14.51 | 0.064 |
| B Errors | 0.21±0.50 | 0.28±0.46 | NS |
| ROCF | |||
| Copy Raw Score | 32.02±3.60 | 31.32±3.92 | NS |
| Delayed Recall Raw Score | 21.46±6.86 | 20.84±5.79 | NS |
| Digit Span | |||
| Forward | 9.89±2.06 | 10.50±4.22 | NS |
| Backward | 9.00±2.49 | 8.78±4.14 | NS |
| Total | 18.89±3.97 | 18.25±4.11 | NS |
| CVLT | |||
| Trial 1 Correct | 7.88±1.56 | 7.35±2.12 | NS |
| Trials 1–5 Correct | 57.81±8.71 | 55.35±8.96 | NS |
| Long Delay Correct | 12.69±2.75 | 12.57±2.97 | NS |
| COWAT | |||
| Corrected Total | 50.16±9.87 | 48.00±16.13 | NS |
| Animals | 25.84±5.90 | 23.00±4.50 | NS |
VIQ = Verbal IQ, PIQ = Performance IQ, THC = Tetrahydrocannabinol, HAM-A = Hamilton Anxiety Rating Scale, BDI = Beck Depression Inventory, PANAS = Positive and Negative Affect Scale, BIS = Barratt Impulsiveness Scale, POMS = Profile of Mood States, WCST = Wisconsin Card Sorting Test, ROCF = Rey-Osterrieth Complex Figure, CVLT = California Verbal Learning Test, COWAT = Controlled Oral Word Association Test
In order to qualify for study entry, MJ smokers had to have smoked MJ a minimum of 2500 times in their lives, used MJ at least five of the last seven days, test positive for urinary cannabinoids, and meet DSM-IV criteria for MJ abuse or dependence. MJ smokers were required to abstain from smoking for at least 12 hours before cognitive testing to ensure that they were not acutely intoxicated at the time of assessment and were told that they would have to give a urine sample upon arrival at the laboratory. To ensure compliance of the 12-hour abstinence, subjects were led to believe that this sample would allow us to detect use of MJ within this time frame, a method we have used previously with success (Gruber et al. 2011). Urine samples were tested for MJ, amphetamines, opioids, phencyclidine, barbiturates, benzodiazepines, and cocaine (Triage® Drugs of Abuse Panel: Immediate Response Diagnostics, San Diego, CA). This procedure was required for three reasons: (1) to exclude subjects who tested positive for other substances of abuse, (2) to determine whether subjects had used MJ recently enough to have a positive urine screen, and (3) to encourage subjects, as requested, to abstain from MJ from the previous evening until arriving at the laboratory to ensure subjects were not acutely intoxicated at the time of the visit. Subjects were repeatedly reminded that they would be tested for MJ use upon their arrival at the lab. A portion of the sample was sent to an outside laboratory for quantification of urinary cannabinoid concentration via gas chromatography–mass spectrometry (GC–MS). Prior to participation, study procedures were explained, and all subjects were required to read and sign an informed consent form approved by the McLean Hospital Institutional Review Board, which described in detail the procedures of the study and explained that participation in the study was voluntary.
Assessments and Procedures
All subjects completed a battery of neuropsychological tests over two visits, which assessed a range of cognitive domains and which were as follows:
Wechsler Abbreviated Scale of Intelligence (WASI): Provides a measure of general intellectual functioning and includes verbal and performance IQ estimates from 4 subtests (vocabulary, block design, similarities, and matrix reasoning (Wechsler, 1999)
Wisconsin Card Sorting Test (WCST): Considered by many to be the ‘gold standard’ measure of executive function, the WCST assesses the ability to shift and maintain set, form abstract concepts, and utilize feedback. In the current investigation, clarification of the rules was given to subjects after the completion of Deck 1, which has been done in previous studies to ensure acquisition of the rule structure before the presentation of Deck 2 (Stuss et al., 1983). Primary variables for analyses include total categories completed (high numbers = better performance), total number of perseverations (low numbers = better performance) and losses of set (low numbers = better performance). (Berg 1948; Lezak, Howieson & Loring, 2004)
Stroop Color Word Test: measures the ability to establish competing response tendencies, inhibit inappropriate responses, and resist interference. Variables for analyses include task accuracy per section and number of commission errors per section; commission errors occur when a given response is incorrect and therefore serve as an indicator of impaired inhibitory processing (MacLeod, 1991).
Trail Making Test: This measure of executive functioning, visual scanning, and cognitive set shifting abilities is comprised of two parts. Trails A measures psychomotor function and attention, while Trails B utilizes an alternative set shifting demand to measure cognitive flexibility and executive function. Performance on the Trail Making Test is measured by the time required to complete each subtest, as well as the number of errors made, which serve as the primary variables for analyses.
