Abstract
Practical and efficient methods have been developed for the diversity-oriented synthesis of isoxazolodihydropyridinones via the 1,3-dipolar cycloaddition of nitrile oxides onto 2,4-dioxopiperidines. A select few of these isoxazolodihydropyridinones were further elaborated with triazoles by copper catalyzed azide-alkyne cycloaddition reactions. A total of 70 compounds and intermediates were synthesized and analyzed for drug likeness. Sixty-four of these novel compounds were submitted to the NIH Molecular Libraries Small Molecule Repository for high-throughput biological screening.
Keywords: Isoxazole; triazole; heterocycle; 1,3-dipolar cycloaddition; Cu(I)-catalyzed azide-alkyne cycloaddition
Introduction
Isoxazoles are an important class of nitrogen containing heterocycles found in many natural products and biologically active compounds.1 While many methods have been developed for isoxazole formation, the cycloaddition of nitrile oxides to alkynes is the most effective.2 Emphasis has been placed on the synthesis of highly substituted isoxazoles by Larock,3 Miyata,4 and others.5 While the reactions of nitrile oxides with cyclic 1,3-diketones6 and β-ketoesters7 have been studied, the cycloaddition of nitrile oxides onto heterocyclic 1,3-dicarbonyl compounds has, to our knowledge, not been reported. Although other routes have been reported for the preparation of isoxazolo[4,5-c]pyridinones,8 our method provides convergent access to a variety of diversity points around the core scaffold.
We recently reported the hydrolytic opening of the amide of a isoxazolodihydropyridinone substrate as a route to orthogonally protected heterocyclic diamino acids.9 As a continuation of this line of research, we report here the synthesis of a library of isoxazolodihydropyridinones via the 1,3-dipolar cycloaddition of nitrile oxides onto 2,4-dioxopiperidine to yield eleven novel 6,7-dihydroisoxazolo[4,5-c]2yridine-4(5H)-ones. Noting the many recent synthetic applications of copper catalyzed azide-alkyne cycloadditon (CuAAC) reactions for the rapid diversification of library collections (→ triazole diversification),10 we also report exploiting the CuAAC reaction to diversify a subset of these isoxazolodihydropyridinones leading to an additional 42 triazole-functionalized isoxazolo-dihydropyridinones. The resulting collection has been added to the NIH Molecular Libraries Small Molecule Repository for high-throughput biological screening.
Results and Discussion
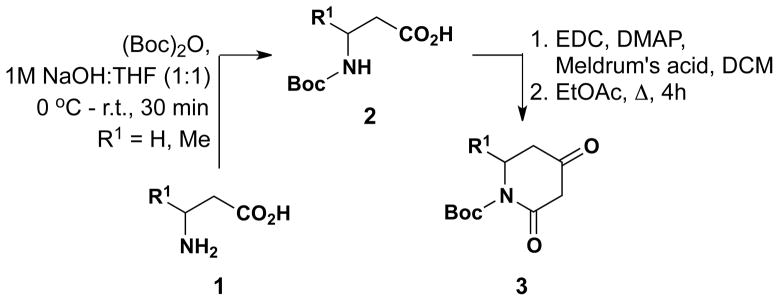
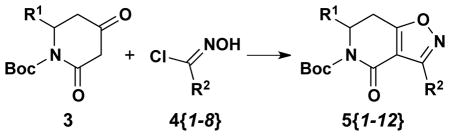
As a starting point, Boc-protected 2,4-dioxopiperidines were synthesized from substituted β-amino acids by a three-step process that commenced with Boc protection of β-alanines (1) following a modified literature procedure (90–95% yield).11 These N-protected amino acids (2) were then coupled to Meldrum’s acid using standard EDC and DMAP coupling conditions9. The resulting conjugates were immediately dissolved in EtOAc and refluxed for four hours to yield Boc-protected dioxopiperidines 3 in 85–90% yield over 2 steps (via the presumed intermediacy of a Meldrum’s acid-derived acylketene, which subsequently underwent intramolecular cyclization with the Boc-protected amine moiety; Scheme 1). These dioxopiperidines were then treated with NaH in THF followed by the slow addition of various N-hydroxybenzimidoyl chlorides 4{1–8} to give isoxazolodihydropyridinones 5{1–11} in 43–76% yield (Table 1). The requisite N-hydroxybenzimidoyl chlorides 4{1–8} were prepared from the corresponding benzaldehydes in 2 steps following well-established literature procedures.12
Scheme 1.
