Abstract
The adiabatic inversion of blood in pseudocontinuous arterial spin labeling (PCASL) is highly sensitive to off-resonance effects and gradient imperfections and this sensitivity can lead to tagging efficiency loss and unpredictable variations in cerebral blood flow (CBF) estimates. This efficiency loss is caused by a phase tracking error between the RF pulses and the flowing spins. This paper introduces a new method, referred to as Optimized PCASL (OptPCASL), that minimizes the phase tracking error by applying an additional compensation RF phase term and in-plane gradients to the PCASL pulse train. The optimal RF phase and gradient amplitudes are determined using a pre-scan procedure, which consists of a series of short scans interleaved with automated post-processing routines integrated to the scanner console. The pre-scan procedure is shown to minimize the phase tracking error in a robust and time efficient manner. As an example of its application, the use of OptPCASL for the improved detection of functional activation in the visual cortex is demonstrated and temporal SNR, image SNR, and baseline CBF measures are compared to those acquired from conventional PCASL.
Keywords: arterial spin labeling, cerebral perfusion, tagging efficiency, pseudocontinuous tagging
INTRODUCTION
Arterial spin labeling (ASL) is an MRI technique that provides a non-invasive and quantitative measure of perfusion (1). In the brain, ASL can be used to measure baseline cerebral blood flow (CBF) and dynamic changes in CBF in functional MRI (fMRI) studies.
Pseudocontinuous ASL (PCASL) (2) is a recently introduced ASL method that provides distinct advantages over pulsed ASL (PASL) and continuous ASL (CASL). When compared to PASL, PCASL provides a higher theoretical SNR and more precise control of the temporal width of the bolus, the latter of which can improve the accuracy of quantified CBF estimates. Continuous ASL (CASL), the original ASL technique, shares the same advantages of PCASL relative to PASL, but has much larger magnetization transfer (MT) related artifacts, and additional hardware requirements make it harder to implement in commercial MRI systems.
Although PCASL appears to be a good choice for ASL acquisition, the theoretical SNR gain is often not attained in the actual implementation due to the method's sensitivity to off-resonance effects and gradient imperfections at the tagging location (3,4). This sensitivity leads to tagging efficiency loss, which may decrease apparent CBF values as well as PCASL's theoretical SNR advantage relative to other methods. The sensitivity of PCASL to these effects comes from its tagging mechanism in which the blood is adiabatically inverted using a series of RF pulses and slice selective gradients (2). The optimal inversion of flowing spins requires that the phase of successive RF pulses matches the phase evolution of the spins. When there is a mismatch between the two, which is referred to here as a phase tracking error, tagging efficiency deteriorates nonlinearly as a function of the error (4). Since off-resonance effects and gradient imperfections can be significantly affected by the B0 field strength, subject variations, and scanner hardware performance, the magnitude of phase tracking error and thus tagging efficiency can result in wide and unpredictable variations in CBF estimates. Consequently, correcting the phase tracking error is a critical step for tapping the full potential of the high SNR advantage offered by PCASL while providing robust estimation of CBF.
The first method to address the measurement and correction of the phase tracking error in PCASL was the multiphase PCASL (MP-PCASL) technique (4). This technique acquires “uncorrected” ASL data using multiple states of blood signal modulation in addition to the conventional control and tag states. The voxel-wise phase tracking error and the “true” magnetization of delivered arterial blood are then estimated from the ASL data using an offline model-based curve fitting procedure, thereby compensating for tagging efficiency loss in the CBF estimation process. One drawback of this technique, however, is that the need to acquire multiple states of blood signal modulation leads to a loss in temporal resolution and SNR efficiency, making it unsuitable for studies that require high temporal resolution, such as functional MRI (fMRI) studies.
Here we propose a complementary technique that addresses the drawback of the MP-PCASL method. In this approach, the phase tracking error is measured using a series of prescans (including a short MP-PCASL scan) and the off-resonance effects and gradient imperfections are corrected by adjusting the pulse sequence parameters at the time of data acquisition. The subsequent ASL scans are then acquired with the optimized control and tag states and thus the acquisition and post processing are identical to that of conventional PCASL. As a result, this approach retains the high temporal resolution necessary for fMRI studies. One additional advantage of this technique relative to MP-PCASL is that while the latter acquires the “uncorrected” ASL signal in which the tagging efficiency loss is compensated for retrospectively, the proposed approach ensures that the ASL signal is collected with the minimal degree of phase tracking error and nearly optimal tagging efficiency. The first implementation of this approach dubbed Optimized PCASL (OptPCASL) was previously presented by our group in abstract form in 2009 (5), with a number of variations subsequently presented by other groups (3,6). However, our initial implementation had a number of limitations. The pre-scan steps were time-consuming, and frequent user interventions were required. In this paper, we provide a more detailed description of the OptPCASL approach and present an improved implementation that addresses the limitations of the original version.
To demonstrate the ability of OptPCASL to reduce phase tracking error, we measured and compared the phase tracking errors before and after the pre-scans in 15 healthy subjects. In addition, 6 of the subjects participated in a pair of functional ASL scans with a visual stimulus paradigm and a pair of baseline scans using both conventional PCASL and OptPCASL. We show that OptPCASL enables the correction of phase tracking errors in a time-efficient manner and has the potential to provide (a) baseline CBF maps with higher SNR and (b) more robust detection of activations in the visual cortex as compared to conventional PCASL.
THEORY
PCASL Tagging Scheme
In the PCASL tagging scheme (2,7), RF pulse trains and gradient fields are applied in order to induce flow driven adiabatic inversion of the magnetization of arterial blood. The tag condition, in which flowing arterial spins are inverted while passing through a tagging plane, is achieved when the RF pulses and spins at the tagging plane are in phase. It should be noted that we have used the “balanced” PCASL tagging approach in this study (7) rather than the “unbalanced” approach (2), i.e. the gradient waveforms are identical in both tag and control states (nonzero mean) and only the RF phase is modulated.
