Abstract
New modalities are available to visualize the small bowel in patients with Crohn’s disease (CD). The aim of this study was to compare the diagnostic yield of magnetic resonance enteroclysis (MRE) and capsule endoscopy (CE) to balloon-assisted enteroscopy (BAE) in patients with suspected or established CD of the small bowel. Consecutive, consenting patients first underwent MRE followed by CE and BAE. Patients with high-grade stenosis at MRE did not undergo CE. Reference standard for small bowel CD activity was a combination of BAE and an expert panel consensus diagnosis. Analysis included 38 patients, 27 (71%) females, mean age 36 (20–74) years, with suspected (n = 20) or established (n = 18) small bowel CD: 16 (42%) were diagnosed with active CD, and 13 (34%) by MRE with suspected high-grade stenosis, who consequently did not undergo CE. The reference standard defined high-grade stenosis in 10 (26%) patients. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value of MRE and CE for small bowel CD activity were 73 and 57%, 90 and 89%, 88 and 67%, and 78 and 84%, respectively. CE was complicated by capsule retention in one patient. MRE has a higher sensitivity and PPV than CE in small bowel CD. The use of CE is considerably limited by the high prevalence of stenotic lesions in these patients.
Keywords: Magnetic resonance imaging, Enteroclysis, Small bowel, Inflammatory small bowel disease, Capsule endoscopy, Crohn’s disease, Balloon-assisted enteroscopy
Crohn’s disease (CD) is a chronic inflammatory disorder associated with both mucosal and transmural inflammation of the bowel wall. CD can affect any part of the gastrointestinal tract, but the small bowel is affected in up to two-thirds of patients, with the distal ileum as the most affected site. Until recently, CD of the small bowel was assessed by upper gastrointestinal endoscopy, ileocolonoscopy, and conventional enteroclysis. However, the armamentarium of imaging techniques for small bowel involvement of CD has increased considerably in recent years with the introduction of capsule endoscopy (CE), balloon-assisted enteroscopy (BAE), and magnetic resonance enteroclysis (MRE).
Already, CE has been shown to provide a significantly higher diagnostic yield than push enteroscopy and conventional enteroclysis in patients with CD [1]. However, capsule retention in stenotic small bowel CD hampers its use in these patients [2]. BAE, first introduced in 2001 [3], combines endoscopic visualization of the entire small bowel with the possibility for endoscopic treatment of fibrotic strictures, and tissue sampling for histological examination [4, 5]. Although BAE can be considered as the reference standard, the lengthy and invasive character of the procedure, the associated discomfort, and need for conscious sedation limit its use [6]. MRE allows optimal visualization of soft tissues with multiplanar imaging capabilities and has already been proven to have additional value compared to endoscopic techniques in CD patients [7–10]. In theory, MRE may be a good alternative for CE and BAE in CD patients with suspected small bowel involvement.
To our knowledge, no study has simultaneously compared CE, MRE, and BAE in one patient population with suspected or established CD. Therefore, the aim of the present study was to compare the diagnostic yield of MRE and CE to BAE as reference standard in patients with suspected or established CD.
Patients and methods
Study population
For this prospective study, patients were recruited at the departments of gastroenterology of the Medical Center Alkmaar and the Erasmus University Medical Center Rotterdam, The Netherlands. Patients were eligible for inclusion if they had suspected or established CD and needed visualization of the small bowel because of suspected small bowel disease activity. Exclusion criteria were age <18 or >75 years, abdominal surgery in the 6 weeks prior to inclusion, clinical suspicion of significant small bowel obstruction, suspicion of an intra-abdominal abscess, pregnancy or breastfeeding, inability to swallow the video capsule, presence of a pacemaker or cardioversion device, or a history of contrast media reaction or allergy. Patients with severe concomitant disease with limited life expectancy or with a psychiatric, addictive, or any disorder compromising the ability to give informed consent were also excluded. The institutional review boards of the participating hospitals approved the study. All patients gave written informed consent prior to inclusion.
From January 2007 to July 2009, 67 patients were eligible for inclusion; 41 agreed to participate and were included after providing informed consent. Three patients were excluded after inclusion: one because of no attendance at examinations and two because of marked deterioration of their clinical condition preventing them from undergoing the subsequent examinations. Thus, 38 consecutive patients were eligible for evaluation: 20 (53%) with suspected and 18 (47%) with established CD. Eleven (29%) patients were male, and the mean age was 36 (range 20–74) years (Table 1). MRE, CE, and BAE were performed within a median of 22 (4–112) days, and in five (13%) patients, this interval was longer than 3 weeks. The median follow-up was 14 (7–36) months.
