Abstract
Objectives
Magnetic resonance imaging (MRI) is not accurate in discriminating T1-2 from borderline T3 rectal tumors. Higher resolution on 3 Tesla-(3T)-MRI could improve diagnostic performance for T-staging. The aim of this study was to determine whether 3T-MRI compared with 1.5 Tesla-(1.5T)-MRI improves the accuracy for the discrimination between T1-2 and borderline T3 rectal tumors and to evaluate reproducibility.
Methods
13 patients with non-locally advanced rectal cancer underwent imaging with both 1.5T and 3T-MRI. Three readers with different expertise evaluated the images and predicted T-stage with a confidence level score. Receiver operator characteristics curves with areas under the curve (AUC) and diagnostic parameters were calculated. Inter- and intra-observer agreements were calculated with quadratic kappa-weighting. Histology was the reference standard.
Results
Seven patients had pT1-2 tumors and six had pT3 tumors. AUCs ranged from 0.66 to 0.87 at 1.5T vs. 0.52–0.82 at 3T. Mean overstaging rate was 43% at 1.5T and 57% at 3T (P = 0.23). Inter-observer agreement was κ 0.50–0.71 at 1.5T vs. 0.15–0.68 at 3T. Intra-observer agreement was κ 0.71 at 1.5T and 0.76 at 3T.
Conclusions
This is the first study to compare 3T with 1.5T MRI for T-staging of rectal cancer within the same patients. Our results showed no difference between 3T and 1.5T-MRI for the distinction between T1-2 and borderline T3 tumors, regardless of expertise. The higher resolution at 3T-MRI did not aid in the distinction between desmoplasia in T1-2-tumors and tumor stranding in T3-tumors. Larger studies are needed to acknowledge these findings.
Keywords: Rectal cancer, MRI, T-stage, 3 Tesla
Besides surgery, neoadjuvant treatment with chemo- and/or radiotherapy has become a crucial part of the treatment of rectal cancer. The choice of the type of neoadjuvant treatment and surgery is based on preoperative imaging. During the past decade magnetic resonance imaging (MRI) has been proven to be the most accurate staging modality for primary rectal cancer [1]. With preoperative imaging the T-stage, involved circumferential resection margin (CRM), nodal involvement, and location of the tumor are evaluated, which determine the type of (pre)operative treatment. So far, MRI has not been able to accurately predict T-stage unless an endorectal coil is used [2, 3]. However, an endoluminal technique leads to less visibility of surrounding structures in the mesorectum because of the limited field of view. Furthermore, it is difficult to apply this technique in high and stenosing tumors. Therefore, endorectal MRI has been less adopted for staging small rectal tumors than endorectal ultrasound (EUS) [4, 5]. EUS has equally good accuracy as endorectal MRI for identification of T1 tumors, is less cumbersome and is therefore the exam of choice for staging superficial rectal cancer [6]. It is, however, less accurate for staging of the more advanced tumors, in specific those with threatened CRM because EUS cannot identify the mesorectal fascia. Therefore, MRI with phased-array coils is widely adopted as the most accurate technique for staging of these bulky T3 and T4 tumors with sensitivities ranging from 74% to 94% and specificities from 74% to 76% [7]. It is the borderline tumor, the T2 or small T3 tumor, that remains a diagnostic challenge. The distinction between T2 and borderline T3 tumors has important implications for clinical decision making. When a tumor is confined to the bowel wall (T1-2) the patient will undergo surgery immediately without neoadjuvant treatment, but when the tumor penetrates the bowel wall (T3) the current trend is to administer preoperative neoadjuvant therapy (radiotherapy with or without chemotherapy) prior to surgery. Furthermore, when a tumor is confined to the bowel wall local excision—leading to less morbidity and mortality than with standard surgery—could be considered [8], whereas in tumors outgrowing the bowel wall this is not possible. A large proportion of the pT2 tumors is overstaged, because desmoplastic benign reactions are mistaken for tumoral extensions associated with a borderline pT3 tumor [9]. Overstaging would thus lead to overtreatment of patients with T1-2 tumors, with increased risk for treatment related morbidity and mortality.