For the tag condition, the phase schedule of the nth RF pulse out of N pulses is:
| [1] |
where γ is the gyromagnetic ratio, G is the mean gradient during the tagging period, T is the time between the centers of successive RF pulses, and d is the distance from the gradient center to the tagging plane.
In contrast, the control condition has an additional phase offset that has an oscillating phase to restore tipped spins back to the longitudinal axis. Hence, the control condition includes an additional alternating phase term in the phase schedule as follows:
| [2] |
Because both tag and control conditions are defined by the phase schedule of RF pulses, the phases of the RF pulses have an essential role in the inversion of arterial spins. If an additional error term is present in both the tag and control phase schedules, the phases of RF pulses will no longer match those of the flowing spins and the theoretical inversion efficiency will not be achieved. Generalizing the above relations and introducing a phase tracking error term (Δθε), we can write the phase (θn,m) of the nth RF pulse in the mth state (m = 0 for the tag state and m = 1 for the control state) as:
| [3] |
Off-resonance fields at the tagging plane and imperfections in the applied gradients are the two main factors that give rise to this error term (Δθε) and the resultant reduction of tagging efficiency in PCASL (3,4). This error term is present to the same extent in both the tag and control states.
PCASL Tagging Optimization
Conventional PCASL, which has two states (see Eqs. 1 and 2), does not provide sufficient information to estimate the phase tracking errors. In MP-PCASL (4), the phase schedules of the control and tag states are replaced by the new schedule as follows:
| [4] |
where M is defined as the total number of phases in the multiphase tagging scheme and N is the total number of RF pulses in the pulse train. In the implementation of MP-PCASL, this new phase offset term increments between 0 and 2π in an interleaved manner and the measured perfusion data from these increments is fitted to the expected inversion response function on a per-voxel basis to estimate the phase tracking error. It is important to note that the phase tracking error is derived from several feeding arteries at the tagging plane and thus is spatially dependent and vessel-specific. For this study, the tagging plane was selected through the internal carotid arteries and vertebral arteries below the confluence to the basilar artery where all arteries were perpendicular to the tagging plane. The MP-PCASL method then provided estimates of the phase tracking errors corresponding to right and left carotid arteries (RCA, LCA) and vertebral arteries (VAs). Since the two vertebral arteries were spatially close at the tagging plane chosen in this study, we treated these two vessels as one vascular source and defined its location as the midpoint of the segment between the two.
The phase tracking error measured from each of the three feeding arteries can be broken down into two components: a global phase error common to all feeding arteries and a local phase error specific to each feeding artery. The former, which represents the common error derived from the mean off-resonance at the tagging plane, can be corrected by adding an additional compensation RF phase term nΔθ (Eq. 8) to the phase schedules of the tag and control conditions (Eqs. 1, 2). The latter, caused by the local off-resonance fields, can be removed by applying additional in-plane gradients between RF tagging pulses and thereby compensating the local errors at each of the feeding arteries. The mathematical relationship between the measured phase tracking errors and the global compensation phase and gradient fields required to correct them can be expressed in matrix form:
| [5] |
where the estimates of the phase tracking error from each feeding artery (i.e. RCA, LCA, and VA) are denoted by Δθε,r, Δθε,l, Δθε,v while their corresponding in-plane locations in x and y are denoted by xr, xl, xv and yr, yl, and yv. As mentioned in the previous paragraph, xv and yv correspond to the spatial coordinates of the midpoint of the line between the two vertebral arteries. With this a priori information, we can analytically calculate the spatial linear errors in x and y, i.e. dΔθε/dx, and dΔθε/dy and the global phase error denoted as Δθε,z
Gradient areas applied and the amount of compensation RF phase can then be expressed by:
| [6] |
| [7] |
| [8] |
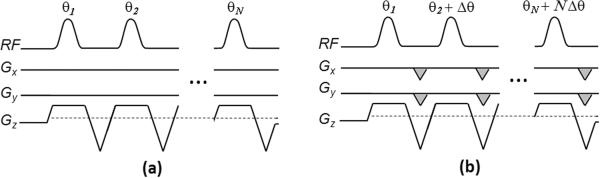
Figure 1 shows the diagram of tagging modulation for conventional PCASL (a) and for OptPCASL (b). Note that OptPCASL includes the additional RF phase offset term (Δθ) as well as the compensation gradients expressed by the shaded areas in Gx and Gy. It should be noted that the sign of the additional RF phase term is identical to that of the global phase tracking error term (Eq. 8) while the sign of the in-plane gradients is opposite to the spatial linear error terms (Eqs. 6, 7).
Figure 1.
Diagram of tagging modulation for (a) conventional PCASL and (b) optimized PCASL. Optimized PCASL includes the additional compensation RF phase (Δθ) term to compensate for the global phase error and in-plane gradients (indicated by the shaded areas in Gx and Gy) to correct the spatially-dependent phase errors from the main feeding arteries.
METHODS
Overview of Pre-scans
The main objective of the OptPCASL technique is to accurately measure the phase tracking errors associated with the main feeding arteries, determine the amount of global compensation RF phase and gradient areas, and to confirm with a follow-up scan that the phase tracking errors are minimized after applying the added specifications of the PCASL tagging scheme. In order to accomplish this objective in a time-efficient and user-friendly manner, we have developed a sequence of pre-scans integrated with post-processing routines that require minimal user-intervention.