Synthesis of 2,4-dioxopiperidines 3 (R1 = H and Me).
Table 1.
1,3-Dipolar cycloaddition of nitrile oxides onto 2,4-dioxopiperidines.
 | ||||
|---|---|---|---|---|
| Entry | Compound | R1 | R2 | Yield |
| 1 | 5{1} | H | 2-MeOC6H4 | 52 |
| 2 | 5{2} | H | 4-BrC6H4 | 64 |
| 3 | 5{3} | H | 2-FC6H4 | 58 |
| 4 | 5{4} | H | 4-NO2C6H4 | 54 |
| 5 | 5{5} | H | 3-NO2C6H4 | 50 |
| 6 | 5{6} | H | 2-NO2C6H4 | 68 |
| 7 | 5{7} | H | 2-NO2,5-ClC6H3 | 50 |
| 8 | 5{8} | H | 4-ClC6H4 | 55 |
| 9 | 5{9} | Me | 2-MeOC6H4 | 76 |
| 10 | 5{10} | Me | 2-NO2,5-ClC6H3 | 43 |
| 11 | 5{11} | Me | 4-ClC6H4 | 64 |
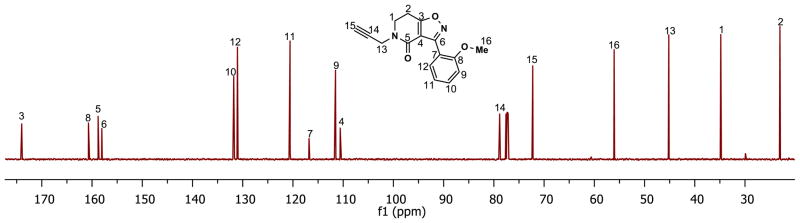
A detailed NMR study of 5{3} was undertaken to verify its structure and completely assign all carbons and protons within the molecule. While many of the assignments could be made from the 1H and 13C NMR spectra, we were unable to assign carbons C3, C4, C5 and C6 (see Figure 1) from these spectra alone. Therefore, several additional NMR experiments were undertaken, including INADEQUATE,13 HSQC,14 HMBC,15 and COSY.16 From the two-dimensional INADEQUATE experiment on 5{3} (Figure 1), we could assign all of its carbons, including C3, C4, C5 and C6. By analogy, the insights gained through this INADEQUATE proved useful in making complete carbon assignments in all of the isoxazolodihydropyridinones reported here; for example, we were able to completely assign carbons for alkyne derivative 6{1} (Figure 2). All of the spectra obtained for 5{3} are available in the Supporting Information.
Figure 1.
INADEQUATE-based assignments of C3, C4, C5 and C6 in 5{3}.
Figure 2.
Assignments for 6{1} based on the INADEQUATE data from 5{3}.