Rey-Osterrieth Complex Figure (ROCF): This test assesses visuo-organizational ability, visual attention, and visual memory. The subject is asked to copy a complex figure, which is then evaluated for strategy, constructional accuracy, and detail reproduction. An immediate and delayed recall are later administered and scored in the same manner. Variables for analyses therefore include copy, immediate recall, and delayed recall scores (Lezak et al., 2004).
Wechsler Adult Intelligence (WAIS-R) Digit Span Subtest: This subtest of the WAIS-R primarily measures the efficiency of attention. Subjects are asked to repeat strings of digits presented by the experimenter in part 1 (Forward), and in part 2 they are asked to repeat strings of digits in reverse order (Backwards). (Wechsler, 1955; 1981). Performance was evaluated by total number of correct trials on each subtest.
California Verbal Learning Test (CVLT): assesses verbal learning and memory using global measures of performance, which include total recall across Trial 1 (initial learning), total recall across Trials 1–5 (overall verbal learning), and long delay free recall (a measure of delayed verbal memory assessed 20 minutes after the initial presentation of material) (Delis, 1987).
Controlled Oral Word Association Test (COWAT): measures the subject's ability to produce individual words under restricted conditions. Words generated in response to a specific letter (F,A, or S) are reflective of executive function, while those generated in response to a semantic category (animals) request are more representative of verbal memory function (Bryan & Luszcz, 2000; Denckla, 1994). Performance is measured by an age and education corrected total of the number of words generated for F, A, and S, while a separate score is given for total number of animals named.
In order to assess clinical state at the time of assessment, subjects also completed a battery of clinical rating scales, including the Positive and Negative Affect Scale (PANAS; Watson, Clark & Tellegen, 1988), which provides a score for both positive and negative affect; the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock & Erbaugh, 1961), which provides a total score for depressive symptoms; the Hamilton Anxiety Scale (HAM-A; Hamilton, 1959), which provides a total score for symptoms related to anxiety, and the Profile of Mood States (POMS; Pollock, Cho, Reker & Volavka, 1979), which yields subscores for the individual domains of vigor, anger, confusion, tension, and depression, as well as a total mood disturbance score. Subjects also completed the Barratt Impulsiveness Scale (BIS; Patton, Stanford & Barratt, 1995), an instrument that measures impulsivity and provides subscales that cover the domains of attention, motor and planning, as well as a total impulsiveness score. The Fagerstrom Test for Nicotine Dependence (FTND) was also administered to all subjects
To assess the potential impact of age of onset on cognitive function, the MJ smokers were divided into early onset (regular MJ use prior to age 16; n=19) and late onset (regular MJ use at age 16 or older; n=15) groups. Although no uniformly accepted definition of early vs late onset exists, a number of studies, including our own, have used age 16 as a cutoff (Ehrenreich et al., 1999; Kempel et al., 2003; Gruber et al., 2011). It is important to note that because groups are created on the basis of age of onset, and not duration of MJ use, subjects with the same age of onset may have different duration of use, depending on their chronological age. Additionally, to assess differences in current use between the early and late onset smokers, frequency (smokes per week), magnitude (grams of MJ used per week), and mode of use were determined over 5 weeks using a time line follow back procedure during weekly check in visits. Lifetime use was also determined using the SCID-P.
Results
Demographics
As seen in Tables 1 and 2, all subject groups were well-matched for age and IQ. With regard to clinical state, no significant between-group differences were detected for the BDI, PANAS, POMS or HAM-A, suggesting the samples were well-matched for clinical state at the time of assessment and that they were affectively stable. Results from the SCID-P revealed that despite current, heavy regular use of MJ, no MJ smoker met full diagnostic criteria for MJ dependence, yet all met for MJ abuse. Further, no subject in either group met criteria for nicotine dependence in either group based on results from the FTND.
Table 2.
