Abstract
Purpose
To describe a video-documented assessment of cataract type in eyes with monocular infantile cataract enrolled in the infant aphakia treatment study (IATS).
Methods
IATS is a randomized clinical trial comparing intraocular lens versus contact lens correction in 114 infants, aged 28 days to <7 months. A total of 83 videos were available for morphological analysis of cataract. Three examiners reviewed all surgical recordings and agreed on the cataract characteristics using a score sheet to record the lens layer or configuration of the opacity.
Results
Nuclear cataract was present in 45 of 83 eyes (54%). Posterior capsule plaque was seen in 73 eyes (88%). All eyes with fetal nuclear cataract had associated posterior capsule plaque. Cortical cataract without nuclear involvement was seen in 21 eyes (25%). Posterior bowing of the posterior capsule was noted in 4 eyes (5%). Evidence of persistent fetal vasculature (PFV) was present in 18 eyes (22%). PFV was the only finding in 5 eyes but was also seen in combination with nuclear (7 eyes) and cortical cataracts (6 eyes). The entire lens was white in 3 eyes (4%), while the lens was partially resorbed in 7 (8%) eyes. Anterior capsule fibrosis was noted in 5 eyes with advanced cataract (1 with total cataract, 4 with partially resorbed lens).
Conclusions
Nuclear opacities were common, but many different cataract types presented in infancy. PFV occurred in isolation or in association with cataract. Posterior capsule plaque was frequently noted, especially when a nuclear cataract was present.
Introduction
Lens opacities in infancy have a wide spectrum of presentations. The nucleus can be the primary structure affected in some eyes. In others the fetal nucleus remains clear but the cortex is involved, displaying different patterns of opacities. Abnormalities in or near the posterior capsule are common in infants with cataract. The visual prognosis may vary according to the morphological type.1,2 The Infant Aphakia Treatment Study (IATS) is a randomized multicenter clinical trial to compare intraocular lens (IOL) with contact lens correction in infants with a monocular cataract who have undergone surgery at 28 days to <7 months of age.3,4 Surgeries throughout the recruitment period were videotaped for verification of protocol adherence. The purpose of our current study is to describe a video-documented assessment of cataract type in eyes with monocular infantile cataract enrolled in the IATS.
Methods
The IATS is a multicenter, randomized, controlled clinical trial comparing IOL and contact lens treatments after cataract surgery performed in children with a unilateral congenital cataract at 1 to 6 months of age. This study was approved by the institutional review boards of all the participating institutions and was in compliance with the Health Insurance Portability and Accountability Act. The off-label research use of the AcrySof SN60AT and MA60AC IOLs (Alcon Laboratories, Fort Worth, TX) was covered by US Food and Drug Administration investigational device exemption G020021.
Of 227 patients screened in the IATS, 113 patients were excluded (81 patients were ineligible patients, 28 eligible patients declined to participate, 4 patients were excluded for other reasons). The remaining 114 were randomized. The process of submitting surgical videos for central review by the steering committee for protocol compliance was initiated after 23 patients (20%) had been enrolled. Videos were submitted for 83 of the remaining 91 patients. After all 114 patients had been enrolled and the review process was completed, videos were found for 6 additional patients, for a total of 89 patients with videos available. For 2 patients there were technical problems with the recordings, and for 4 patients the videos were of insufficient quality to determine the cataract morphology. For the remaining 83 patients, the videos were reviewed and the features of the cataract identified.
Three of the authors (MEW, RHT, DGM) reviewed all surgical recordings and documented cataract characteristics using a standardized score sheet (Table 1) to record the lens layer or configuration of the opacity. For each video, reviewers checked all characteristics that applied to the cataract being classified. For score sheets where reviewers did not agree on morphological characteristics, the three reviewers reevaluated videos simultaneously to reach a consensus. Table 1 summarizes the definitions of each characteristic used in the scoring. All applicable diagnostic characteristics were marked for each video viewed. After tabulation, we attempted to fit each eye into the following major cataract categories: nuclear, cortical, persistent fetal vasculature (PFV), posterior lentiglobus, isolated posterior capsule plaque, and total cataract. These categories are defined in Table 2.
Table 1.