Table 1.
Patient characteristics
| Total (n = 38) | Suspected CD (n = 20) | Known CD (n = 18) | |
|---|---|---|---|
| Age (years) | 36 (20–74) | 31 (20–54) | 43 (28–74) |
| Male (%) | 11 (29) | 6 (30) | 5 (28) |
| Duration of CD (months) | 68 (1–204) | 44 (1–204) | 91 (24–192) |
| CDAI | 73 (22–147) | 78 (22–147) | 66 (34–134) |
CD Crohn’s disease, CDAI Crohn’s disease activity index
Patient disease activity was determined using the CD activity index at the time of inclusion. Currently, no validated enteroscopic small bowel CD severity scale exists, so small bowel lesions were defined as (1) absent: no disease activity; (2) mild: erythematous and/or edematous mucosa and/or small ulcerative lesions (<0.5 mm) within otherwise normal appearing mucosa; (3) moderate: larger ulcerative lesions (≥0.5 mm and <20 mm); or (4) severe: large ulcerative lesions (≥20 mm) and/or significant stenotic lesions, with or without macroscopic signs of inflammation. Changes in medical therapy, surgery, or other therapeutic measures were documented.
Study modalities
For evaluation, the small bowel was divided into four segments; duodenum, jejunum, proximal ileum, and distal (last 30 cm) ileum. For the qualitative assessment, image quality was graded on a three-point scale (non-diagnostic study, diagnostic study albeit with artifacts, diagnostic study of good quality). Investigators performing the examinations received the same clinical information but were blinded to the results of the other diagnostic procedures performed for the study.
All patients first underwent MRE, followed by CE and BAE with the aim of having all investigations completed within 3 weeks. For early diagnosis of high-grade small bowel stenosis, MRE was performed first. During the whole study, high-grade stenosis was defined as a small bowel lumen of <10 mm with maximal bowel distention, and these patients did not undergo CE. If MRE defined a luminal stenosis of 10–14 mm, a patency capsule (Agile Patency Capsule, Given Imaging Limited) was applied before CE. Failure of the patency capsule to pass the small bowel in <16 h based on plain abdominal X-ray was considered compatible with the presence of high-grade stenosis, and CE was subsequently not performed.
MRE
MRE was performed as previously described [11]. In brief, after bowel preparation, 1000–3000 ml 0.5% methylcellulose solution was infused at a rate of 60–150 ml/min via a nasoduodenal catheter for optimal small bowel distension. The MR protocol consisted of MR fluoroscopy, fat-saturated True FISP, and HASTE sequences as pre- and post-contrast T1-weighted VIBE sequences after intravenous administration of 0.1 mmol/kg of body weight of gadobutrol (Gadovist, Bayer Schering, Berlin, Germany). Butylscopolamine bromide (Buscopan, Boehringer, Ingelheim, Germany), 20 mg, used as a spasmolytic, was injected before the injection of gadobutrol. MRE studies were evaluated on a Picture Archiving and Communications System station (PACS, AGFA IMPAX version 4.5 service pack 5, Mechelen, Belgium) by a radiologist with an experience of >200 MRE studies. Electronic calipers were used for measurements. Bowel distension was graded as either insufficient (i.e., collapsed bowel loops) or sufficient for diagnosis (small bowel lumen measuring > 0.5 cm). For MRE, the duodenum was defined as the first 20 cm of the small bowel; jejunum was considered as small bowel loops left of an imaginary line from the liver dome to the roof of the left acetabulum; all bowel loops located right of this imaginary line were regarded as ileum; and the terminal ileum was defined as the last 30 cm of the ileum. Disease activity was based on the presence or absence of bowel wall thickness >4 mm, intramural and mesenteric edema, mucosal hyperemia, wall enhancement and enhancement pattern and transmural ulcerations and fistula formation.