Three-way ANOVA Results
| Demographic Data | Controls | Early MJ Onset |
Late MJ Onset |
3-way ANOVA Significance |
|---|---|---|---|---|
| N | 28(19M, 9F) | 19(16M, 3F) | 15(13M, 2F) | - |
| Handedness | 26R, 2L | 17R, 2L | 14R, 1L | - |
| Age | 24.32±6.65 | 22.79±7.22 | 22.73±5.89 | NS |
| WASI (four-factor) | ||||
| VIQ | 122.00±11.45 | 115.67±16.76 | 124.86±8.82 | NS |
| PIQ | 118.93±15.08 | 116.67±16.77 | 121.21±11.07 | NS |
| MJ-Related Variables | ||||
| Urinary THC/creatinine level (ng/ml) | - | 509.16±674.42 | 293.75±341.17 | NS |
| Age of Onset | - | 14.11±1.33* | 17.33±1.59* | <0.001 |
| Smokes/Week | - | 24.80±24.33* | 12.19±6.87* | 0.061 |
| Grams/Week | - | 14.80±18.80* | 5.88±4.97* | 0.084 |
| Duration of Use (yrs) | - | 8.68±8.22 | 5.40±5.68 | NS |
| Clinical Data | ||||
| BDI | 2.19±2.86 | 1.33±2.14 | 2.64±4.20 | NS |
| PANAS | ||||
| Positive | 32.59±5.79 | 31.47±6.26 | 33.27±4.60 | NS |
| Negative | 11.37±2.12 | 12.11±3.26 | 13.27±3.69 | NS |
| POMS | ||||
| Vigor | 20.56±3.95 | 18.78±3.08 | 20.00±5.64 | NS |
| Anger | 3.12±3.91 | 3.89±3.43 | 5.67±5.18 | NS |
| Confusion | 4.77±3.94 | 4.78±2.37 | 5.67±2.85 | NS |
| Tension | 5.79±5.17 | 5.06±3.40 | 6.00±4.02 | NS |
| Fatigue | 4.21±3.40 | 3.67±2.43 | 4.67±2.87 | NS |
| Depression | 3.75±3.87 | 2.44±2.71 | 4.13±5.10 | NS |
| Total Mood Disturbance | 1.08±17.72 | 1.06±11.43 | 6.13±18.52 | NS |
| HAM-A | 1.81±2.48 | 1.84±1.74 | 2.50±2.65 | NS |
| BIS | ||||
| Attention | 15.32±3.85 | 16.87±2.47 | 17.00±2.32 | NS |
| Motor | 19.60±4.89* | 21.88±4.65 | 23.36±3.20* | 0.040 |
| Non-Planning | 24.28±4.73 | 27.56±5.70 | 25.86±4.47 | NS |
| Total | 59.20±10.28* + | 66.31±10.42* | 66.21±6.89+ | 0.031 |
| Neurocognitive Data | ||||
| WCST | ||||
| Deck 1 Categories | 4.45±1.14* | 3.47±1.42* | 4.36±1.08 | 0.039 |
| Deck 2 Categories | 4.64±0.58* | 3.94±1.14* | 4.57±0.76 | 0.032 |
| Total Categories | 9.09±1.38* | 7.59±2.32* | 8.93±1.59 | 0.029 |
| Deck 1 Perseverations | 3.41±2.84* | 10.29±8.01* + | 4.36±3.54+ | <0.001 |
| Deck 2 Perseverations | 2.27±2.21 | 4.29±4.30 | 2.36±2.44 | NS |
| Total Perseverations | 5.68±3.55* | 14.59±10.57* + | 6.71±4.97+ | <0.001 |
| Loss of Set Deck 1 | 0.09±0.29 | 0.29±0.47 | 0.07±0.27 | NS |
| Loss of Set Deck 2 | 0.09±0.29* | 0.53±0.51* | 0.21±0.43 | 0.006 |
| Total Losses of Set | 0.18±0.40* | 0.82±0.81* + | 0.29±0.47+ | 0.003 |
| Stroop Color Word Test | ||||
| Color Naming % Accuracy | 93.89±6.04 | 92.82±7.05 | 95.17±5.82 | NS |
| Color Naming Commissions | 2.00±2.42 | 2.39±2.12 | 2.07±2.43 | NS |
| Word Reading % Accuracy | 99.08±1.81 | 97.97±3.33 | 98.06±2.95 | NS |
| Word Reading Commissions | 0.29±0.54* | 1.56±2.18* | 1.33±1.80 | 0.014 |
| Interference % Accuracy | 96.63±5.61* | 90.03±13.22* | 96.48±7.83 | 0.041 |
| Interference Commissions | 1.46±2.87* | 3.67±4.10* | 1.40±2.61 | 0.054 |
| Trail Making Test | ||||
| A Time (sec) | 21.25±5.96 | 22.18±4.85 | 22.87±6.68 | NS |
| A Errors | 0.11±0.32 | 0.29±0.59 | 0.20±0.41 | NS |
| B Time (sec) | 42.61±14.37 | 51.06±15.09 | 48.07±14.16 | NS |
| B Errors | 0.21±0.50 | 0.41±0.51 | 0.13±0.35 | NS |
| ROCF | ||||
| Copy Raw Score | 32.02±3.60 | 30.65±4.18 | 32.14±3.55 | NS |
| Delayed Recall Raw Score | 21.46±6.86 | 19.94±5.44 | 21.93±6.22 | NS |
| Digit Span | ||||
| Forward | 9.89±2.06 | 9.94±1.77 | 11.21±6.12 | NS |
| Backward | 9.00±2.49 | 7.83±2.62 | 10.00±5.39 | NS |
| Total | 18.89±3.97 | 17.78±3.74 | 18.86±4.62 | NS |
| CVLT | ||||
| Trial 1 Correct | 7.88±1.56 | 6.94±2.05 | 7.86±2.18 | NS |
| Trials 1–5 Correct | 57.81±8.71 | 54.06±7.63 | 56.93±10.43 | NS |
| Long Delay Correct | 12.69±2.75 | 12.75±2.52 | 12.36±3.50 | NS |
| COWAT | ||||
| Corrected Total | 50.16±9.87 | 39.38±17.64* | 54.90±11.41* | 0.035 |
| Animals | 25.84±5.90 | 22.63±4.93 | 23.30±4.37 | NS |
&
indicate significant differences between means using Bonferroni post hoc analyses at p ≤ 0.10
VIQ = Verbal IQ, PIQ = Performance IQ, THC = Tetrahydrocannabinol, HAM-A = Hamilton Anxiety Rating Scale, BDI = Beck Depression Inventory, PANAS = Positive and Negative Affect Scale, BIS = Barratt Impulsiveness Scale, POMS = Profile of Mood States, WCST = Wisconsin Card Sorting Test, ROCF = Rey-Osterrieth Complex Figure, CVLT = California Verbal Learning Test, COWAT = Controlled Oral Word Association Test