It is important to note that while the phase tracking errors are derived from the feeding arteries located in the tagging plane where the flow-driven adiabatic inversion occurs, they are measured in the imaging plane. The phase tracking errors therefore have a spatial dependence and it is important to map the vascular distribution of these feeding arteries at the imaging plane in order to estimate the phase tracking error corresponding to each feeding artery. We have used vascular territory imaging (VTI) to tag blood in the feeding arteries with spatial modulations and to map the vascular distribution of these arteries (7). The calculated ASL signals from the three (RCA, LCA, VA) territories were then converted into respective regional masks, which were used to calculate the corresponding mean phase tracking errors (Δθε,r, Δθε,l, and Δθε,v in Eq 5).
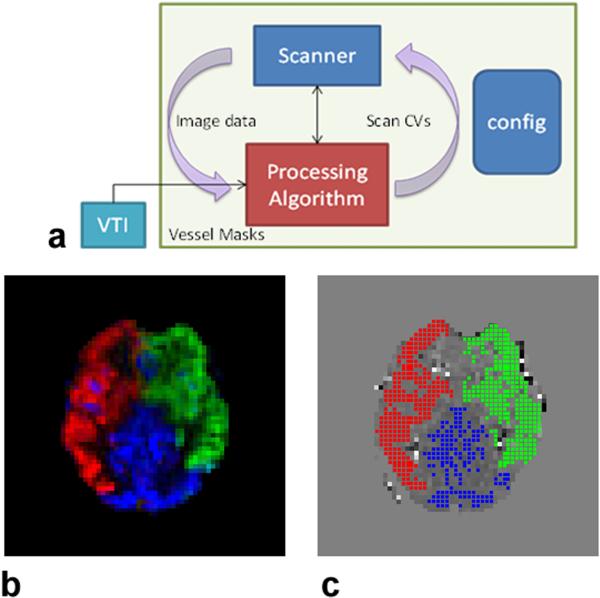
Overall, the pre-scans consisted of (a) a time-of-flight (TOF) scan to define the tagging plane and to determine the spatial coordinates of the three feeding arteries needed for the VTI scan; (b) a VTI scan followed by an automatically invoked post-processing routine to generate the three (RCA, LCA, VA) regional masks; (c) an MP-PCASL scan followed by a post-processing routine to estimate the phase tracking errors from the three vascular territories and to calculate the global compensation RF phase and the in-plane gradient fields; and (d) a follow-up MP-PCASL scan with an automated update of relevant scanner control variables (CVs) to adjust the RF phase offset and gradient fields, followed by the second instance of the MP-PCASL post-processing routine to confirm that the territory-specific phase tracking errors are minimized. Figure 2a shows the schematic of the pre-scan processes. Excluding the TOF and structural scans, the total processing time needed for the completion of pre-scans (VTI, pre and post MP-PCASL scans, and post-processing routines) was roughly 5 minutes.
Figure 2.
(a) Schematic of the pre-scans. The VTI sequence (box in cyan) reads in the location of the tagging plane and coordinates of the major feeding arteries from the configuration file. The post processing routine at the end of the scan automatically generates the binary territory masks in panel (c), which are passed onto the post-processing script (red box) associated with the MP-PCASL scan. Using the image data from the MP-PCASL scan and the binary territory masks, this script calculates the global RF phase term and in-plane gradient fields and saves them as scanner control variables (CVs) in the configuration file. The second MP-PCASL scan acquires new image data with the updated CVs and the post-processing script recalculates the phase tracking errors from the three feeding arteries. (b) A vascular territory map generated using the VTI post-processing routine. The calculated ASL signals from the three territories, i.e. RCA, LCA, and VA are shown in red, green, and blue, respectively. (c) The corresponding binary RCA, LCA and VA maps generated by the post-processing routine.
The subsequent ASL scans were then carried out using the conventional PCASL tagging scheme, i.e. the tag and control conditions, with the optimized set of compensation RF phase and gradient fields applied.
Participants and Study Design
Fifteen healthy subjects [9 males, 6 females, age: 29.0 ± 5.9 yrs, weight: 68.5 ± 10.7 kg] participated in this study. The study was approved by the institutional review board at the University of California, San Diego, and written informed consent was obtained from the subjects. All subjects underwent the pre-scans and the phase tracking errors from RCA, LCA, and VAs were compared before and after the pre-scans.
From six of the fifteen subjects [3 males, 3 females, age: 26.8 ± 3.7 yrs, weight: 66.1 ± 15.0 kg], two functional scans with a visual stimulus paradigm and two baseline scans were performed using both conventional PCASL and OptPCASL. The visual stimulus paradigm consisted of a block design with 60s off, 4 cycles of 20s on/40s off using 8-Hz flickering checkerboard visual stimulus. Cardiac pulse and respiratory data were monitored using a pulse oximeter (InVivo) and a respiratory belt (BIOPAC), respectively. The recorded cardiac and respiratory data sampled at 40Hz were used to calculate the physiological noise regressors (8,9).
MR Imaging
The study was executed on a 3T GE Discovery MR750 scanner with a body transmit coil and an 8-channel receive-only coil (GE Healthcare, Waukesha, WI). For all PCASL scans, the image prescriptions were kept the same: a single-shot spiral readout, 20 axial slices, 5mm slice thickness with no gap, and 240×240mm FOV. The PCASL tagging parameters (10) also remained the same: Hanning-shaped RF pulses of 375us duration, B1max = 0.1G, Gmax = 1.6G/cm, Gmean = 0.09G/cm, and an RF-to-RF spacing of 998us.
Time of Flight
The TOF scan provides a high resolution view of the main feeding arteries, i.e. RCA, LCA, and VAs. The tagging plane used for the pre-scans and all subsequent ASL scans was determined based on the anatomy and orientation of these vessels. The midline of the graphic prescription slab was aligned to the bottom of cerebellum to set the tagging plane between the vertebral crossing into the brain and the confluence to the basilar artery. The scan parameters were: 1mm-thick 52 axial slices, FOV = 220×170mm, TR = 20ms, TE = 3ms, FA = 15°, and scan time = 1:43 minutes. Parallel imaging with acceleration factor of 2 was used to minimize scan time.