Most studies evaluating the accuracy of phased-array MRI for rectal cancer staging have focused on 1.5 Tesla (1.5T) MR units. MR imaging at higher field strength (3 Tesla) could improve the diagnostic performance of phased-array MRI for T-stage prediction. Three Tesla (3T) MRI has a theoretical two-fold increase of signal-to-noise ratio compared to 1.5T MRI [10]. This could allow for better resolution and enhanced image quality, resulting in a more accurate distinction between T2 and borderline T3 tumors. Only few studies exist on 3T MRI for T-stage prediction in rectal cancer. To the best of our knowledge, no report exists on the intra-patient comparison of the diagnostic performance of 3T MRI with that of 1.5T MRI [9, 11–16].
Therefore, the aim of this study is to determine, with an intra-patient comparison, whether 3T MRI compared with 1.5T MRI leads to improvement of accuracy for the differentiation between tumors confined to the bowel wall (T1-2) and tumors penetrating the bowel wall (T3) and to evaluate reproducibility.
Materials and methods
Patients
Between November 2007 and July 2008, 13 patients with primary non-locally advanced rectal cancer who underwent MR imaging of the pelvis at 1.5 and 3T (with a mean interval of 19.8 ± 10.8 days) were included. The 3T MRI scans were performed as part of another MRI study protocol, which was approved by the local institutional review board. Inclusion criteria were: (1) patients had to have undergone pelvic MRI at 1.5 and 3T, (2) had non-locally advanced rectal cancer and underwent 5 × 5 Gray followed by immediate surgery, which has been shown to have no downstaging effect on the tumor [17]. Non-locally advanced rectal cancer was defined as tumors with a T-stage up to borderline T3 and uninvolved CRM, based on histology after surgery, which was the reference standard.
MRI techniques
Patients were placed in feet first supine position. No antiperistaltic agent or endorectal filling was administered prior to imaging. Phased-array multichannel coils were used for signal reception. The 1.5T MR unit was either an Intera unit (n = 5, Philips Medical Systems, Best, The Netherlands) or an Avanto unit (n = 8, Siemens Medical Solutions, Erlangen, Germany). The MR sequences followed the standard rectal MR protocol as described in the literature and consisted of 2D T2-weighted fast spin echo (FSE) sequences (TR/TE 3500-4780/94-150 ms, with in plane resolution ranging from 1.14 × 0.78 to 1.09 × 1.56 mm2, slice thickness 3–5 mm) in two planes: sagittal and axial. Acquisition time per sequence ranged from 3:17 to 5:08 min. All patients underwent a second scan at a 3T MRI unit (Intera Achieva (software release 1.5.4.0); Philips Medical Systems, Best, The Netherlands) with standard 2D T2-weighted FSE sequences (TR/TE 3107/150 ms, in plane resolution 0.65 × 1.06 mm2, slice thickness 4 mm) in two planes: sagittal and axial. Acquisition time per sequence was 2:52 min. This sequence was based partially on the sequence as published in the literature by Kim et al. [14].
Image analysis
Three readers with different experience in reading rectal cancer MRI retrospectively evaluated the images, independently and blinded for each other’s and histological results. Image evaluation was performed according to earlier published criteria [18, 19]. Reader 1 has 7 years of specific experience in reading rectal MR in a referral hospital. Reader 2 is an experienced MR rectum reader who has 5 years of experience in a referral hospital. Reader 3 is a general radiologist who works in a general hospital, without specific MR rectum experience.
All readers evaluated the MR images for the T-stage as follows: the likelihood for tumor confined to the bowel wall (T1-2) was scored using a confidence level system ranging from: 0 = definitely outgrowing wall, 1 = probably outgrowing wall, 2 = possibly confined to the wall, 3 = probably confined to wall, and 4 = definitely confined to wall. All readers evaluated the images at the same Picture Archiving and Communication System.