CE
CE was performed as previously described [12]. In brief, after bowel preparation with 1 l Klean-Prep (Norgine Ltd., Marburg, Germany), the patient swallowed the capsule (PillCam type SB, Given Imaging Limited, Yokneam, Israel). After 8 h, the belt with the hard disk and the sensor array were removed. In case of doubt about passage of the capsule through the whole bowel, an X-ray of the abdomen was performed 1 week after ingestion. CE recordings were evaluated on a dedicated workstation (Rapid 4, Given Imaging Limited, Yokneam, Israel) by a gastroenterologist with extensive experience (>500 CE procedures). Gastric transit time, small intestinal transit time, and viewing time were recorded for each procedure. For CE, the duodenum was defined as the first 20 min of the small bowel; the time between start jejunum and start terminal ileum was divided into two for the transition between jejunum and ileum; and the terminal ileum was defined as the last 30 min of the ileum.
BAE
After an overnight fast and bowel preparation with 4 l of Klean-Prep (Norgine Ltd., Marburg, Germany), BAE was performed (Fujinon EN-450P5 or EN-450T5, Saitama, Japan) by one of two experienced enteroscopists, both having performed >200 BAE procedures. During the procedure, conscious sedation was applied using midazolam (Dormicum, Roche, Woerden, Netherlands), with or without fentanyl (Janssen-Cilag, Tilburg, Netherlands), and on withdrawal, butylscopalamine (Buscopan, Boehringer, Ingelheim, Germany) was administered. Most BAE procedures were performed via the anal approach; in selected cases with clinical suspicion of proximal small bowel pathology, first an oral approach was performed during the same procedure. Insertion depths were estimated using the method described by May et al. [4]. The complete studies were taped on digital video and the duration of each procedure noted. For BAE, the duodenum was defined as the first 20 cm distal from the bulb; jejunum as 20–200 cm distal from the bulb by oral and 130–230 cm proximal from the ileocecal valve or ileostoma by anal approach; proximal ileum as 200–300 cm from the bulb by oral and 30–130 cm from the ileocecal valve or ileostoma by anal approach; and the terminal ileum as the last 30 cm of the ileum. Biopsy sampling was performed in a standard fashion. Samples were taken if lesions were found during BAE or to rule out inflammation in endoscopically normal appearing small bowel segments.
Reference standard and expert panel
The reference standard consisted of (1) small bowel findings at BAE in those small bowel segments visualized by BAE and (2) an expert panel diagnosis for the remaining small bowel segments not visualized by BAE. The expert panel also re-evaluated segments for which BAE was negative and MRE and/or CE diagnosed small bowel lesion(s). This expert panel consisted of two experienced gastroenterologists, not involved in primary reading of the examinations evaluated in this study nor in the management of the patients included. Separately, both experts were presented with the anonymized, full patient medical history, clinical status and the written reports of BAE, MRE and CE with the most important images. Consensus was subsequently reached on the bowel segments scored discordantly. The patient medical history and clinical status included the indication for the diagnostic work-up, laboratory findings and, if available, results of histopathological examination together with the results of the three diagnostic procedures. For all cases, both experts came to a final consensus diagnosis.
Statistical analysis
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for MRE and CE were calculated compared to the reference standard.
No direct comparative data for the different techniques in the studied patient populations were available, so we estimated that the difference for detection of disease activity in CD would be 30% in favor of the reference standard. To detect a significant difference with a power of an alpha of 0.05 and beta of 0.20 required a study population of at least 33 patients.
Results
BAE and reference standard diagnosis
All patients underwent BAE, which was performed by a combined proximal and distal approach in 19 (50%), and by distal approach only in the other 19 patients. All studies were of good diagnostic quality. The median small bowel insertion depth with BAE was proximal 335 (200–460) cm and distal 98 (5–240) cm. The mean duration of BAE was 70 (30–150) min. BAE visualized 98/152 (65%) segments of the small bowel. The duodenum and jejunum were both visualized in 19/38 (50%) patients, the proximal ileum in 22/38 (58%) patient, and the distal ileum in 38/38 (100%) patients. No complications were noted during or after the BAE procedures. BAE revealed small bowel lesions in 16 (42%) patients (Table 2), confirmed as active CD by the expert panel. In the remaining 22 patients defined as normal by BAE, the expert panel did not change this diagnosis based on the data provided by MRE and CE. BAE diagnosed non-CD-related pathology in two patients; a pseudomelanotic colon and a Trichuris infection.
Table 2.