At the end of the scan, a custom-made JAVA program was invoked on the scanner console to review the images and to choose the location where all arteries were perpendicular to the image plane. This plane was set as the tagging plane, and the location and the coordinates of the RCA, LCA, and VA (midpoint of the line between the two vertebral arteries) were automatically saved into a configuration file (Fig. 2a). This step was carried out during the acquisition of the structural scan (next section) for efficient use of the scan time.
Structural Scan
High resolution (1mm isotropic), anatomical data were acquired using a magnetization prepared 3D fast spoiled gradient recalled (FSPGR) sequence with the following parameters: 172 sagittal slices, TI = 600ms, TR = 7.9ms, TE = 3.1ms, flip angle = 8°, parallel imaging acceleration factor = 2, and scan time = 3:45 min. The data were used to create a whole-brain gray matter (GM) mask down-sampled to the resolution of the functional and baseline ASL data.
Vascular Territory Imaging
The mapping of vascular territories was achieved using a vessel-encoded arterial spin labeling technique proposed by Wong (7). Specifically, we used the six-vessel encoding scheme with a total of 5 averages for each of the six encoding cycles (30 repetitions total). The six encoding steps were: 1. non-selective tag, 2. non-selective control, 3. tag LCA, control RCA, and saturate VA, 4. control LCA, tag RCA, and saturate VA, 5. tag LCA, tag RCA, and control VA, 6. control LCA, control RCA, and tag VA. The spatial coordinates of RCA, LCA, and VA and the tagging location needed for the VTI sequence were automatically imported from the previously saved configuration file at the start of the scan (Fig. 2a). The scan parameters were: TR = 3200ms, TE = 3.3ms, tag duration = 2000ms, post labeling delay = 400ms, and scan time = 1:36 minutes. A short post labeling delay was used to maximize SNR in territory maps and to minimize TR and scan time (11). A tagging duration of 2000ms was used to achieve maximum SNR efficiency based on theoretical calculations with the general kinetic ASL model (12) using the assumed T1 of 1664ms for blood at 3T (13).
At the end of the scan, the vascular territory map was automatically generated using a post-processing routine based on the method described by Wong et al (14). In this approach, a cluster analysis was utilized to get accurate estimates of the tagging efficiency of each tagged vessel (RCA, LCA, VA). These relative tagging efficiencies were then used in the construction of the encoding matrices for linear decoding of territory information. Figure 2b shows a sample vascular territory map. The calculated ASL signals from the three territories, i.e. RCA, LCA, and VA are presented as the intensity of the red, green, and blue channel of the RGB color scale, respectively. The signal sum was generated by adding the calculated ASL signals from the three territories. The regional masks required to estimate Δθε,r,Δθε,r, Δθε,1, and Δθε,v, in Eq. 5 were then created by normalizing the ASL signal from each territory by the signal sum and thresholding the normalized value at 0.8. Thresholding ensured that each territory mask excluded voxels with significant mixing of blood from the other two feeding arteries. In order to exclude low signal voxels from appearing in these masks, the voxels were multiplied by a rough GM mask. This binary GM mask was generated by 1) calculating a conventional ASL image via subtraction of non-selective tag from non-selective control, 2) setting a threshold level at half the intensity of the 99th percentile in this image. The three regional masks confined within the GM were automatically saved by the post-processing routine, which were called by the post-processing routine during the MP-PCASL pre-scan step.
Multiphase PCASL
A MP-PCASL scan was acquired using 8 RF phase offsets (M = 8 in Eq. 4) with the following sequence parameters: TR = 3200ms, TE = 3.2ms, tag duration = 2000ms, post labeling delay = 400ms, and scan time = 1:40 min. At the end of the scan, a post-processing routine was automatically invoked to estimate the RCA, LCA, and VA phase tracking errors using the saved territory masks described in the previous section. We used a signal demodulation algorithm (15) to estimate the phase tracking errors since it reduced the processing time by two orders of magnitude (5 minutes vs. 5 seconds), as compared to the nonlinear approach used in the original MP-PCASL paper (4), while providing comparable estimates of phase tracking errors. The postprocessing routine additionally calculated the global compensation RF phase and the gradient fields according to Eqs. 5–8, which were saved into the configuration file in the form of scanner CVs (Fig. 2a). After automatically loading the CVs, the MP-PCASL scan was repeated and the post-processing routine measured the new phase tracking errors.
Functional and Baseline ASL
For the functional and baseline ASL scans acquired with both conventional PCASL and OptPCASL, the common scan parameters used were: TR = 4000ms, tag duration = 2000ms and post labeling delay = 1400ms. The baseline scans used 60 repetitions with the total scan time of 4:00 minutes. The functional scans used 75 repetitions with the total scan time of 5:00 minutes to accommodate the visual stimulus paradigm as described in the Participants and Study Design section.
Calibration Scans for CBF quantification
A 36-second spiral scan with TR = 4s, TE = 3.4ms, NEX = 9 was acquired to obtain an estimate of the equilibrium magnetization of cerebral spinal fluid (16–18), which was used to convert the perfusion signal into calibrated CBF units (ml blood/100 ml tissue-min). The 90° excitation RF pulse was turned off for the first 8 repetitions of this scan.