All readers evaluated the 1.5T scans first. After a 30 min interval, the 3T images were evaluated at random patient order, besides blinding for each others’ and histology results, the readers were also blinded for the 1.5T results. These evaluations were used for analysis and comparison of the accuracy between 1.5 and 3T MRI for T-stage prediction.
Furthermore, for reader 2 an additional scoring procedure of both 1.5 and 3T scans was organized to determine intra-observer agreement (with an interval of 2 weeks between scoring procedures).
Histopathologic examination
Results for T-stage prediction by all readers at both field strengths were compared with histopathological staging of the surgical specimen, which was the reference standard. Surgery was performed directly subsequent to the short course of radiation. The surgical specimens were handled according to standard clinical practice as advocated by Quirke et al. [20] and were pathologically examined in accordance with the Tumor Node Metastasis staging system.
Statistical analysis
For statistical analyses Statistical Package for the Social Sciences (SPSS, version 15, Inc., Chicago, IL) was used. Receiver operator characteristics (ROC) curve analyses were performed and areas under the curve (AUC) with 95% confidence intervals (CI) were calculated. AUCs were compared according to the method as described by DeLong [21] using Stata software (Stata, release 9.2; StataCorp, College Station, Texas) to determine whether the difference in diagnostic performance between both field strengths was statistically significant. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95%CI were calculated for all readers on both field strengths. For these analyses, it was decided at the start of the study to use a cut-off level between confidence level 2 (possibly confined to the bowel wall) and 3 (probably confined to the bowel wall), with tumor confined to the bowel wall as the positive outcome measure. Mean overstaging rate of tumors limited to the bowel wall and understaging rate of tumors outgrowing the bowel wall were calculated for all the three readers. The paired samples t test was used to test whether the difference in mean over- and understaging rates between 1.5T MRI and 3T MRI was statistically significant. Intra- and inter-observer agreements were analyzed by using weighted kappa values with quadratic weighting. Degree of agreement was stated as follows: κ value 0.00–0.20 poor agreement; κ value 0.21–0.40 fair agreement; κ value 0.41–0.60 moderate agreement; κ value 0.61–0.80 good agreement, and κ value 0.81–1.00 excellent agreement. P values smaller than 0.05 were considered statistically significant.
Results
Patient characteristics
Median age of the 13 included patients was 67 years (range 53–78), 10 were male and 3 were female. Of the 13 patients, 3 had pT1 tumors, 4 had pT2 tumors, and 6 had pT3 tumors, thus 7 patients had tumor confined to the bowel wall (pT1-2) and 6 had tumor outgrowing the bowel wall (pT3). Total mesorectal excision was performed in all 13 patients. Median interval between final radiation dose and surgery was 1 day (range 1–5).
Diagnostic performance
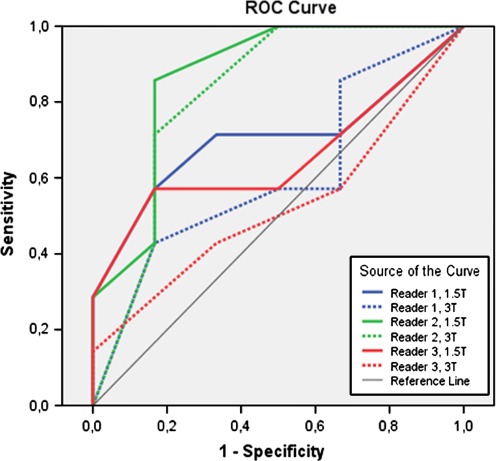
Sensitivity, specificity, PPV, NPV, AUC, overstaging, and understaging rates for all readers at both field strengths are shown in Table 1. ROC curves for all readers at both field strengths are shown in Figure 1. There was no difference in AUC for 1.5 and 3T between the readers except between reader 1 (0.821) and reader 3 (0.524) at 3T MRI (P = 0.04).