Crohn’s disease activity of MRE and CE
| Reference standarda n (%) | MRE diagnosis n (%) | CE diagnosis n (%) | |
|---|---|---|---|
| No disease activity | 19 (50) | 22 (58) | 19 (50) |
| Mild CD | 7 (18) | 3 (8) | 4 (11) |
| Moderate CD | 7 (18) | 6 (16) | 2 (5) |
| Severe CD | 5 (13) | 7 (18) | 0 (0) |
| Not performed | 0 | 0 (0) | 13 (34) |
| Total | 38 (100) | 38 (100) | 38 (100) |
a The reference standard consisted of (1) small bowel findings at BAE in those small bowel segments visualized by BAE and (2) an expert panel diagnosis for the remaining small bowel segments not visualized by BAE
MRE
The mean duration of MRE was 53 (37–91) min. All studies were of good diagnostic quality with sufficient small bowel distension. The MRE procedure was complicated in four (11%) patients by vomiting and in one (3%) patient by a mild allergic rash after intravenous contrast injection. In two patients, vomiting resulted from the presence of a high-grade small bowel stenosis and in one patient from a low-grade small bowel stenosis. The mean evaluation time was 9 (6–20) min. Visualization and evaluation of the four pre-defined small bowel segments was possible in all patients. MRE found evidence for CD small bowel disease activity in 16 (42%) patients (Table 2). This activity was confirmed by the gold diagnostic standard for 14 (88%) (Table 3). MRE revealed extramural abnormalities in four (11%) patients: a mesenteric abscess in two, an abdominal-enteral fistula in one, and intra-abdominal adhesions in one patient. MRE did not reveal non-CD-related pathology.
Table 3.
Crohn’s disease diagnosis by MRE and CE per patient in comparison with reference standard
| MRE diagnosis n (%) | CE diagnosis n (%) | |
|---|---|---|
| True positive | 14 (37) | 4 (16) |
| True negative | 17 (45) | 16 (64) |
| False positive | 2 (5) | 2 (8) |
| False negative | 5 (13) | 3 (12) |
| Total | 38 (100) | 25 (100) |
CE
MRE raised the suspicion of small bowel stenosis in 14 (37%) patients. In 11 of them, this stenosis was defined as high grade, and consequently these patients were excluded from CE. The remaining three patients had a suspected low-grade stenosis at MRE and underwent patency capsule testing. This patency capsule passed in one patient and was retained in two. Thus, 13 (34%) patients were excluded from CE because of small bowel stenosis and 25 (66%) underwent CE. CE visualized the complete small bowel in all but one patient (4%; capsule retention). The stenosis in this patient had not been detected during MRE, and the patient therefore had not been tested with a patency capsule. The capsule could not be removed during subsequent BAE and was removed surgically. The capsule retention was caused by a high-grade ileal stenosis. All CE procedures were of good diagnostic quality. The mean evaluation time was 23 (14–48) min. CE found evidence for CD small bowel disease activity in 6 (24%) patients (Table 2). This activity was confirmed by the gold diagnostic standard for 4 (67%) (Table 3). CE revealed presumed non-CD-related pathology in one patient with erosive gastritis.
MRE and CE compared with reference standard
MRE showed a higher rate of detection of moderate to severe CD activity compared to CE (17 vs. 3%). However, the exclusion of patients with suspected stenotic disease for subsequent CE (Figs. 1, 2) largely influenced this outcome. CE showed a higher detection rate of lesions in mild CD activity patients (Table 2; Fig. 3). These results (Table 3) correspond with a sensitivity of 74 and 57%, specificity of 90 and 89%, PPV of 88 and 67%, and NPV of 78 and 84%, for detection of small bowel CD lesions by MRE and CE, respectively.
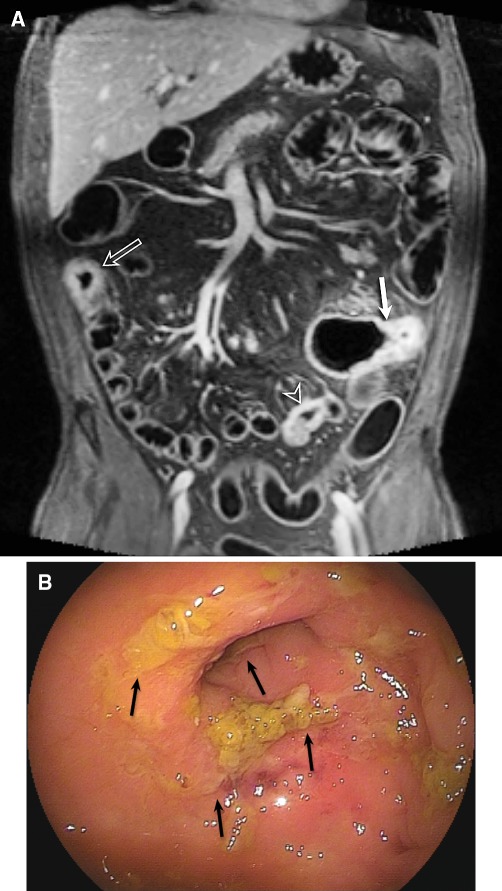
Fig. 1.