Overall, MJ smokers had higher BIS scores for all domains relative to the control subjects, which reached statistical significance for the Motor (p=0.017) and Total (p=0.008) subscores. Similarly, the late onset smokers had significantly higher BIS Motor subscores than controls, and both the early and late onset smokers had significantly higher Total BIS scores as compared to controls. (Motor: p=0.04; BIS Total: p=0.03; See Table 1).
MJ Use Characteristics
Interestingly, relative to the late MJ onset group, the early onset MJ group reported smoking more than twice as many times per week (24.8 vs 12.2; p=0.06) and used nearly three times as much MJ per week (14.8g vs 5.9g/wk; p=0.08), both of which approached statistical significance. The early onset group also reported a longer duration of MJ use and had higher urinary cannabinoid concentration relative to the late onset group, which did not approach statistical significance. In order to isolate the specific contribution of age of onset of MJ use on the neurocognitive measures, we completed ANCOVAs which individually covaried for each MJ use variable that approached statistical significance between the early and late MJ onset groups (i.e. frequency (smokes/week) and magnitude (grams/week)). ANCOVA analyses revealed no significant differences from the original ANOVA analyses, suggesting that the differences between the early and late MJ onset groups were not due to the MJ use differences noted between the groups. Data from the original ANOVA analyses are therefore reported in Tables 1 and 2.
Neurocognitive Performance
Controls vs. MJ Smokers
As hypothesized, we detected several significant between-group differences in neurocognitive performance between healthy controls and MJ smokers. On the WCST, MJ smokers made significantly more perseverative errors on Deck 1 (p=0.01) and the deck 1 and 2 combined total (p=0.01) relative to control subjects. Additionally, MJ smokers had significantly more losses of set for deck 2 (p=0.02) and the combined deck 1 and deck 2 total losses of set (p=0.02). MJ smokers made significantly more errors of commission during the Word Reading subtest of the Stroop test (p=0.004) relative to controls, and although results did not reach statistical significance, controls had higher task accuracy scores and lower commission errors relative to MJ smokers on the interference condition of the test. For the Trail Making Test, despite similar performance on Trails A, MJ smokers showed a trend for slower completion times for Trails B (p=0.06). No significant differences were noted between controls and MJ Smokers for the remainder of the tasks, which included the ROCF, Digit Span, CVLT, and COWAT.
Controls, Early Onset MJ smokers, Late Onset MJ Smokers
In order to assess the specific contribution of age of MJ onset on cognitive performance, we completed analyses comparing controls, early and late onset smokers. As seen in Table 2, three-way ANOVA results revealed significant differences on the performance of the WCST, including categories completed for deck 1 (p=0.04), deck 2 (p=0.03), and total (p=0.03); perseverative errors for deck 1 (p<0.001), and total perseverative errors (p<0.001), losses of set on deck 2 (p=0.006), and total losses of set (p=0.003). Post hoc analyses using a Bonferroni correction revealed that early onset smokers performed significantly more poorly than control subjects on each of these measures. Early onset smokers also made significantly more perseverative errors for deck 1, total perseverations, and total losses of set relative to their late onset cohorts. For the Stroop Color Word Test, the groups performed similarly during the Color Naming condition of the task, and a significant between-group difference in Word Reading commission errors was noted for the controls compared to the early onset smokers (p=0.01). Performance of the Interference condition, which measures the ability to inhibit inappropriate responses, revealed a significant between-group difference for both accuracy (p=0.04) and commission errors (p=0.05) for the early onset MJ smokers relative to the control subjects. Additionally, while no between group difference was detected between controls and MJ smokers as a group on the COWAT, age of onset analyses revealed that early onset smokers generated significantly fewer words than late onset smokers (p=0.04) despite no significant difference in semantic category scores. No significant between-group differences were detected on the Trail Making Test, Rey-Osterrieth Complex Figure test, WAIS-R Digit Span subtest, or CVLT between the three groups.