Table 1.
Overview of results for all readers at 1.5 and 3T
| 1.5 Tesla MRI | 3 Tesla MRI | |||||||
|---|---|---|---|---|---|---|---|---|
| Reader 1 (RFAV) | Reader 2 (MJL) | Reader 3 (MOJ) | Overall (3 readers) | Reader 1 (RFAV) | Reader 2 (MJL) | Reader 3 (MOJ) | Overall (3 readers) | |
| Sensitivity (%) | 57 (4/7) (33–69) | 43 (3/7) (20–54) | 57 (4/7) (33–69) | 52 (11/21) (39–61) | 57 (4/7) (35–78) | 43 (3/7) (20–54) | 29 (2/7) (10–40) | 43 (9/21) (29–55) |
| Specificity (%) | 83 (5/6) (55–97) | 83 (5/6) (55–97) | 83 (5/6) (55–97) | 83 (15/18) (67–94) | 50 (3/6) (24–75) | 83 (5/6) (55–97) | 83 (5/6) (62–97) | 72 (13/18) (56–86) |
| PPV (%) | 80 (4/5) (46–96) | 75 (3/4) (36–95) | 80 (4/5) (46–96) | 79 (11/14) (58–92) | 57 (4/7) (35–78) | 75 (3/4) (ateria36–95) | 75 (3/4) (24–94) | 64 (9/14) (44–82) |
| NPV (%) | 63 (5/8) (41–73) | 56 (5/9) (38–65) | 63 (5/8) (41–73) | 60 (15/25) (48–67) | 50 (3/6) (37–58) | 56 (5/9) (38–65) | 50 (5/10) (37–58) | 52 (13/25) (40–62) |
| AUC | 0.70 (0.40–1.00) | 0.87 (0.65–1.00) | 0.66 (0.34–0.97) | 0.73 (0.57–0.89) | 0.61 (0.29–0.93) | 0.82 (0.57–1.00) | 0.52 (0.20–0.85) | 0.64 (0.46–0.81) |
| Accuracy (%) | 69 (9/13) (43–82) | 62 (8/13) (37–74) | 69 (9/13) (43–82) | 67 (26/39) (52–76) | 54 (7/13) (30–77) | 62 (8/13) (37–74) | 54 (7/13) (34–66) | 56 (22/39) (42–69) |
| Overstaging (%) | 43 (3/7) | 29 (2/7) | 57 (4/7) | 43 (9/21) | 43 (3/7) | 43 (3/7) | 86 (6/7) | 57 (12/21) |
| Understaging (%) | 17 (1/6) | 17 (1/6) | 0 (0/6) | 11 (2/18) | 33 (2/6) | 17 (1/6) | 0 (0/6) | 17 (3/18) |
Positive outcome measure is pT1-2
PPV positive predictive value, NPV negative predictive value, AUC area under ROC curve
Fig. 1.
ROC curves for T-stage prediction for all readers at 3 and 1.5T.
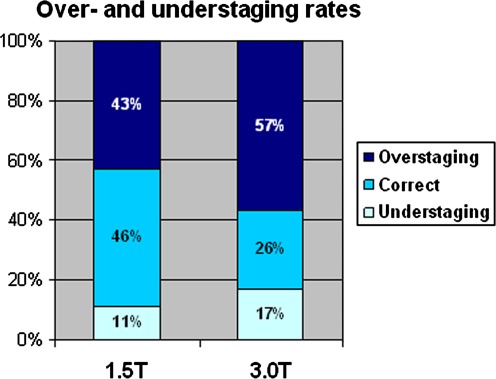
Mean rates of overstaging and understaging for all readers were comparable at both field strengths (Fig. 2). Mean overstaging was 43% at 1.5T vs. 57% at 3T (P = 0.23). Mean understaging was 11% at 1.5T vs. 17% at 3.0T (P = 0.42).