48-Year-old male patient with known CD for 20 years and postoperative ileocecal resection. Patient complained of abdominal pain. MRE showed on coronal T1 3d fat-sat image (A) after contrast injection, three active segments of CD (arrows) with bowel wall thickening, increased contrast enhancement, irregular mucosa, high-grade stenosis, and increased mesenterial vascularization (comb sign). CE was not performed because of the high-grade small bowel stenosis. BAE (B) showed ulcerations (arrows) in the terminal ileum. The proximal segments could not be visualized because of the high-grade small bowel stenosis.
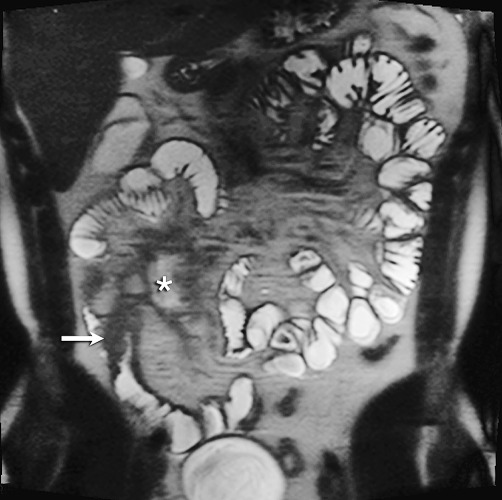
Fig. 2.
35-Year-old male patient without medical history and with suspected CD. Patient complaints were abdominal pain in the right lower quadrant. MRE showed coronal T2 HASTE image with small bowel thickening in the terminal ileum with high-grade stenosis (arrow). Extramural abscess medial of the terminal ileum (asterisk). CE was not performed because of the high-grade small bowel stenosis. BAE (not shown) showed swollen terminal ileum, without the possibility of cannulation.
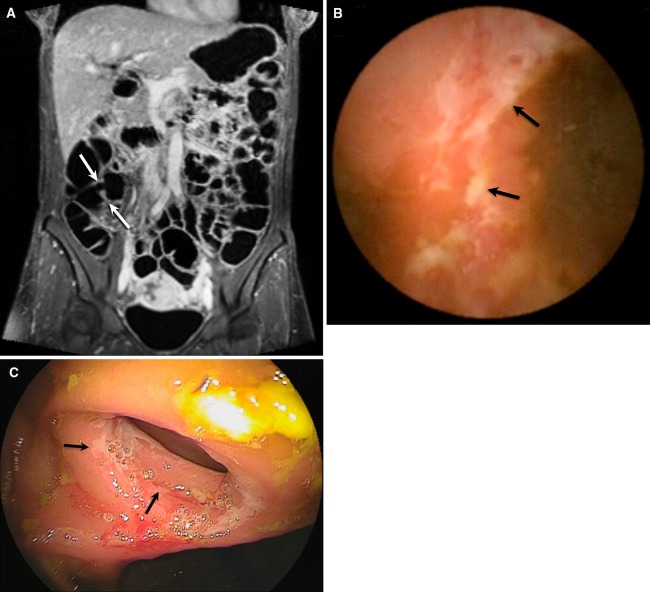
Fig. 3.
39-Year-old female patient with known CD and postoperative ileocecal resection 3 years earlier. Patient complaints were abdominal pain and diarrhea. MRE showed on coronal T1 3d fat-sat image (A) after contrast injection, normal anastomosis (arrows) of the neo-ileocecal junction without bowel wall thickening or increased contrast enhancement. CE (B) and BAE (C) both indicated superficial ulcerations on the level of the anastomosis (arrows). No other abnormalities were diagnosed.