As used in previous studies (17,18), a 32-second minimum contrast scan was acquired using a 8-shot spiral acquisition with TR = 2000ms, TE = 1.1ms, NEX = 2 to estimate the combined transmit and receive coil inhomogeneities. The optimal parameters for an image with minimal contrast between tissue types were determined by experiment, starting with the parameters used by (16) for the spin echo case. We used the 8-shot spiral acquisition to minimize the intensity variations arising from signal drop outs as well as from T2* blurring. The two images were averaged to create the minimum contrast image, which then was used to generate a normalized surface fit within a brain mask to remove any residual contrast between tissue types. The brain mask was used to avoid fitting values outside the brain since doing so would lead to underestimated sensitivity values at the edges of the brain. Finally, the ASL image was divided by the normalized surface fit to correct for transmit and receive coil inhomogeneities during the CBF quantification step.
Both scans used the same transmit and receive gains as the functional and baseline scans.
Field Map Acquisition
To correct for blurring in spiral images due to off-resonance fields, a field map was acquired using a spoiled gradient echo sequence with parallel imaging acceleration factor 2, which generated two sets of images consecutively, each set at a specific TE. The scan parameters were TR=500ms, TE1 = 6.5ms, TE2 = 8.5ms, FA = 45°, scan time = 1:16 minutes. The acquired field map was then used for offline reconstruction of all ASL data using a k-space based fast iterative image reconstruction algorithm (19).
Post Processing and Statistical Analysis
For calibration of ASL data into absolute CBF units (ml blood/100 ml tissue-min), we used the standard ASL kinetic model from Buxton et al (12) with the assumption that the outflow effect is negligible (residue function is a constant equal to 1) and the magnetization decay function is governed by T1 of blood. The first four images from both functional and baseline scans were excluded from data analysis to ensure that the MR signal reached the steady state. All scans (PCASL and OptPCASL) were motion corrected and registered to the respective first functional image using AFNI software (20). Statistical analysis of the two functional runs was performed using a general linear model (GLM), which included the measured cardiac and respiratory fluctuations as the GLM regressors (8). Clusters of voxels showing CBF activation were detected using an overall p-value significance threshold of 0.05 where the correction for multiple comparisons was performed using the AFNI AlphaSim program. To exclude spurious voxels outside the visual cortex, Brodmann parcellation (area 17) was used to generate a regional mask. A 12-parameter affine transformation matrix was first estimated by registering the subject-specific anatomical volume to the T1 template in AFNI (TT_avg152T1+tlrc). The matrix was then applied to warp the Brodmann parcellation into the space of the anatomical volume. The intersection of the “active” clusters from PCASL and OptPCASL was used to generate a common ROI for each subject. The F values within the common ROIs were compared between PCASL and OptPCASL for the group of 6 subjects.
Temporal SNR (tSNR) maps were calculated for the two functional scans first by creating CBF time-series from the running subtraction of the control and tag image series (21) followed by computing the mean signal per voxel divided by the standard deviation over time.
Image SNR maps were calculated from the two baseline scans by voxelwise mean of the difference images (control minus tag) divided by standard deviation of the signal obtained from a separate scan with the PCASL tagging module and the 90° excitation RF pulse turned off. We used the correction factor described by Henkelman et al (22) to remove the contribution of noise from the magnitude signal amplitudes before SNR was calculated.
For each of the 6 subjects, the mean GM tSNR, image SNR, and baseline CBF were calculated for PCASL and OptPCASL. Two-tailed paired t-tests were used to assess the effect of phase tracking error correction on tSNR, image SNR, and baseline CBF.
RESULTS
Figure 2b shows a perfusion territory map from a representative subject generated by the post-processing routine following the VTI scan. The calculated ASL signal from each of the three vessel territories (RCA, LCA, and VA) is presented as the intensity of the red, green, and blue of the RGB color scale. The corresponding binary masks generated by the post-processing routine can be seen as the red, green, and blue areas in Fig. 2c.
Representative phase tracking error maps calculated after the first and second MPPCASL scans, i.e. with the added compensation RF phase and gradient fields are shown in Fig. 3a. For this particular subject, the combined mean phase tracking error from all three territory masks was 25.01° before and 1.53° after the phase error correction. Figure 3b shows the vessel-specific (RCA, LCA, VA) phase tracking errors before and after correction for all 15 subjects. In all three regions there was a statistically significant reduction in phase tracking errors (paired two-tailed t-test, tRCA = −4.63, tLCA = −7.21, tVA = −11.43, dof = 14, p < 0.05), with the group average error reduced to less than 5 degrees in all three regions (RCA = 3.3 ± 3.4°, LCA = 3.1 ± 2.9°, VA = 4.1 ± 3.6°). The phase tracking errors presented in Fig. 3a and b are the magnitude of the original values.
Figure 3.
(a) Voxel-wise phase tracking errors measured from a representative subject using MP-PCASL before and after correction. (b) RCA, LCA, and VA phase tracking errors before and after correction for 15 subjects. In all three regions, there was a statistically significant reduction in phase tracking errors (p < 0.05)*. The phase tracking errors are presented as the magnitude of the original values.
Repeated measures ANOVA determined that the mean initial phase tracking error differed significantly between the three vessels (F(2,28) = 6.07, p = 0.006). Post hoc tests using the Bonferroni correction revealed that there were regional differences in the phase tracking error, i.e. RCA vs. LCA (16.6 ± 9.9° vs. 20.6 ± 8.6°) and RCA vs. VA (16.6 ± 9.9° vs. 22.4 ± 5.8°), which were statistically significant (p < 0.05).