Fig. 2.
Mean over- and understaging rates for 1.5 and 3T MRI.
Agreement
At 1.5T, inter-observer agreement was moderate to good (κ 0.502–0.708). Inter-observer agreement was lower at 3T MRI (κ 0.153–0.681). Intra-observer agreement (reader 2) was good: at 1.5T weighted κ was 0.710 (95% CI: 0.544–0.875) and at 3T weighted κ was 0.762 (95% CI: 0.662–0.862). Results for agreement are presented in Table 2.
Table 2.
Inter-observer and intra-observer agreement
| κ | ||
|---|---|---|
| 1.5 Tesla MRI | 3 Tesla MRI | |
| Inter-observer | ||
| Reader 2 vs. reader 1 | 0.544 (0.132–0.956) | 0.301 (0–0.744) |
| Reader 2 vs. reader 3 | 0.708 (0.458–0.958) | 0.681 (0.408–0.953) |
| Reader 1 vs. reader 3 | 0.502 (0.057–0.947) | 0.153 (0–0.587) |
| Intra-observer | ||
| Reader 2 | 0.710 (0.544–0.875) | 0.762 (0.662–0.862) |
Discussion
This intra-patient comparison study between 3 and 1.5T MRI does not show an improved diagnostic performance for T-staging with 3T MRI regardless of the experience of the reader, with sensitivities from 29% to 57% and specificities from 50% to 83%. For the reader from a general hospital, prediction at 3T MRI was least accurate. Overstaging was higher at 3T MRI and highest for the general reader: 86% at 3T compared to 57% at 1.5T. Inter-observer agreement was moderate to good at 1.5T and poor to good at 3T MRI. Intra-observer agreement was measured for reader 2 and was good.
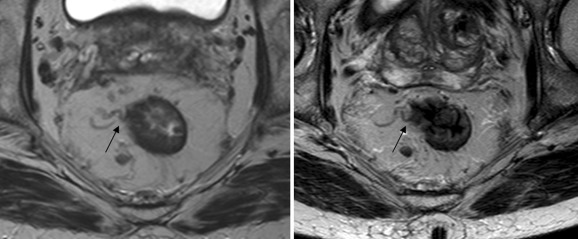
Our finding that 3T is not better than 1.5T MRI for T2 vs. T3 differentiation might be surprising; nevertheless, it could be explained given the fact that with MR the differentiation between T1-2 and T3 tumors is associated with interpretation difficulties in the distinction between desmoplastic benign reactions in a T2 tumor and malignant tumor stranding in a T3 tumor (Fig. 3). This results in substantial overstaging errors in this group of borderline tumors, confirming the findings of other authors [18, 22]. Overstaging rates at 1.5T MRI are reported to range from 25% to 46% [9]. In our study at 1.5T, overstaging occurred in 29%–57%, slightly higher figures than in the literature because of our study population, with mainly patients with borderline T1-2 and T3 tumors. At 3T, it was higher ranging from 43% to 86%. This confirms the hypothesis that despite the inherent higher resolution and the more detailed depiction of the rectal wall, 3T does not help us to obtain a better performance because the interpretation difficulties of desmoplastic reactions with or without tumor cells remain.
Fig. 3.
Stranding (arrows) around a pT2 tumor which cannot be distinguished from tumor extension in a T3 tumor (left 1.5T and right 3T).
Our results are in contrast to other publications. Only small studies on 3T MRI have been reported and these showed controversial findings with overstaging rates ranging from 15% to 30% [11]. In the largest study to date by Kim et al. [13] in 109 patients who were evaluated at 3T MRI, only equal overstaging rates were found as earlier reported with 1.5T MRI. Their findings suggest that 3T MRI is, unlike our results, not worse than 1.5T MRI.