MJ-Related Variables Affect Task Performance
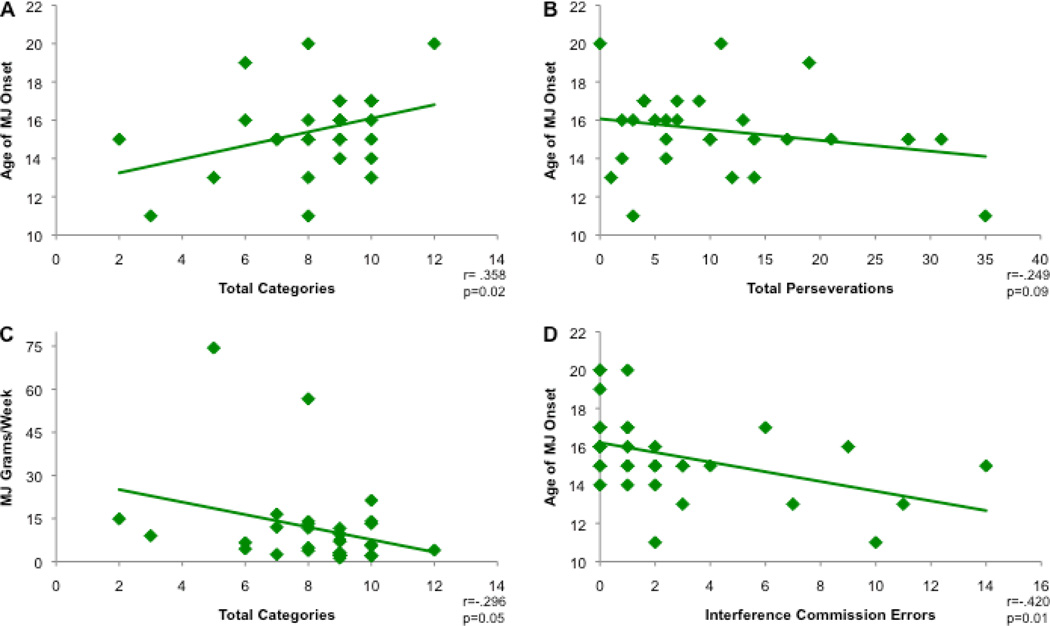
In order to determine the potential relationship between MJ use and cognitive performance, we completed correlation analyses for the MJ smokers as a group. Earlier MJ onset was significantly associated with the completion of fewer categories for deck 1 (r=0.30; p=0.05), deck 2 (r=0.37; p=0.02) and the combined deck total (r=0.36; p=0.02; see Figure 1A), while the relationship between earlier MJ onset and higher rates of perseverative errors for both deck 1 and total approached significance (r=−0.25; p=0.08; r=−0.25;p=0.09; see Figure 1B). Total grams of MJ used per week was also related to WCST performance; those who smoked greater amounts of MJ each week completed fewer total categories (r=−0.30; p=0.05; see Figure 1C).
Figure 1.
Age of onset of MJ use and (1A) Total Categories completed on the WCST; (1B) Total Perseverations on the WCST; Marijuana Grams per week and Total Categories completed on the WCST(1C); and Age of Onset of MJ use and Commission Errors on the Stroop Interference subtest; (1D)
Age of MJ onset as well as frequency and magnitude of MJ use were also related to impairments on the Interference condition of the Stroop test. Earlier age of MJ onset correlated with increased errors of omission (r=−0.32; p=0.03) and commission (r=−0.42; p=0.007; see Figure 1D) as well as lower performance accuracy (r=0.41; p=0.01) on the Interference condition of the Stroop. Higher grams of MJ used per week was also associated with poorer performance, including increased omission (r=0.30; p=0.05) and commission (r=0.66; p<0.001) errors, as well as lower performance accuracy (r=−0.50; p=0.002), and increased MJ smoking episodes per week were associated with more commission errors (r=0.54; p=0.001) and lower performance accuracy (r=−0.41; p=0.008).