Temporal SNR, image SNR, and baseline CBF maps generated from a representative female subject are shown in Fig. 4. The tSNR values confined within the GM mask were significantly greater (paired two-tailed t-test, t = 4.11, dof = 4060, p < 0.01) in the OptPCASL scan, with mean tSNR values of 2.80 ± 1.05 and 3.10 ± 1.13 for PCASL and OptPCASL, respectively. The mean GM SNR was 14.20 ± 5.02 for PCASL and 15.67 ± 5.41 for OptPCASL. The GM SNR values were significantly higher in OptPCASL (paired two-tailed t-test, t = 24.66, dof = 4060, p < 0.01). Lastly, the whole-brain GM CBF values for PCASL and OptPCASL were 84.61 ± 29.80 and 94.69 ± 31.08 ml/100 ml tissue-min, respectively. This difference was also statistically significant (paired two-tailed t-test, t = 13.12, dof = 4060, p < 0.01).
Figure 4.
(a) Temporal SNR, (b) image SNR, and (c) baseline CBF maps between PCASL and OptPCASL from a representative subject.
Mean gray matter tSNR, SNR, and baseline CBF are graphed for 6 subjects in Fig. 5. Each scatter plot shows the measured metrics under both PCASL and OptPCASL with the solid line representing equality between the two. Mean GM tSNR (t = 2.74, dof = 5, p = 0.041), image SNR (t = 2.65, dof = 5, p = 0.046), and baseline CBF (t = 2.85, dof = 5, p = 0.036) measured from OptPCASL were all significantly higher than those from PCASL based on paired two-tailed t-test.
Figure 5.
(a) Scatter plots of the mean GM (a) tSNR, (b) image SNR, and (c) baseline CBF for 6 subjects (PCASL vs. OptPCASL). The solid black lines represent equality between PCASL and OptPCASL. The calculated p-values from the paired t-test are also shown in the panels.
Figure 6 shows functional CBF correlation maps overlaid on high-resolution anatomical images for 6 subjects. A representative slice is shown from each subject. Within the common ROIs defined between PCASL and OptPCASL, OptPCASL generated significantly higher F values (paired two-tailed t-test, t = 10.52, dof = 553, p < 0.001) within the visual cortex.
Figure 6.
Functional CBF correlation maps (p < 0.05) overlaid on high-resolution anatomical images. From each of the 6 subjects (columns), a representative slice including the visual cortex is shown.
Since the total phase tracking error in a given region is the summation of the global error and the in-plane errors in the x and y directions (Eq. 5), we calculated the contribution of each component as a percentage. Figure 7 shows the amount of phase tracking error correction contributed by the global RF phase offset term and the transverse gradients (x and y) for all three vessels (RCA, LCA, VA). The horizontal axis represents 6 individual subjects. Subscripts x and y denote right-left and anterior-posterior directions, respectively.
Figure 7.
Percentage of phase error correction contributed by the global RF phase offset term and the transverse gradients for a given regional phase tracking error across 6 subjects. Subscripts x and y denote right-left and anterior-posterior directions, respectively.
DISCUSSION
Across 15 subjects, the pre-scans successfully reduced the group mean phase tracking error to less than 5° in all three vessel locations (RCA, LCA, VA). Researchers at our facility have found the integrated and streamlined calibration procedure (Fig. 2a) to be easy to use and efficient. Relative to conventional PCASL, OptPCASL requires roughly 5 minutes of additional scan time.
While we did not directly measure the inversion efficiency at the individual arteries, indirect estimate of the inversion efficiency using the changes in whole brain baseline CBF values (Fig. 4c and 5c) can be made between PCASL and OptPCASL. For all 6 subjects, OptPCASL produced significantly higher whole brain CBF (t = 2.85, dof = 5, p = 0.036). Since the two scans were performed in the same session consecutively and the CBF quantification step was identical between them, it can be inferred that the higher CBFs produced with OptPCASL were the direct result of the improved inversion efficiency. The mean CBF was 60.9 ± 18.5 and 68.7 ml/100 ml tissue-min for PCASL and OptPCASL, respectively. As a rough group estimate, this translates to 13% improvement in the inversion efficiency as the direct result of the calibration procedure.
In the original implementation of this method (5), the VTI scan and the definition of the territory maps using the automated post processing routine were lacking. Instead, a manual delineation of the vascular territories was performed by the scan operator. Although manual delineation had the benefit of not requiring additional scan time, the outcome of the phase error calibration step was highly variable, and in some cases the resultant phase errors were even higher than the initial levels. In a territory mapping study, Van Laar et al (23) reported large variations in flow territories across a sample of 115 subjects, underscoring the difficulty in accurate delineation of vascular territories using visual inspection alone. Therefore, the VTI scan is a crucial improvement to the original implementation of this technique. Furthermore, the use of thresholding in the generation of the three territory masks is also a critical post processing step since it ensures that each mask excludes regions of significant mixing of blood from the remaining two feeding arteries, which can be caused by vascular anatomy or poor separation of vascular components in post processing.
In this study, the two vertebral arteries, which were located close to each other at the tagging plane, were treated as one vascular source. This was based on the assumption that they have similar phase tracking errors and that the phase tracking error estimate from the VA territory mask represents the mean of the two errors. This assumption is built into Eq. 5. Specifically, we could have expanded the third line of Eq. 5 into two lines, i.e., replace xv, yv (currently defined as the midpoint of the segment between the two vertebral arteries), and Δθε,v with xvr, yvr, Δθε,v (third line) and xvl, yvl, Δθε,v (fourth line). Adding the two lines (third and fourth) and dividing by 2 would then yield the third line in Eq. 5. Across 15 subjects, the average distance between the two vertebral arteries measured at the tagging plane was 1.55 ± 0.33 cm while that between the two carotids was 5.40 ± 0.39 cm. We estimated the phase tracking error difference between the two vertebral arteries using the measured distance and the actual gradient field applied in the right-to-left direction during the calibration. The mean error difference was 1.48 ± 1.29°. More importantly, we demonstrated statistically significant and consistent reduction in the regional phase tracking error as measured by the VA mask (tVA = 11.43, dof = 14, p < 0.05) across 15 subjects, a strong indication that the phase errors at both vertebral arteries were effectively minimized using the assumption that they were one vascular source. While defining the tagging plane at the level of the basilar artery would have eliminated the need for this assumption, we chose the tagging plane to occur before the two vertebral arteries fused to form the basilar artery for two reasons. First, the level at which the vertebral arteries merge into the basilar artery tends to correspond to the petrous segment of the internal carotid arteries. Within this segment, the carotid arteries are not oriented along the vertical axis, which would significantly degrade the tagging efficiency. Second, placing the tagging plane below the confluence ensured that there was a spatial gap between the plane and the most proximal imaging slice, thereby minimizing the potential for aliasing from the tagging plane. Across 15 subjects, the average gap between the tagging plane and the most proximal imaging slice was 1.23 ± .88 cm. Setting the tagging plane above the confluence would have caused an overlap between the tagging plane and the most proximal imaging slice for some of the subjects. In practice, tagging above the confluence can be implemented without any modifications to our method and this may be preferable on a per subject basis as long as the perpendicularity requirement of the carotids is satisfied and the spatial gap is greater than zero. The latter requirement can be readily satisfied if the prescribed coverage of the imaging slab is smaller than the whole brain coverage used in this study.