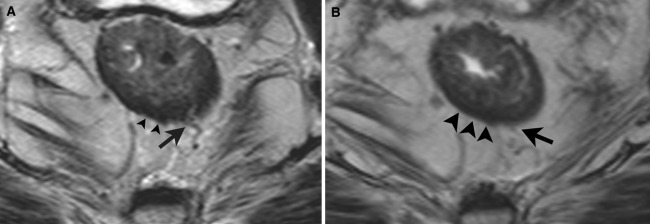
The inter-observer agreement was lower at 3T MRI compared to 1.5T MRI, which indicates higher degrees of uncertainty between readers. This may have been caused by the more detailed depiction of the tumor and of the normal anatomy. Improved visibility at 3T MRI of small vessels penetrating the rectal wall may cause more interpretation difficulties (Fig. 4A, B). Increased variation between readers just by chance would be less likely because of the relatively high intra-observer agreement at both field strengths, although tested in only one of the readers (reader 2). We believe that the solid intra-observer agreement at both field strengths can be explained by the type of case mix that reader 2 is exposed to in the referral hospital, which had resulted in a stable and consistent learning curve, making him more flexible to handle different and new techniques.
Fig. 4.
A, B A small vessel penetrating the bowel wall is depicted in more detail on the 3T image (A) than on the 1.5T image (B). This could lead to more interpretation difficulties and thus overstaging of T2 tumors.
The highest overstaging rate was found in the reader from a non referral center (86% at 3T). The fact that this reader reads rectal MRI on a less frequent basis than the others might explain the inferior results compared to the others. This trend may be even aggravated by the more detailed depiction at 3T MRI which can lead to more interpretation difficulties in the less experienced reader in particular. The PPV for staging of T2 tumors with MRI is known to be high [23], because the existence of a hypo-intense bowel wall on T2W FSE sequences (Fig. 5) is a reliable predictive criterion for a tumor limited to the bowel wall. Yet this sign is subject to some learning curve. Therefore, readers from a referral center are more confident to identify a tumor that is restricted to the bowel wall based on this bowel wall sign.
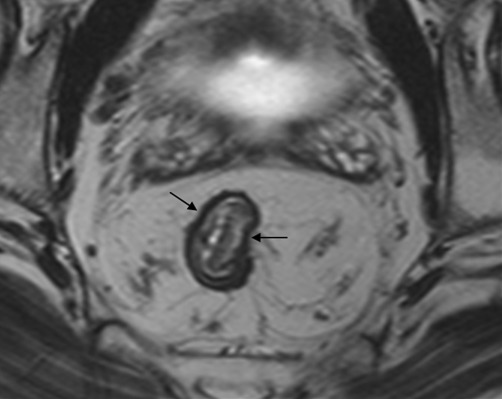
Fig. 5.
An intact hypo-intense line (arrows) is seen surrounding the tumor, which indicates that the muscularis propria is intact (1.5T image). Therefore, the tumor is confined to the bowel wall (pT1-2).
There are some limitations to this study. First, the sample size is small. Nevertheless, this is the first study performing an intra-patient comparison between 1.5 and 3T. Thus, the results are important and suggest that it is not so obvious that 3T MRI would be better than 1.5T MRI. Second, it can be argued that other published studies had a higher resolution at 3T than our study [11, 13–15]. Furthermore, it would have been ideal to have a similar acquisition time at 3T as at 1.5T. We acknowledge that our 3T protocol could be improved; however, due to the retrospective nature of the study this was not possible. Nevertheless, compared to our 1.5T standard rectal MR protocol, our 3T protocol was still superior in resolution and we could not achieve better results with 3T MRI. Third, we performed the 1.5T scans on two different MR units and the 3T scans on one unit. If this would have influenced our findings, we would expect an advantage for the diagnostic performance of 3T MRI (because of a uniform image acquisition at 3T for all patients), which we did not find in this study.