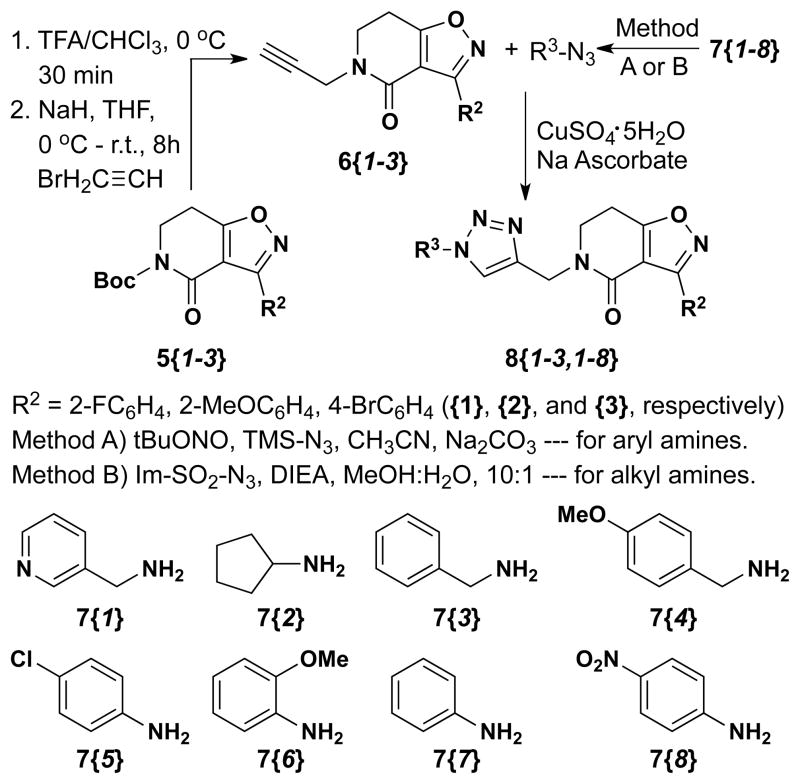
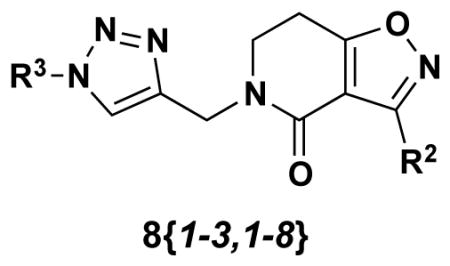
Three of the isoxazolodihydropyridinones delineated in Table 1 (5{1–3}) were selected for diversification through CuAAC reactions by first installing an alkyne moiety on the dihydropyridinone nitrogen. As outlined in Scheme 2, this chemistry commenced by first removing the Boc group with TFA in CHCl3 (30 min) to yield the secondary amide in quantitative yield. Each amide was then deprotonated with NaH in dry THF and N-propargylated with propargyl bromide to yield the alkyne- containing scaffold 6{1–3) in 50–55% yield. Subsequent copper-mediated 1,3-dipolar cycloaddition to these substrates with eight azides (7{1–8}) delivered triazole products 8{1–3, 1–8} in good to excellent yields (Table 2). The azides employed in these CuACC reactions were generated in situ from the corresponding amines following one of two protocols: (i) alkyl 1°-amines were treated with the diazo-transfer reagent imidazole-SO2N3 to yield the azide as reported by Stick17 and (ii) aryl 1°-amines were treated with t-BuONO and subsequent ArN2+ → ArN3 conversion was effected with TMS-N3 as reported by Moses.18 These in situ prepared azides, the alkynes (6{1–3}), CuSO4, and sodium ascorbate were combined and the ensuing 1,3-dipolar cycloaddition reactions yielded triazole-substituted products 8{1–3,1–8} (Table 2).
Scheme 2.
CuAAC of the isoxazolodihydropyridinones.
Table 2.
CuAAC diversification of isoxazolodihydropyridinones.
 | ||||
|---|---|---|---|---|
| Entry | Azide | 8{1,1–8} % yield | 8{2,1–8} % yield | 8{3,1–8} % yield |
| 1 | {1} | 28 | 85 | 89 |
| 2 | {2} | 571 | 95 | 92 |
| 3 | {3} | 52 | 76 | 79 |
| 4 | {4} | 46 | 92 | 88 |
| 5 | {5} | 76 | 74 | 76 |
| 6 | {6} | 78 | 74 | 94 |
| 7 | {7} | 84 | 79 | 77 |
| 8 | {8} | 70 | 89 | 92 |
Decomposed upon isolation.