For research studies that utilize multiple functional ASL scans, temporal SNR is critical and the additional time required for the OptPCASL pre-scans is readily justified. In contrast, for routine fMRI protocols that acquire a short baseline ASL scan as a secondary measure to aid the interpretation of fMRI studies, the additional 5 minutes of pre-scans required for OptPCASL may not be acceptable. Under this scenario, researchers may opt for alternate PCASL techniques that retrospectively compensate for the phase tracking errors and the related loss in tagging efficiency in the computation of CBF estimates (4,24,25). However, these approaches only compensate for the quantitation errors and do not provide gains in SNR. Researchers will need to take into account the trade-off between additional pre-scan time and SNR requirements when choosing a PCASL technique for a given study.
Regarding the sensitivity of the proposed method to subject motion between the pre-scans and the following OptPCASL scans, the regional phase tracking error estimates are expected to be quite robust against head motion since each regional error is acquired based on the mean of errors from a fairly large vascular territory area. On the other hand, the effectiveness of phase tracking error correction is likely to be adversely affected by any type of head motion that causes displacement of tagged vessels in the tagging plane, particularly the carotid arteries that are positioned away from the isocenter where the applied in-plane gradient fields are larger.
It is possible but unlikely that the previous calibration settings determined from a specific subject can be reused without the need for a repeated pre-scan procedure. As a rough estimate based on a small number of subjects, intra-subject variations ranging from 10 to 15% were observed in the measured phase tracking error between sessions. This range is plausible given that the local field environment can vary due to changes in various factors between sessions including the relative position of the subject inside the magnet isocenter, the auto shimming levels set by prescans, the positioning of the coil/headphone on the subject, and the location of the tagging plane.
While this study focused on the minimization of the phase tracking error for three feeding arteries, the proposed method can be extended to a case where the tagging plane contains more than three vessels, provided that the phase tracking error for each of the additional arteries is known. Under this scenario, the determination of the in-plane gradients and the global compensation phase terms (Eq. 5) becomes an overdetermined problem and the solutions can be found by the method of least squares. However, the practical limitation may be the difficulty in obtaining more than three phase tracking errors. For example, given the tagging location selected in this study, the number of regional phase tracking errors that could be identified was limited to RCA, LCA, and VA based on the VTI scan and its post processing method.
Jahanian et al (6) recently proposed a similar technique to OptPCASL for restoring tagging efficiency in PCASL. In contrast to using phase tracking errors, it employs a field map at the tagging plane to model the local field inhomogeneities along the z-direction as a constant frequency shift plus a linear gradient, which are then compensated by modulating the RF phase term (similar to Δθ in Eq. 8) and area of the refocusing lobe of the slice-selective gradient. While the two methods share the same objective, i.e. restoring tagging efficiency, there are a few notable differences in their approaches. First, the technique by Jahanian et al confines the correction of off-resonance effects to the z-direction. As measured by the initial phase tracking errors from 15 subjects, our results have shown that there are significant regional differences in the off-resonance effects between RCA, LCA, and VAs, which underscore the importance of in-plane corrections using Gx and Gy gradients. We also found that while the RF phase correction made a majority contribution to the overall phase tracking error correction (mean = 78.5 ± 13.1%), the gradient-based corrections were not insignificant, i.e. the percent contribution of the transverse gradients ranged from 0.1 to 42.8% with the mean contribution of the anterior-posterior and right-left gradients being 14.8 ± 11.5% and 6.7 ± 6.4%, respectively across subjects and vascular sources (Fig. 7). Second, while field maps provide information regarding local field inhomogeneities, phase tracking errors used in OptPCASL reflect error contributions from gradient imperfections of the slice selective gradient in addition to the local field inhomogeneities, allowing both sources to be corrected. Third, the repeated and automated measurements of phase tracking errors collected as part of the pre-scans in OptPCASL provide quantifiable measure of improvement in tagging efficiency before baseline and/or functional ASL scans are performed.
The phase tracking error for a given off-resonance field is directly proportional to the duration of the RF-to-RF spacing of the PCASL pulse train. Based on a Bloch simulation study, we chose an optimized tagging scheme that minimized the RF-to-RF spacing (998μs) while preserving high tagging efficiency over a wide range of flow velocities, B1 inhomogeneities, and gradient errors (10). However, we still observed phase errors greater than 30° in some of our subjects prior to the phase error correction, suggesting that reducing the RF-to-RF spacing alone is not sufficient to minimize the errors. This finding is also consistent with a recent study by Wu et al (24) who reported a significant improvement in the repeatability of CBF measures after tagging efficiency variations were accounted for. That study used a similar tagging scheme with a sub-millisecond RF-to-RF spacing.