Clinical relevance
Accurate staging of tumors limited to the bowel wall vs. tumors outgrowing the rectal wall is important because it determines treatment stratification. When tumors are confined to the bowel wall (T1-2), patients are referred for immediate TME. When the tumor penetrates the bowel wall (T3-4), the patient will be stratified for a more intensive treatment arm with a long course of chemoradiation treatment followed by resection. In the Netherlands and Northern Europe, patients with T3 tumors without CRM involvement are given preoperative 5 × 5 Gray radiation treatment (instead of a long course of chemoradiation) followed by immediate TME. Overstaging of T2 tumors by MRI would lead to a substantial overtreatment of patients with a T1-2 tumor, who will receive unnecessary preoperative (chemo)radiotherapy. Furthermore, the recent trend toward organ sparing treatment propagates local excision for tumors that are limited to the bowel wall [8]. When local excision becomes standard treatment for T1-2 tumors, the high overstaging rate leads to overtreatment of these patients as well, with not only unnecessary neoadjuvant treatment, but also an unnecessary TME.
Conclusions
In this first intra-patient comparison between 3T MRI with 1.5T MRI for the clinically relevant distinction between T2 and borderline T3 tumors, no improvement was found with 3T MRI, regardless of the expertise of the readers. Even though there is a better visibility of the rectal wall at 3T, it does not aid in the distinction between T2 tumors with desmoplasia and borderline T3 tumors with tumor stranding in the mesorectal fat, a distinction which is crucial for the differentiation and known to be difficult on MRI. The lower inter-observer agreement at 3T MRI reflects less certainty about the prediction of the T-stage, most prominent in the reader from a general hospital. Future studies have to validate these findings in a larger sample size by comparing local staging of rectal cancer at 3T with 1.5T MRI in one patient group.
Acknowledgments
Conflicts of interest
There are no conflicts of interest or acknowledgements associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Max J. Lahaye, Phone: 0031-43-3876957, FAX: 0031-43-3876909
Roy F. A. Vliegen, Phone: 0031-45-5766666, FAX: 0031-45-5766055
Margreet Osinga-de Jong, Phone: 0031-88-4597777.
Joachim E. Wildberger, Phone: 0031-43-3881863, FAX: 0031-43-3881890
Regina G. H. Beets-Tan, Phone: 0031-43-3876957, FAX: 0031-43-3876909, Email: beetstan@me.com
References
- 1.Valentini V, Aristei C, Glimelius B, et al. Multidisciplinary rectal cancer management: 2nd european rectal cancer consensus conference (EURECA-CC2) Radiother Oncol. 2009;92(2):148–163. doi: 10.1016/j.radonc.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Blomqvist L, Machado M, Rubio C, et al. Rectal tumor staging: MR imaging using pelvic phased-array and endorectal coils vs endoscopic ultrasonography. Eur Radiol. 2000;10(4):653–660. doi: 10.1007/s003300050979. [DOI] [PubMed] [Google Scholar]
- 3.Vogl TJ, Pegios W, Mack MG, et al. Accuracy of staging rectal tumors with contrast-enhanced transrectal MR imaging. AJR Am J Roentgenol. 1997;168(6):1427–1434. doi: 10.2214/ajr.168.6.9168702. [DOI] [PubMed] [Google Scholar]
- 4.Hunerbein M, Pegios W, Rau B, et al. Prospective comparison of endorectal ultrasound, three-dimensional endorectal ultrasound, and endorectal MRI in the preoperative evaluation of rectal tumors. Preliminary results. Surg Endosc. 2000;14(11):1005–1009. doi: 10.1007/s004640000345. [DOI] [PubMed] [Google Scholar]