Discussion
As hypothesized, results of this study demonstrate that chronic, heavy MJ smokers perform more poorly on several measures of cognitive performance relative to healthy non-MJ smoking control subjects, specifically on measures of executive function. Further, we found that individuals who started smoking MJ regularly prior to the age of 16 performed these tasks significantly more poorly than controls and smoked twice as often and nearly three times as much MJ per week than their later smoking counterparts. This is especially important, given the relationship detected between age of onset of MJ use, frequency and magnitude of MJ use and performance on measures of executive function. Moreover, while differences in frequency and magnitude of MJ use were noted between early and late onset groups, ANCOVA analyses confirmed that the observed differences in neuropsychological performance between the groups remained, even after separately co-varying for these MJ use-related variables. Taken together, these findings suggest that earlier onset of MJ smoking may have lasting effects on neurocognitive performance as well as the frequency and magnitude of MJ use, data that have important public policy implications.
Overall, these findings are in agreement with previously published studies, which have reported MJ-associated alterations in frontal function, most notably during tasks that require executive control, inhibition, and decision-making (Eldreth, Matochik, Cadet, & Bolla, 2004; Gruber & Yurgelun-Todd, 2005). Tapert et al. (2007) reported increased brain processing effort during a Go/No-go task as compared to non-using controls despite similar task performance. Further, both earlier age of onset and more lifetime MJ use episodes were associated with reduced inhibitory response. Using the same task, Hester, Nestor, & Garavan (2009) reported that earlier onset MJ use may be associated with poorer inhibitory control. Becker, Wagner, Gouzoulis-Mayfrank, Spuentrup, & Dauman (2010) reported a significant association between age of onset of MJ use and incongruent trial accuracy on a Stroop test, also suggesting that earlier regular MJ use is related to poorer inhibitory control.
Findings from the current study suggest that MJ smokers have more difficulty than controls in the ability to inhibit inappropriate responses and maintain cognitive set, two primary components of executive function, and that the early MJ onset group performed more poorly than their later MJ onset smoking counterparts. Specifically, on the WCST, widely considered a ‘gold standard’ of executive function, as a group, MJ smokers achieved fewer categories and made significantly more perseverative errors and losses of set than control subjects. Once separated by early and late MJ onset groups, analyses revealed that these differences were primarily attributed to the early onset MJ group, as they completed significantly fewer categories, made significantly more perseverative errors and had higher losses of set overall. It is of note that these differences persisted into Deck 2 of the task, despite an explanation of the sorting principles used which was given to subjects between Decks 1 and 2, suggesting that early onset MJ smokers were not able to adequately utilize feedback to improve their performance. Correlation analyses revealed a significant relationship between age of onset of MJ use and performance on this task, suggesting that earlier use, in addition to higher frequency and magnitude of use, compromised task performance. During the Stroop Interference condition, a measure of the ability to inhibit inappropriate response tendencies and cognitive control, MJ smokers had lower task accuracy scores and higher errors of commission relative to control subjects, which was statistically significant when the early onset group was directly compared to control subjects. This finding suggests that early MJ onset smokers are significantly more impaired than late MJ onset smokers on this measure, which is further underscored by the associations detected between MJ use variables and performance on the task. While MJ smokers did not differ from controls in the time required to complete Trails A, they took longer to complete Trails B, which approached statistical significance, suggesting that MJ smokers have difficulty with the set-shifting demands of Trails B and not the pure psychomotor component of Trails A. Finally, early onset smokers generated significantly fewer total words relative to late onset smokers on the COWAT but had similar semantic category scores, suggesting difficulty with the executive function component of the task relative to late onset smokers. Overall, these findings confirm our hypothesis of decreased executive function in MJ smokers relative to control subjects, and significantly worse performance in those who begin regular, heavy MJ use prior to age 16 as compared to those who begin after age 16.
It is of note that while this investigation revealed significant between-group differences on several measures of executive function, a number of measures which assess other cognitive domains did not, specifically those that assessed visuospatial construction and memory, attention, and verbal memory. These findings are consistent with previous reports which have demonstrated that not all cognitive domains are equally impacted by MJ use, and highlight the importance of utilizing a cognitive test battery which assesses multiple domains of function (Hart et al. 2010; Pope & Yurelun-Todd, 1996).