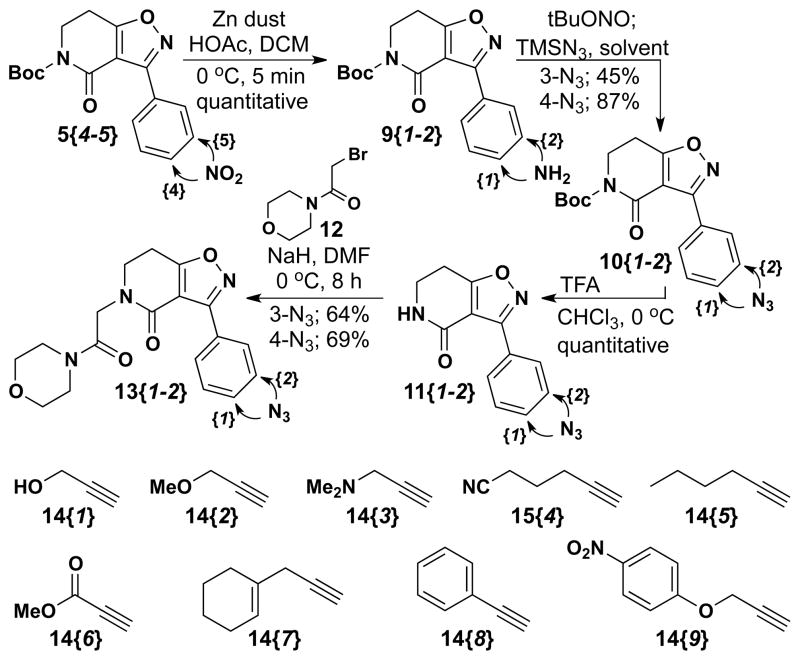
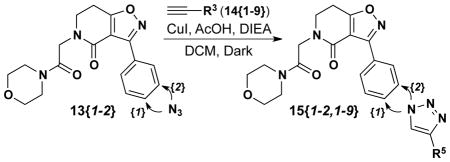
Two additional isoxazolodihydropyridinones delineated in Table 1 (5{4–5}) were selected for diversification through CuAAC reactions by taking advantage of the nitro group for azide entry. Isoxolopyridinones 5{4–5} were treated with Zn/AcOH to deliver the m- and p-anilines 9{1–2} in quantitative yields (Scheme 3). These anilines were then treated with tBuONO and the targeted azides were obtained by displacement of the diazonium salt with TMS-N3 to yield 10{1–2} in 45% and 87% yield (meta and para), respectively. The Boc group in 10{1–2} was then removed by TFA treatment in CHCl3 to give 11{1–2} in quantitative yield. At this juncture, N-alkylation of the 2°-amide in 11{1–2} proceeded smoothly by deprotonation with NaH in dry THF and subsequent treatment with 2-bromo-1-morpholinoethanone (12) to give 13{1–2} in 64% and 69% yield, respectively. 2-Bromo-1- morpholinoethanone 12 was prepared by dropwise addition of morpholine to a solution of bromoacetyl bromide in dry DCM under nitrogen (clear oil; 82% yield).19 Nine alkynes (Scheme 3) were then used to diversify this azide set to give 16 additional triazole products (15{1–2, 1–9}; Table 3).
Scheme 3.
Azide synthesis for CuAAC.
Table 3.
Diversification of the alkylated isoxazolodihydropyridinones.
 | |||
|---|---|---|---|
| Entry | Alkyne | m-N3 yield | p-N3 yield |
| 1 | {1} | 28 | 79 |
| 2 | {2} | 69 | 47 |
| 3 | {3} | 51 | 99 |
| 4 | {4} | 89 | 99 |
| 5 | {5} | 36 | 32 |
| 6 | {6} | 57 | 93 |
| 7 | {7} | 68 | 99 |
| 8 | {8} | 47 | 65 |
| 9 | {9} | 85 | 62 |
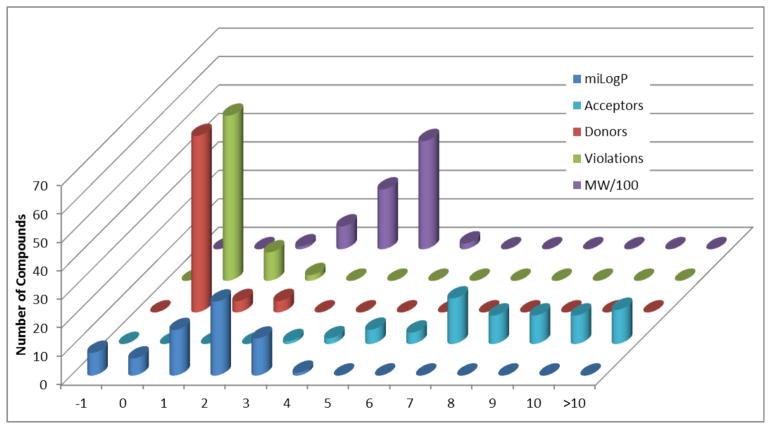
A Lipinski rule-of-five analysis20 was performed to evaluate the drug likeness of all compounds and intermediates reported (Figure 3). Of the seventy compounds analyzed, fifty-eight were within the parameters set by Lipinski. Of the remaining, nine from chemset 15 had >10 hydrogen bond acceptors and the remaining two compounds (also from chemset 15) had two violations each (>10 acceptors and a mass >500 Daltons; both had molecular weights of ~560 g/mol). The other chemsets combined had only one violation (e.g., 8{2,8}), suggesting that this collection of compounds is very appropriate for high-throughput screening to discover drug leads and biological probes.