- 5.Slater A, Halligan S, Taylor SA, Marshall M. Distance between the rectal wall and mesorectal fascia measured by MRI: effect of rectal distension and implications for preoperative prediction of a tumor-free circumferential resection margin. Clin Radiol. 2006;61(1):65–70. doi: 10.1016/j.crad.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Ptok H, Marusch F, Meyer F, et al. Feasibility and accuracy of TRUS in the pre-treatment staging for rectal carcinoma in general practice. Eur J Surg Oncol. 2006;32(4):420–425. doi: 10.1016/j.ejso.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Bipat S, Glas AS, Slors FJ, et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 8.Tytherleigh MG, Warren BF, Mortensen NJ. Management of early rectal cancer. Br J Surg. 2008;95(4):409–423. doi: 10.1002/bjs.6127. [DOI] [PubMed] [Google Scholar]
- 9.Kim CK, Kim SH, Chun HK, et al. Preoperative staging of rectal cancer: accuracy of 3-Tesla magnetic resonance imaging. Eur Radiol. 2006;16(5):972–980. doi: 10.1007/s00330-005-0084-2. [DOI] [PubMed] [Google Scholar]
- 10.Merkle EM, Dale BM, Paulson EK. Abdominal MR imaging at 3T. Magn Reson Imaging Clin N Am. 2006;14(1):17–26. doi: 10.1016/j.mric.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Chun HK, Choi D, Kim MJ, et al. Preoperative staging of rectal cancer: comparison of 3-T high-field MRI and endorectal sonography. AJR Am J Roentgenol. 2006;187(6):1557–1562. doi: 10.2214/AJR.05.1234. [DOI] [PubMed] [Google Scholar]
- 12.Futterer JJ, Yakar D, Strijk SP, Barentsz JO. Preoperative 3T MR imaging of rectal cancer: local staging accuracy using a two-dimensional and three-dimensional T2-weighted turbo spin echo sequence. Eur J Radiol. 2008;65(1):66–71. doi: 10.1016/j.ejrad.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Lim JS, Choi JY, et al. Rectal cancer: comparison of accuracy of local-regional staging with two- and three-dimensional preoperative 3-T MR imaging. Radiology. 2010;254(2):485–492. doi: 10.1148/radiol.09090587. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Lee JM, Lee MW, et al. Diagnostic accuracy of 3.0-Tesla rectal magnetic resonance imaging in preoperative local staging of primary rectal cancer. Invest Radiol. 2008;43(8):587–593. doi: 10.1097/RLI.0b013e31817e9083. [DOI] [PubMed] [Google Scholar]
- 15.Winter L, Bruhn H, Langrehr J, et al. Magnetic resonance imaging in suspected rectal cancer: determining tumor localization, stage, and sphincter-saving resectability at 3-Tesla-sustained high resolution. Acta Radiol. 2007;48(4):379–387. doi: 10.1080/02841850701196914. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XM, Zhang HL, Yu D, et al. 3-T MRI of rectal carcinoma: preoperative diagnosis, staging, and planning of sphincter-sparing surgery. AJR Am J Roentgenol. 2008;190(5):1271–1278. doi: 10.2214/AJR.07.2505. [DOI] [PubMed] [Google Scholar]
- 17.Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001;19(7):1976–1984. doi: 10.1200/JCO.2001.19.7.1976. [DOI] [PubMed] [Google Scholar]
- 18.Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumor-free resection margin in rectal cancer surgery. Lancet. 2001;357(9255):497–504. doi: 10.1016/S0140-6736(00)04040-X. [DOI] [PubMed] [Google Scholar]
- 19.Brown G, Radcliffe AG, Newcombe RG, et al. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90(3):355–364. doi: 10.1002/bjs.4034. [DOI] [PubMed] [Google Scholar]
- 20.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumor spread and surgical excision. Lancet. 1986;2(8514):996–999. doi: 10.1016/S0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 22.Kim NK, Kim MJ, Park JK, et al. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol. 2000;7(10):732–737. doi: 10.1007/s10434-000-0732-3. [DOI] [PubMed] [Google Scholar]
- 23.Lahaye M, Beets G, Engelen S, et al. Accuracy of USPIO-enhanced MRI for local staging of rectal cancer: a multicenter study in expert and 3 regional centers. Eur Radiol. 2008;18(suppl 2):B44. [Google Scholar]