Importantly, data from this investigation revealed that early onset smokers smoked MJ twice as often and nearly three times as much MJ per week relative to the late onset group, a finding that has particular relevance given the trend of a growing population of younger users. While this finding approached statistical significance using a conservative statistical approach (ANOVA), correlations between age of onset of MJ use, magnitude of MJ used per week, and executive task performance provide further evidence that age of MJ onset as well as magnitude of use are associated with compromised ability to perform tasks requiring cognitive control and inhibition. Taken together, these findings suggest that earlier onset of MJ smoking is related to poorer executive function as well as increased frequency and magnitude of MJ use relative to late onset, an important finding given the widespread use of MJ within the US. It is important to note that although earlier MJ onset is associated with higher rates of frequency and magnitude of current MJ use, analyses controlling for these variables still show significant task performance differences between early and late onset groups, suggesting that neuropsychological deficits are more impacted by age onset of MJ than current level of MJ usage.
While intriguing, results from the current study should be interpreted in light of several limitations. First, the data sample consisting of 34 and 28 subjects per study group is moderate in size, which may limit the overall generalizability of the study findings. Further, the significantly higher BIS scores noted in the MJ smokers relative to control subjects suggests a relationship between MJ smoking and impulsivity, however, the current study design does not allow us to determine cause from effect. Larger, longitudinal studies designed to assess the relationship between age of onset of MJ use, frequency and magnitude of use, behavioral measures and neurocognitive performance would be an important next step to clarify the precise impact of early exposure to MJ on the brain. In addition, while subjects in the current investigation were told to abstain from MJ for a minimum of 12 hours prior to neurocognitive testing and led to believe that we could determine if in fact they had smoked more recently via their urine screen, we cannot be certain that all subjects fulfilled this requirement. It is unlikely, however, that our results are due to acute marijuana intoxication. All study subjects reported being abstinent from marijuana use for a minimum of 12–16 hours prior to their scanning session, were matched for time since last MJ use and fully expected that the investigators would be able to tell if they had used the drug since the previous evening upon their arrival at the lab, underscoring the likelihood that subjects were not intoxicated at the time of testing. Indeed, no subject appeared intoxicated or even vaguely altered at the time of assessment, and all subjects were able to complete the tasks with minimal effort. While all of our subjects in the MJ smoking group were chronic, heavy users of MJ, and were required to smoke daily or a minimum of 4 of the last 7 days, none of those included in the current investigation met diagnostic criteria for MJ dependence, while all met for MJ abuse, Our findings may therefore be limited to individuals who do not endorse the more negative effects of marijuana use (i.e. psychological issues, inability to stop or cut down on use, withdrawal effects), and to those who do not meet for dependence, despite frequent, heavy use. In addition, while we did not find any statistically significant difference between the subject groups on any measure of clinical state or demographic variable, it is possible that the groups differed on measures which we did not assess, including social style and personality traits. It is of note, however, that our sample is comprised of fairly young adults, all of whom are clinically stable, are functioning at a fairly high level in either their academic or employment settings and who primarily belong to extended social groups, and we therefore do not believe that our findings are related to differences in social interactive style between the groups. Finally, both the control and MJ smoking groups were largely comprised of male subjects, which raises the question of generalizability of findings across gender. In order to ensure that findings were not due to the inclusion of primarily male subjects, we completed analyses with and without our female subjects, and did not see any significant difference in results.
The present study reports decrements in executive functioning in early onset MJ smokers, which are also associated with increased frequency and magnitude of MJ use. Fundamental neuromaturational processes have been shown to be regulated by the endocannabinoid system (Harkany et al., 2007), and a number of longitudinal studies have demonstrated that frontal brain regions responsible for higher-order cognitive processes, including cognitive control and behavioral inhibition are among the last to mature (Gogtay et al., 2004; Sowell et al., 2004; Blakemore and Choudhury, 2006). Exposure to MJ during a period of known neurodevelopmental vulnerability, such as adolescence, may therefore result in altered brain development and enduring neuropsychological changes. Given the recent decrease in perceived risk among adolescents and the increased prevalence of MJ use among adolescents in the United States, these findings underscore the importance of establishing effective educational and intervention strategies to prevent and/or decrease MJ use during these critical developmental years.
Acknowledgements
This project was supported by the National Institute of Drug Abuse (NIDA) [Grant number 5 R21 DA021241].
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/adb.
The authors declare that there is no conflict of interest.