BAE diagnosed ten high-grade stenotic lesions in 10 (26%) patients: eight stenoses in distal ileum, one in jejunum, and one in proximal ileum. MRE reported 15 stenotic segments in 13 patients (34%). The detection of high-grade small bowel stenosis by MRE had a sensitivity of 91%, 99% specificity, 77% PPV, and 96% NPV. MRE was false negative in one patient, leading to capsule retention resulting from a high-grade stenosis in the distal jejunum.
Discussion
The results of this prospective study demonstrate that MRE has a higher overall sensitivity and PPV, for small bowel lesions as compared to CE in patients with suspected or established CD with suspected small bowel activity. In these patients, the use of CE is limited by the risk of capsule retention. The findings of a higher sensitivity and PPV in staging more advanced CD and reduced accuracy in staging mild CD activity for MRE as compared to CE seem to accord with an earlier study by Tillack et al. [ 13]. However, in the present study, CE was prohibited in one-third of patients because of suspected high-grade small bowel stenotic disease, contributing to the result that MRE performed better compared to CE in patients with more advanced CD.
The high prevalence of high-grade stenoses is in line with the findings of Voderholzer et al. in a CE study of 15 patients (27%) with established CD [14]. The frequency of high-grade stenosis varied depending on CD stage and ranged from 1.4% in patients with suspected CD to 13% in known CD [15, 16]. The possible presence of high-grade small bowel stenosis is the major drawback of CE, necessitating the exclusion of functional stenoses prior to CE. Nevertheless, even with pre-exclusion of high-grade small bowel stenosis, capsule retention may occur, as illustrated by one of our patients.
Capsule retention in these patients is a major adverse event, requiring additional intervention that is a burden and a risk for the patient and that generates extra costs [17]. In our opinion, the increased risk of capsule retention in these patients outweighs the higher accuracy of CE in staging mild CD. Although MRE has a high diagnostic yield for stenotic CD, it can overstage this condition. MRE indicated small bowel stenoses in 13 patients, but in three patients, BAE did not confirm this. However, in one patient with clinically suspected small bowel stenosis, the patency capsule was retained and a high-grade ileal stenosis identified during surgery at 1 year of follow-up.
An advantage of MRE compared to the other two modalities is the visualization of extramural small bowel disease, which can be of additional value in staging CD and determining therapeutic options. In this study, MRE detected significant extramural abnormalities in 8% of patients. A disadvantage of MRE is that some patients do not tolerate the oral preparation and/or small bowel distension, especially those with high-grade small bowel stenosis. This intolerance may lead to insufficient small bowel distension and visualization, and consequently to reduced sensitivity of MRE for small bowel pathology. We performed enteroclysis to obtain optimal distension of the small bowel. Masselli et al. [18] concluded that MRE was superior to MR enterography in detection of milder superficial pathology. Another study presented by Negaard et al. [19] showed comparable results of MRE to MR enterography in the terminal ileum, but the study defined MRE as MR of the small bowel after transportation of the patient to the MR unit following conventional enteroclysis. In our experience, bowel distention decreases rapidly with reduction or termination of the infusion rate, and small bowel wall thickening therefore can be missed with this type of MR procedure.
This study has some limitations. First, the order of the examinations was pre-determined, and MRE was used to rule out high-grade stenosis before patients had CE to prevent capsule retention. Second, the study population was relatively small, and a proportion of patients did not undergo CE because of a high-grade stenosis. Because of this limited number, significance could not be calculated. Third, BAE could not visualize all small bowel segments in all patients, and an expert panel was therefore asked to establish a final diagnosis in a subset of patients. The bias such a panel may introduce can have two effects: (1) weakening the study because of subjectivity or (2) strengthening the study because the diagnosis of CD, or disease activity assessment, is often based on multiple diagnostic tests. The major strength of the study is the head-to-head comparison of MRE and CE to a reference standard including BAE, suggesting that an expert panel is an acceptable reference standard. A fourth limitation is the fact that not all procedures were performed within the three-week time frame. The prolonged interval between the investigations might have influenced the findings in some cases.
From the results, we conclude that MRE could be a first-choice non-invasive diagnostic procedure in patients with suspected small bowel CD, followed by CE or BAE, depending on (1) the outcome with MRE and/or (2) the need for histopathological findings. The high incidence of small bowel stenosis in these patients prohibits the use of CE as a diagnostic modality.