Figure 3.
Lipinski rules analysis for 70 compounds and intermediates; calculated using Molinspirations Online Molecular Properties Calculator [http://www.molinspiration.com/cgi-bin/properties?textMode=1].
Conclusions
A short, reliable, and efficient convergent method has been developed to produce a diverse array of novel isoxazolodihydropyridinones starting from commercially available beta-amino acids and benzaldehydes. Eleven isoxazolpyridinones were synthesized and five of these were diversified further with CuAAC chemistry to give 42 additional isoxazolodihydropyridinone-triazole products. In all, 64 of the 70 compounds and intermediates synthesized were acceptable for submission to the NIH Molecular Libraries Small Molecule Repository for high-throughput biological screening; the unsubmitted 6 compounds contained excluded aromatic azides.
Supplementary Material
Acknowledgments
The authors thank the National Institutes of Health (GM0891583 and RR1973) and the National Science Foundation [CHE-0910870; and CHE-0443516, CHE-0449845, CHE-9808183, and DBIO 722538 for NMR spectrometers] for their generous support.
Footnotes
Supporting Information Available.
Detailed experimental procedures, characterization data, and 1H and 13C NMR spectra of all compounds, including COSY, INADEQUATE, HSQC, and HMBC for compound 5{3}. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Jawalekar AM, Reubsaet E, Rutjes FPJT, van Delft FL. Synthesis of Isoxazoles by Hypervalent Iodine-Induced Cycloaddition of Nitrile Oxides to Alkynes. Chem Comm. 2011;47:3198–3200. doi: 10.1039/c0cc04646a. [DOI] [PubMed] [Google Scholar]; (b) Sperry J, Wright D. Furans, Thiophenes and Related Heterocycles in Drug Discovery. Curr Opin Drug Discovery Develop. 2005;8:723–740. [PubMed] [Google Scholar]; (c) Shin KD, Lee MY, Shin DS, Lee S, Son KH, Koh S, Paik YK, Kwon BM, Han DC. Blocking Tumor Cell Migration and Invasion with Biphenyl Isoxazole Derivative KRIBB3, a Synthetic Molecule That Inhibits Hsp27 Phosphorylation. J Biol Chem. 2005;50:41439–41448. doi: 10.1074/jbc.M507209200. [DOI] [PubMed] [Google Scholar]; (d) Rozman B, Praprotnik S, Logar D, Tomsic M, Hijnik M, Kos-Golja M, Dolenc P. Leflunomide and Hypertension. Ann Rheum Dis. 2002;61:567–569. doi: 10.1136/ard.61.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lee YS, Park SM, Kim BH. Synthesis of 5-Isoxazol-5-yl-2′-deoxyuridines Exhibiting Antiviral Activity Against HSV and Several RNA Viruses. Med Chem Lett. 2009;19:1126–1128. doi: 10.1016/j.bmcl.2008.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lawrence SL, Roth V, Slinger R, Toye B, Gaboury I, Lemyre B. Cloxacillin Versus Vancomycin for Presumed Late-Onset Sepsis in the Neonatal Intensive Care Unit and the Impact Upon Outcome of Coagulase Negative Staphylococcal Bacteremia: a Retrospective Cohort Study. BMC Pediatrics. 2005;5:49. doi: 10.1186/1471-2431-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For review of isoxazole synthesis see: Margaretha P. Synthesis of Isoxazoles. Sci of Syn. 2010;1:109–131.Pinho e Melo TMVD. Recent Advances on the Synthesis and Reactivity of Isoxazoles. Curr Org Chem. 2005;9:925–958.Wakefield BJ. Isoxazoles. Sci of Syn. 2001;11:229–288.. Other sources: Huisgen R. 1,3-Dipolar Cycloaddition – Introduction, Survey, Mechanism. In: Padwa A, editor. 1,3-Dipolar Cycloaddition Chemistry. Vol. 1. Wiley; NewYork: 1984. pp. 1–176.Jaeger V, Colinas PA. Nitrile Oxides. In: Padwa A, editor. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Towards Heterocycles and Natural Products. Vol. 59. Wiley; Hoboken: 2002. pp. 361–472.Mukaiyama T, Hoshino T. The Reactions of Primary Nitroparaffins with Isocyanates. J Am Chem Soc. 1960;82:5339–5342.