References
- 1.Barker LA, Andrade J, Morton N, Romanowski CAJ, Bowles DP. Investigating the ‘latent’ deficit hypothesis: Age at time of head injury, implicit and executive functions and behavioral insight. Neurospsychologia. 2010;48:2550–2563. doi: 10.1016/j.neuropsychologia.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology. 2010;212:613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 4.Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Dauman J. The impact of early-onset cannabis use on functional brain correlates of working memory. Progress in Neuro-psychopharmacology & Biolological Psychiatry. 2010;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Berg EA. A simple objective technique for measuring flexibility in thinking. The Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 6.Blakemore S, Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 7.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 8.Bryan J, Luszcz MA. Measurement of executive function: Considerations for detecting adult age differences. Journal of Clinical and Experimental Neuropsychology. 2000;22:40–55. doi: 10.1076/1380-3395(200002)22:1;1-8;FT040. [DOI] [PubMed] [Google Scholar]
- 9.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Copeland J, Swift W. Cannabis use disorder: epidemiology and management. International Review of Psychiatry. 2009;21:137–143. doi: 10.1080/09540260902782745. [DOI] [PubMed] [Google Scholar]
- 11.Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Wells JE. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: Findings from the WHO World Mental Health Surveys. PLoS Medicine. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delis DC, Karmer JH, Kaplan E, Ober BA. California verbal learning test: Adult version. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 13.Denckla MB. Measurement of Executive Function. In: Reid L, editor. Frames of reference for the assessment of learning disabilities: New views on measurement issues. Baltimore, MD: Paul H Brookes Publishing; 1994. pp. 117–142. [Google Scholar]
- 14.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schiling L, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 15.Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, Satz P. Cognitive correlates of long-term cannabis use in Costa Rican men. Archives of General Psychiatry. 1996;53:1051–1057. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- 17.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Science. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 18.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8147–8149. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber SA, Rogowska J, Yurgelun-Todd Altered affective response in marijuana smokers: An fMRI study. Drug and Alcohol Dependence. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd DA. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and Clinical Psychopharmacology. 2011;19:231–242. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psycholology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 23.Harkany T, Guzman M, Glave-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends in Pharmacological Sciences. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Hart CL, Ilan AB, Gevins A, Gunderson EW, Role K, Colley J, Foltin RW. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacology, Biochemistry, and Behavior. 2010;96:333–341. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey MA, Sellman JD, Porter RJ, Frampton DM. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26:309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- 26.Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan AM, Vargha-Khadem F, Kirkham FJ, Baldeweg T. Maturation of action monitoring from adolescence to adulthood: an ERP study. Developmental Science. 2005;8:525–534. doi: 10.1111/j.1467-7687.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2010. Ann Arbor: Institute for Social Research, The University of Michigan; 2011. [Google Scholar]
- 29.Kempel P, Lampe K, Parnefjord R, Hennig J, Kunert HJ. Auditory-evoked potentials and selective attention: different ways of information processing in cannabis users and controls. Neuropsychobiology. 2003;48:95–101. doi: 10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- 30.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 31.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 32.McHale S, Hunt N. Executive function deficits in short-term abstinent cannabis users. Human Psychopharmacology. 2008;23:409–415. doi: 10.1002/hup.941. [DOI] [PubMed] [Google Scholar]
- 33.Monti PM, Miranda R, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, Crews FT. Adolescence: Booze, brains and behavior. Alcoholism, Clinical and Experimental Research. 2005;29:207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- 34.Novaes MA, Guindalini C, Almedia P, Jungerman F, Bolla K, Laranjeira R, Bressan RA. Cannabis use before age 15 years is associated with poorer attention and executive function. Biological Psychiatry. 2008;63:18S–19S. [Google Scholar]
- 35.Patton JM, Stanford MS, Barratt ES. Factor Structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Pollock V, Cho DW, Reker D, Volavka J. Profile of Mood States: the Factors and Their Physiological Correlates. The Journal of Nervous and Mental Disease. 1979;167:612–614. doi: 10.1097/00005053-197910000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd DA. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 38.Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd DA. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 39.Pope HG, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. [PubMed] [Google Scholar]
- 40.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addiction Biology. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 41.Solowij N, Pesa N. Cognitive abnormalities and cannabis use. Revista Brasileira de Psiquiatria. 2010;32(Suppl1):S31–S40. [PubMed] [Google Scholar]
- 42.Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 43.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Substance Abuse and Mental Health Services Administration. Rockville, MD: 1998. Analyses of substance abuse and treatment need issues: adolescent self-reported behaviors and their association with marijuana use. (Office of Applied Studies, OAS Analytic Series #A-7, DHHS Publication No. SMA 98-3227) [Google Scholar]
- 45.Substance Abuse and Mental Health Services Administration. Rockville, MD: 2010. Results from the 2009 national survey on drug use and health: volume I. Summary of National Findings. (Office of Applied Studies, NSDUH Series H-34A, DHHS Publication No. SMA 10-4586 Findings) [Google Scholar]
- 46.Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;94:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D. Manual for the Wechsler Adult Intelligence Scale. New York, NY: The Psychological Corporation; 1955. [Google Scholar]
- 49.Wechsler D. Manual for the Wechsler Adult Intelligence Scale - Revised. New York, NY: The Psychological Corporation; 1981. [Google Scholar]
- 50.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]