The extent to which the correction for phase tracking errors is required may vary across sites since MRI systems from different vendors can exhibit varying levels of shimming and gradient performance. However, it is likely that the correction of phase tracking errors will remain an important component for PCASL, especially at high field strengths (e.g. 7T and higher) where the off-resonance effects are far more significant (3).
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Ajit Shankaranarayanan at GE Healthcare for providing the custom-made JAVA application on the GE scanner console, which was used for viewing of TOF images and defining the location of arterial vessels and tagging plane.
The authors also thank Jia Guo in the Bioengineering Department at University of California, San Diego for his help on the VTI post-processing and Dr. Gary H. Glover at Stanford University for providing the pulse sequence used for field map acquisition.
This study was supported by National Institutes of Health 1R01MH084796.
REFERENCES
- 1.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion Imaging. Magnetic Resonance in Medicine. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 2.Dai WY, Garcia D, de Bazelaire C, Alsop DC. Continuous Flow-Driven Inversion for Arterial Spin Labeling Using Pulsed Radio Frequency and Gradient Fields. Magnetic Resonance in Medicine. 2008;60(6):1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luh W, Li T, Wong C, Bandettini PA. Pseudo-continuous Arterial Spin Labeling at 7T. Toronto, Canada: 2008. p. 3339. [Google Scholar]
- 4.Jung Y, Wong EC, Liu TT. Multiphase Pseudocontinuous Arterial Spin Labeling (MP-PCASL) for Robust Quantification of Cerebral Blood Flow. Magnetic Resonance in Medicine. 2009;64(3):799–810. doi: 10.1002/mrm.22465. [DOI] [PubMed] [Google Scholar]
- 5.Jung Y, Rack-Gomer AL, Wong EC, Buracas GT, Liu TT. Pseudo-Continuous Arterial Spin Labeling with Optimized Tagging Efficiency for Quantitative ASL FMRI. Honolulu, Hawaii; 2009. p. 1578. [Google Scholar]
- 6.Jahanian H, Noll DC, Hernandez-Garcia L. B(0) field inhomogeneity considerations in pseudo-continuous arterial spin labeling (pCASL): effects on tagging efficiency and correction strategy. NMR Biomed. 2011 doi: 10.1002/nbm.1675. doi: 10.1002/nbm.1675. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magnetic Resonance in Medicine. 2007;58(6):1086–1091. doi: 10.1002/mrm.21293. [DOI] [PubMed] [Google Scholar]
- 8.Restom K, Behzadi Y, Liu TT. Physiological noise reduction for arterial spin labeling functional MRI. Neuroimage. 2006;31(3):1104–1115. doi: 10.1016/j.neuroimage.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Shin DD, Wong EC, Jung Y, Liu H-L, Liu TT. Optimization of pseudo continuous ASL tagging for robust inversion efficiency - A Bloch simulation and in vivo study at 3T. Montreal, Canada: 2011. p. 231. [Google Scholar]
- 11.Luh W, Talagala SL, Bandettini PA. Robust Prescan for Pseudo-Continuous Arterial Spin Labeling at 7T: Estimation and Correction for Off-Resonance Effects. Stockholm, Sweden: 2010. p. 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magnetic Resonance in Medicine. 1998;40(3):383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- 13.Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52(3):679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- 14.Wong EC, Kansagra A. Mapping middle cerebral artery branch territories with vessel encoded pseudo-continuous ASL: sine/cosine tag modulation and data clustering in tagging efficiency space. Toronto, Canada: 2008. p. 31. [Google Scholar]
- 15.Jung Y, Liu TT. Fast CBF Estimation in Multi-Phase Pseudo-Continuous Arterial Spin Labeling (MPPCASL) Using Signal Demodulation. Stockholm, Sweden: 2010. [Google Scholar]
- 16.Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31(3):680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- 17.Liu TT, Behzadi Y, Restom K, Uludag K, Lu K, Buracas GT, Dubowitz DJ, Buxton RB. Caffeine alters the temporal dynamics of the visual BOLD response. Neuroimage. 2004;23(4):1402–1413. doi: 10.1016/j.neuroimage.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 18.Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB. Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: a calibrated BOLD fMRI study. Neuroimage. 2008;40(1):237–247. doi: 10.1016/j.neuroimage.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noll DC, Fessler JA, Sutton BP. Conjugate phase MRI reconstruction with spatially variant sample density correction. IEEE Trans Med Imaging. 2005;24(3):325–336. doi: 10.1109/tmi.2004.842452. [DOI] [PubMed] [Google Scholar]
- 20.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 21.Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24(1):207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 22.Henkelman RM. Measurement of signal intensities in the presence of noise in MR images. Med Phys. 1985;12(2):232–233. doi: 10.1118/1.595711. [DOI] [PubMed] [Google Scholar]
- 23.van Laar PJ, Hendrikse J, Golay X, Lu H, van Osch MJ, van der Grond J. In vivo flow territory mapping of major brain feeding arteries. Neuroimage. 2006;29(1):136–144. doi: 10.1016/j.neuroimage.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Wu WC, Jiang SF, Yang SC, Lien SH. Pseudocontinuous arterial spin labeling perfusion magnetic resonance imaging-A normative study of reproducibility in the human brain. Neuroimage. 2011;56(3):1244–1250. doi: 10.1016/j.neuroimage.2011.02.080. [DOI] [PubMed] [Google Scholar]
- 25.Shin DD, Liu H-L, Shankaranarayanan A, Liu TT. Tagging Efficiency Corrected Pseudo Continuous Arterial Spin Labeling – A New Approach for Correction of Phase Tracking Errors. Montreal, Canada: 2011. p. 231. [Google Scholar]