Definition and occurrence of individual cataract diagnosis categories among 83 patients
| Diagnostic categories | Definition | N (% of 83 patients) | |
|---|---|---|---|
| 1 | Entire lens white | A uniform total white color lens with no red reflex visible even in the periphery of the lens | 3 (4%) |
| 2 | Lens partially resorbed | An appearance of reduced central lens thickness leaving little if any cortex between the central anterior and posterior capsule | 7 (8%) |
| 3 | Anterior capsular fibrosis | Presence of a dense, white fibrous- appearing opacity adherent to the anterior capsule | 5 (6%) |
| 4 | Anterior and/or posterior cortical opacity not involving nucleus | Opacity located within any of the cortex peripheral to the Y-sutures with clear nuclear sparing | 21 (25%) |
| 5 | Opacity between Y-sutures (fetal nucleus) | Opaque lens material located between the anterior and posterior Y-sutures | 45 (54%) |
| 6 | Posterior bowing of posterior lens capsule | Clear video evidence of bowing, such as posterior capsular defects associated with a lentiglobus lens | 4 (5%) |
| 7 | Opacity of posterior lens capsule | Presence of opacity of the posterior capsule after the entire cortex had been surgically aspirated | 73 (88%) |
| 8 | Retrolental membrane with or without visible vessels | An opacity or vascular network on the posterior aspect of the lens that was associated with a fetal remnant and appeared to be distinct from a standard posterior capsular plaque | 12 (14.5%) |
| 9 | Patent persistent hyaloid vessel | Appearance of blood within the persistent hyaloid vessel | 9 (11%) |
| 10 | Nonpatent persistent hyaloid vessel | Absence of visible blood within the persistent hyaloid vessel | 2 (2%) |
| 11 | Ciliary processes stretched | Visible stretched ciliary process | 2 (2%) |
Table 2.
Morphological classification of infantile cataract
| Classification | |
|---|---|
| Fetal nuclear | Opaque lens material between anterior and posterior Y-suture that may spread into the surrounding (especially posterior) cortex and often associated with posterior capsule plaque |
| Cortical | Anterior and/or posterior cortical opacity not involving the fetal nucleus and often associated with posterior capsule plaque |
| Persistent fetal vasculature | A combination of one or more of the following: retrolental membrane with or without visible vessels, patent or nonpatent persistent hyaloid vessel, or stretched ciliary processes |
| Isolated posterior capsule plaque (posterior polar) | Opacity of the posterior capsule without overlying opacity in the cortex or nucleus |
| Posterior lentiglobus | Posterior bowing of the posterior capsule with or without a preexisting posterior capsule defect |
| Total | Entire lens white |
Results
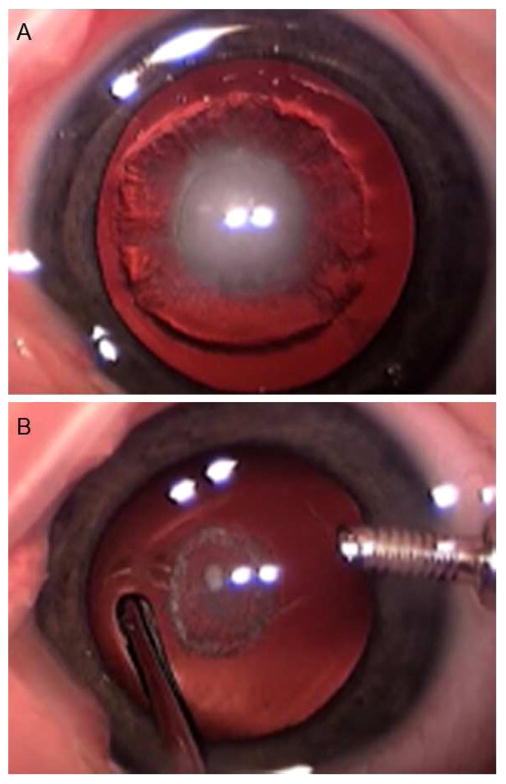
Nuclear cataract was present in 45 of 83 eyes (54%). Of those, nuclear cataract was associated with extension into the surrounding lens cortex (Figure 1A) in 41. All (45/45) nuclear cataracts had an associated posterior capsule plaque (Figure 1B). In 4 eyes with nuclear cataract, an isolated persistent hyaloid stalk was seen without a retrolental membrane or stretched ciliary processes. In another 3 eyes, a retrolental membrane was present with 1 eye also having stretched ciliary processes.
FIG 1.
A, Fetal nuclear cataract with spread into the surrounding cortex. B, Posterior capsule plaque visible after the cataract was aspirated.