- 3.Waldo JP, Mehta S, Neuenswander B, Lushington GH, Larock RC. Solution Phase Synthesis of a Diverse Library of Highly Substituted Isoxazoles. J Comb Chem. 2008;10:658–663. doi: 10.1021/cc800055x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueda M, Sato A, Ikeda Y, Miyoshi T, Naito T, Miyata S. Direct Synthesis of Trisubstituted Isoxazoles Through Gold-Catalyzed Domino Reaction of Alkynyl Oxime Ethers. Org Lett. 2010;12:2594–2597. doi: 10.1021/ol100803e. [DOI] [PubMed] [Google Scholar]
- 5.Tang S, He J, Sun Y, He L, She X. Efficient and Regioselective One-Pot Synthesis of 3-Substituted and 3,5-Disubstituted Isoxazoles. Org Lett. 2009;11:3982–3985. doi: 10.1021/ol901626n. [DOI] [PubMed] [Google Scholar]
- 6.(a) Kaminski, Jerzy, Eckstein Zygmunt. 1,3-Dipolar Cycloaddition of some Diphenylacethydroximic Acid Chlorides. Pol J Chem. 1982;56:221–228. [Google Scholar]; (b) Bode JW, Hachisu Y, Matsuura T, Suzuki K. Facile Construction and Divergent Transformations of Polycyclic Isoxazole: Direct Access to Polyketide Architectures. Org Lett. 2003;5:391–394. doi: 10.1021/ol027283f. [DOI] [PubMed] [Google Scholar]; (c) El-Badri MH, Kurth MJ. Synthesis of Thiazolo- and 7,8-Dihydrothiazolo[4,5-e]benzoisoxazoles. J Comb Chem. 2009;11:228–238. doi: 10.1021/cc800141s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones RCF, Bhalay G, Carter PA, Duller KAM, Dunn SH. 1,3-Dipolar Cycloaddition Route to Nitrogen Heterocyclic Triones. J Chem Soc, Perkins Trans. 1999;1:765–776. [Google Scholar]
- 8.(a) Jones RCF, Bhalay G, Carter PA, Duller KAM, Vulto SIEA. 1,3-Dipolar Cycloaddition Route to 3-Acyl-4-hydroxy-pyridin-2-ones and –pyran-2-ones. Synlett. 1995:149–150. [Google Scholar]; (b) Weis R, Schweiger K, Fabian WMF. Synthesis of Isomeric Isoxazolopyridinones. Monatsh Chem. 1998;129:1285–1292. [Google Scholar]
- 9.Butler JD, Coffman KC, Ziebart KT, Toney MD, Kurth MJ. Orthogonally Protected Thiazole and Isoxazole Diamino Acids: An Efficient Synthetic Route. Chem Eur J. 2010;16:9002–9005. doi: 10.1002/chem.201001492. [DOI] [PubMed] [Google Scholar]
- 10.For recent reviews of CuAAC chemistry see; Meldal M, Tornoe CW. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479.Hein JE, Fokin VV. Copper-Catalyzed Azide-Alkyne Cycloaddition and Beyond: New Reactivity of Copper(I) Acetylides. Chem Soc Rev. 2010;39:1302–1315. doi: 10.1039/b904091a.