Acknowledgment
Jeroen Doodeman, Yvonne Afman, Mai Thieme and Tjeerd van der Ploeg are acknowledged for their assistance during the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407–2418. doi: 10.1111/j.1572-0241.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Terano A. Capsule endoscopy: past, present, and future. J Gastroenterol. 2008;43:93–99. doi: 10.1007/s00535-007-2153-6. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Sekine Y, Sato Y, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216–220. doi: 10.1067/mge.2001.112181. [DOI] [PubMed] [Google Scholar]
- 4.May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62–70. doi: 10.1016/S0016-5107(05)01586-5. [DOI] [PubMed] [Google Scholar]
- 5.Heine GD, Hadithi M, Groenen MJ, et al. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42–48. doi: 10.1055/s-2005-921188. [DOI] [PubMed] [Google Scholar]
- 6.Domagk D, Bretthauer M, Lenz P, et al. Carbon dioxide insufflation improves intubation depth in double-balloon enteroscopy: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:1064–1067. doi: 10.1055/s-2007-966990. [DOI] [PubMed] [Google Scholar]
- 7.Wiarda BM, Kuipers EJ, Houdijk LP, Tuynman HA. MR enteroclysis: imaging technique of choice in diagnosis of small bowel diseases. Dig Dis Sci. 2005;50:1036–1040. doi: 10.1007/s10620-005-2700-z. [DOI] [PubMed] [Google Scholar]
- 8.Negaard A, Sandvik L, Mulahasanovic A, Berstad AE, Klow NE. Magnetic resonance enteroclysis in the diagnosis of small-intestinal Crohn’s disease: diagnostic accuracy and inter- and intra-observer agreement. Acta Radiol. 2006;47:1008–1016. doi: 10.1080/02841850600979071. [DOI] [PubMed] [Google Scholar]
- 9.Ochsenkuhn T, Herrmann K, Schoenberg SO, et al. Crohn disease of the small bowel proximal to the terminal ileum: detection by MR-enteroclysis. Scand J Gastroenterol. 2004;39:953–960. doi: 10.1080/00365520410003218. [DOI] [PubMed] [Google Scholar]
- 10.Paoloantonio P, Tomei E, Rengo M, et al. Adult celiac disease: MRI findings. Abdom Imaging. 2007;32:433–440. doi: 10.1007/s00261-006-9089-9. [DOI] [PubMed] [Google Scholar]
- 11.Wiarda BM, Heine DG, Rombouts MC, Kuipers EJ, Stoker J. Jejunum abnormalities at MR enteroclysis. Eur J Radiol. 2008;67:125–132. doi: 10.1016/j.ejrad.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Van Tuyl SA, Van Noorden JT, Kuipers EJ, Stolk MF. Results of videocapsule endoscopy in 250 patients with suspected small bowel pathology. Dig Dis Sci. 2006;51:900–905. doi: 10.1007/s10620-006-9351-6. [DOI] [PubMed] [Google Scholar]
- 13.Tillack C, Seiderer J, Brand S, et al. Correlation of magnetic resonance enteroclysis (MRE) and wireless capsule endoscopy (CE) in the diagnosis of small bowel lesions in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1219–1228. doi: 10.1002/ibd.20466. [DOI] [PubMed] [Google Scholar]
- 14.Voderholzer WA, Beinhoelzl J, Rogalla P, et al. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369–373. doi: 10.1136/gut.2004.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourreille A, Ignjatovic A. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED–ECCO consensus. Endoscopy. 2009;41:618–637. doi: 10.1055/s-0029-1214790. [DOI] [PubMed] [Google Scholar]
- 16.Legnani P, Abreu MT. Use of capsule endoscopy for established Crohn’s disease. Gastrointest Endosc Clin N Am. 2006;16:299–306. doi: 10.1016/j.giec.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006;101:2218–2222. doi: 10.1111/j.1572-0241.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 18.Masselli G, Casciani E, Polettini E, Gualdi G. Comparison of MR enteroclysis with MR enterography and conventional enteroclysis in patients with Crohn’s disease. Eur Radiol. 2008;18:438–447. doi: 10.1007/s00330-007-0763-2. [DOI] [PubMed] [Google Scholar]
- 19.Negaard A, Paulsen V, Sandvik L, et al. A prospective randomized comparison between two MRI studies of the small bowel in Crohn’s disease, the oral contrast method and MR enteroclysis. Eur Radiol. 2007;17:2294–2301. doi: 10.1007/s00330-007-0648-4. [DOI] [PubMed] [Google Scholar]