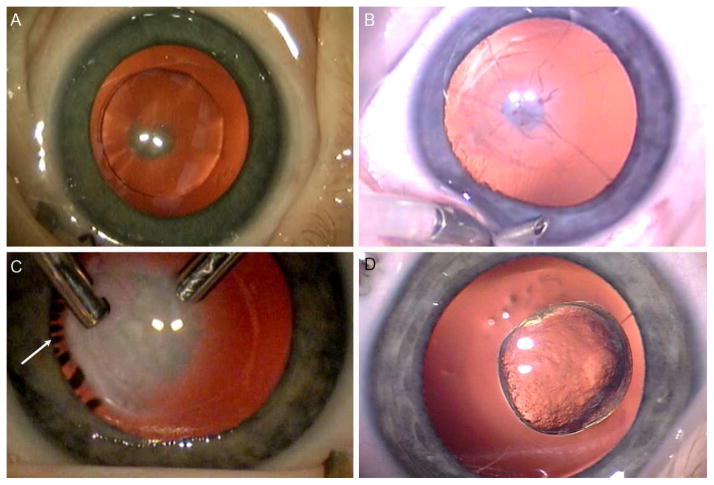
Cortical cataract without nuclear involvement was seen in 21 of 83 eyes (25%). Of these, 16 eyes also had a posterior capsule plaque (Figure 2A). In 6 eyes, evidence of PFV was also present (4 eyes with a retrolental membrane and 2 eyes with an isolated persistent hyaloid stalk).
FIG 2.
A, Cortical cataract with small central posterior plaque. B, Persistent fetal vasculature: mild involvement with a small plaque, a few vessels and a nonpatent hyaloid remnant. C, Persistent fetal vasculature: dense retrolenticular plaque with stretched ciliary processes (arrow) that became visible after cataract aspiration. D, Posterior lentiglobus.
Evidence of PFV (retrolental membrane [Figure 2B] or persistent hyaloid stalk [Figure 2C]) was present in 18 of 83 eyes (22%). As noted above, 7 eyes had nuclear cataract and 6 eyes had cortical cataract. In 5 additional eyes, a retrolental membrane was present without any nuclear or cortical cataract. One of those eyes also had a posterior capsule plaque and one was noted to have anterior capsule fibrosis with partially resorbed lens cortex.
Posterior lentiglobus was diagnosed when bowing of the posterior capsule was noted (Figure 2D). This finding was present in 4 of 83 eyes (5%). Of those, cortical cataract was present in 3 eyes; the remaining eye had PFV as well as a bowing of the posterior capsule.
An isolated posterior capsule plaque was present in 6 of 83 eyes (7%). In these eyes, no nuclear, cortical, PFV, or lentiglobus characteristics could be seen. The entire lens was white (Figure 3A) in 3 of 83 eyes (4%), while the lens was partially resorbed in 7 of 83 eyes (8%). Anterior capsule fibrosis (Figure 3B) was noted in 5 eyes with advanced cataract (1 with total cataract, 4 with partially resorbed lens).
FIG 3.
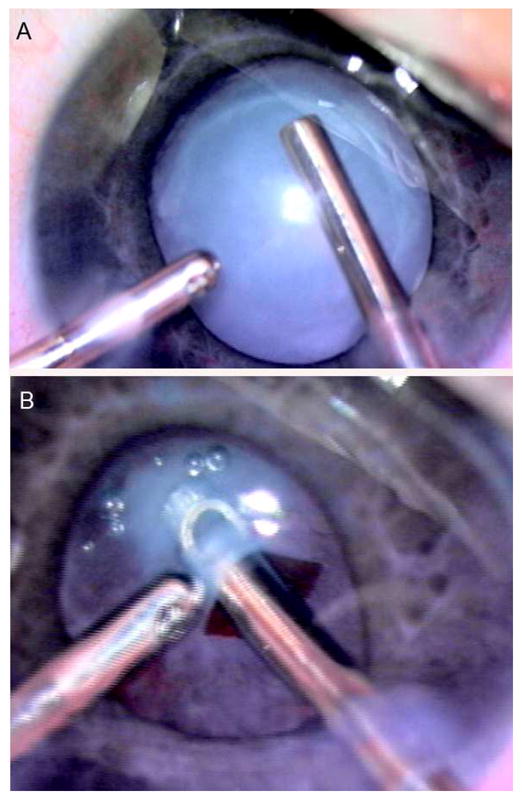
A, Complete cataract. B, Anterior capsule fibrosis (same eye as 3A).