- 11.Guha S, Drew MGB, Banerjee A. A New Molecular Scaffold for the Formation of Supramolecular Peptide Double Helices: The Crystallographic Insight. Org Lett. 2007;9:1347–1350. doi: 10.1021/ol0701870. [DOI] [PubMed] [Google Scholar]
- 12.Dixon SM, Milinkevich KA, Fujii J, Liu R, Yao N, Lam KS, Kurth MJ. A Spiroisoxazolinoproline-Based Amino Acid Scaffold for Solid Phase and One-Bead-One-Compound Library Synthesis. J Comb Chem. 2007;9:143–157. doi: 10.1021/cc060090p. [DOI] [PubMed] [Google Scholar]
- 13.Bourdonneau M, Ancian B. Rapid-Pulsing Artifact-Free Double-Quantum-Filtered Homonuclear Spectroscopy. J Magn Reson. 1998;132:316–327. doi: 10.1006/jmre.1998.1392.(b) Our significant parameters: Acquisition time 98msec, sw = 200ppm, carrier = 100ppm, recycle rate = 11.1 sec, total time = 63 hours.
- 14.(a) Palmer AG, III, Cavanagh J, Wright PE, Rance M. Sensitivity Improvement in Proton-Detected Two-Dimensional Heteronuclear Correlation NMR Spectroscopy. J Magn Reson. 1991;93:151–170. [Google Scholar]; (b) Kay LE, Keifer P, Saarinen T. Pure Absorption Gradient Enhanced Heteronuclear Single Quantum Correlation Spectroscopy with Improved Sensitivity. J Am Chem Soc. 1992;114:10663–10665. [Google Scholar]; (c) Schleucher J, Schwendinger M, Sattler M, Schmidt P, Schedletzsky O, Glaser SJ, Sorensen OW, Griesinger C. A General Enhancement Scheme in Heteronuclear Multidimensional NMR Employing Pulsed-Field Gradients. J Biomol NMR. 1994;4:301–306. doi: 10.1007/BF00175254. [DOI] [PubMed] [Google Scholar]
- 15.(a) Bax A, Summers MF. Complete 1H and 13C Assignments of Coenzyme B12 through the Use of New Two-Dimensional NMR Experiments. J Am Chem Soc. 1998;108:2093–2094. [Google Scholar]; (b) Wagner R, Berger S. ACCORD-HMBC: A Superior Technique for Structural Elucidation. Magn Reson Chem. 1998;36:S44–S46. [Google Scholar]
- 16.(a) Shaw AA, Salaun C, Dauphin J-F, Ancian B. Artifact-Free PFG-Enhanced Double-Quantum-Filtered COSY Experiments. J Magn Reson. 1996;A 120:110–115. [Google Scholar]; (b) Ancian B, Borgeois I, Dauphin J-F, Shaw AA. Artifact-Free Pure Absorption PFG-Enhanced DQF-COSY Spectra Including a Gradient Pulse in the Evolution Period. J Magn Reson. 1997;A 125:348–354. [Google Scholar]
- 17.Goddard-Borger ED, Stick RV. An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-sulfonyl Azide Hydrochloride. Org Lett. 2007;9:3797–3800. doi: 10.1021/ol701581g. [DOI] [PubMed] [Google Scholar]
- 18.Barral K, Moorhouse AD, Moses JE. Efficient Conversion of Aromatic Amines into Azides: A One-Pot Synthesis of Triazole Linkages. Org Lett. 2007;9:1809–1811. doi: 10.1021/ol070527h. [DOI] [PubMed] [Google Scholar]
- 19.Conrad WE, Fukazawa R, Haddadin MJ, Kurth MJ. The Davis-Beirut Reaction: N1, N2-Disubstituted-1H-Indazolones via 1,6-Electrophilic Addition to 3-Alkoxy-2H-Indazoles. Org Lett. 2011;13:3138–3141. doi: 10.1021/ol2010424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv Drug Delivery Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.