The most common single finding in this series was opacity of the posterior capsule, which was found in 73 of 83 eyes (88%) (Table 1).
Discussion
The morphology of a cataract is largely determined by the anatomy of the lens and the timing and nature of the insult that caused the abnormality. It is common to classify childhood cataracts based on the appearance of the lens at the time of clinical presentation using biomicroscopy or retinoscopy or during a preoperative examination under anesthesia using the operating microscope.1,5 This approach is likely to miss lens-layer involvement that can only be seen during the active aspiration of the lens. Posterior capsule plaque, for instance, is not often visible until after the overlying opacified nucleus is removed. Persistent hyaloid artery remnants may become visible only after removal of the entire lens. Although not truly three-dimensional, video evaluation allows a visualization of lens involvement as each layer is removed during the surgical procedure. This video-documented assessment of cataract type in infancy demonstrates a higher-than-previously-realized rate of posterior capsule involvement in monocular congenital cataracts.
Forster and colleagues6 collected data from the infantile cataract literature and reported the prevalence of nuclear involvement as 10%. Haargaard and colleagues7 reviewed medical records of children 0–17 years of age and reported that in eyes with unilateral cataract, nuclear cataract accounted for 34% of all cases and was the most common morphology in all major etiological groups. In our series, nuclear cataract was present in 54% of eyes and associated with a posterior capsule plaque in all cases. This relatively higher proportion of nuclear cataract can be explained by the fact that our cohort included infants up to only 7 months of age and with monocular cataract only. In all but 4 eyes, nuclear cataracts also had an opacity extending into the surrounding cortex. Thus nuclear cataract with cortical spread and posterior capsule plaque as a combination was noted in 49% of eyes.
For an isolated layer of the lens, posterior capsule plaque was the most common finding in our study. Posterior capsule plaque is described as a dense, white opacity adherent to the internal surface of the posterior lens capsule. These are often referred to as posterior polar opacities when they are confined to the center of the posterior capsule. Jain and colleagues8 reported a combination of nuclear cataract with posterior polar opacities as the most frequent combination found in a study of 146 eyes examined in northern India. In our series, posterior capsule plaque was seen in 88% of eyes. Praveen and colleagues9 reported posterior capsule plaque in only 90 of 670 eyes (13%); however that study included patients with unilateral or bilateral cataracts up to 15 years of age. In our cohort, all eyes with nuclear cataract and more than three-quarters (16 of 21) of those with cortical cataracts had associated posterior capsule plaque. Isolated posterior capsule plaque with no other opacity was present in 6 of 83 of the eyes. We avoided the term lamellar when describing cortical cataracts in this study, which most often refers to a specific type of developmental cortical cataract that is most often bilateral, is slowly progressive over the course of years, and is not associated with posterior capsule plaque.
PFV, as defined in this study, was seen in 22% of eyes. Of the 81 patients found to be ineligible for IATS, 29 patients (36%) were excluded due to severe PFV (stretched ciliary process or posterior involvement). Thus the prevalence of PFV in all eyes with monocular infantile cataract is higher than we found in the IATS-eligible study group. Haargaard and colleagues7 reviewed the medical records of infants 0–17 years of age and reported that in eyes with unilateral cataract, PFV was noted in 56 of 99 eyes (57%). Forster and colleagues6 collected data from the literature and reported the prevalence of PFV as 20%.
Using a broader definition of PFV, Mullner-Eidenbock and colleagues10 found that 100% of 31 eyes with congenital unilateral cataracts showed signs of PFV. These authors prospectively searched for evidence of PFV during surgery using high magnification at the operating microscope. They searched specifically for vascular remnants in the area of the posterior capsule/anterior hyaloid face during and after lens aspiration. In addition to the features of PFV that we identified in the current study, they identified findings they considered to be minimal fetal vascular remnants. These included nonperfused spidery ghost vessels within a posterior capsule plaque, an abnormally thickened hyaloid face, or a membranelike structure continuous with the posterior capsular lens opacity. They hypothesize that all unilateral posterior lens opacities in young children are caused by and are a variant of PFV. Using this definition of PFV, a much higher percentage of the patients in our series would have been considered to have PFV.
We noted in our video reviews that the fetal nuclear opacities often appeared to spread toward the posterior capsule into the more posterior layers of the lens cortex with relative sparing of the anterior cortical layers. There was also often a more opaque cataract in the posterior aspect of the nucleus as compared to the anterior portion. All fetal nuclear cataracts in the current study had an associated posterior capsule plaque. Most (84%) did not have persistent hyaloid vessels or a vascularized retrolental membrane. Four additional eyes had only an isolated hyaloid stalk without any retrolenticular membrane or stretching of the ciliary processes. In these eyes, the isolated hyaloid stalk did not appear to contribute to the vision loss produced by the opacity of the lens nucleus. In 3 eyes with nuclear cataract, a vascularized retrolenticular membrane was also present. It could be argued that these eyes should be classified as PFV and not as a nuclear cataract. Since we do not have any evidence of causality of the nuclear cataract by the PFV, we counted these eyes in both categories; however, Mullner-Eidenbock and colleagues10 asserted that classical evidence of retrolenticular membranes or hyaloid remnants may not be present in all forms of PFV cataracts. These authors argue that fetal vessels have invaded the posterior lens capsule and anterior hyaloid face resulting in an abnormally strong attachment site between the posterior capsule, cortex, and nucleus prior to dissipating. Thus in infants with unilateral cataracts, they contend that the posterior capsule plaques and the associated opacities in the nucleus and cortex may occur as a result of a mild form of PFV. The finding of an unexpectedly high incidence of posterior capsule plaque in our series of unilateral congenital cataracts supports this theory.
In the current series, the lens was partially resorbed in 7 eyes (8%). Four of these had a fibrotic appearing central anterior capsule plaque and all had posterior capsule plaque. This fibrotic plaque of the anterior capsule was noted in a total of 5 eyes, 4 with partially resorbed lens and one with total cataract. The association of an anterior capsule plaque with total cataract has been reported.11
We found a total cataract in only 4% of eyes. Forster and colleagues6 collected data from 3 studies and calculated a similar prevalence of total cataract at 3%. Nontraumatic total cataracts are not very common in industrialized countries. In an additional published series of pediatric cataracts from the United States, only 4 of 199 cases (2%) were classified as total12; however, in the developing countries, total cataracts are more common. Johar and colleagues13 noted that total cataract was present in 39 of 85 of infant eyes (46%) presenting with cataract in western India. In a series from Nepal, 1804 of 2633 of the lens opacities (68%) were described as total cataract.14
Posterior bowing of the posterior capsule was observed in 5% of eyes. Forster and colleagues6 collected data from the literature and reported a prevalence of posterior lenticonus/lentiglobus as 10%. Haargaard and colleagues7 reviewed medical records of children from 0 to 17 years of age and reported that in eyes with unilateral cataract, posterior lenticonus was noted in 7 of 99 of eyes (7%).
In Table 2 we have proposed a classification for unilateral infantile cataracts. A few of the categories are mutually exclusive. For instance, nuclear cataract and cortical cataract, based on the definitions we propose, are mutually exclusive; however, other categories can occur in combination. PFV, as discussed above, was often found along with a nuclear or cortical lens opacity. Posterior capsule plaque, while found in isolation in a 7% of eyes, was usually seen in combination with nuclear or cortical cataract. In this series, it is noteworthy that every nuclear cataract had posterior capsule involvement yet only a few had a persistent hyaloid stalk. It should be noted that we analyzed only unilateral opacities so these data do not necessarily predict what would be found in eyes with bilateral involvement.
Questions remain when categorizing infantile cataracts. Should a dense nuclear opacity with an isolated, bloodless, hyaloid stalk be classified as a nuclear cataract or as a PFV? If an eye with a vascularized retrolental membrane shows a central posterior capsule bulge, does the eye have a posterior lentiglobus or merely another of the many manifestations of PFV? In the eyes we analyzed, one component of the cataract was sometimes obviously responsible for most of the visual acuity reduction in that eye. Other findings, while present, were less important to the visual status of the eye. However, in some eyes, the white posterior cortical opacity and the opaque posterior capsule plaque gave the initial impression that the entire fetal nucleus was involved. When we carefully watched the surgical videos, the cataract was located in the posterior portion of the fetal nucleus and the cortex between the posterior Y-suture and the opaque posterior capsule. Sometimes a video was studied several times before all reviewers could agree as to whether the opacity was located between the Y-sutures or entirely posterior to the nucleus. We believe that unedited surgical video is an excellent way to sort out these details. It was also very helpful to have three graders simultaneously reanalyze any cases where there was disagreement on the original score sheets. The consistent finding of posterior capsule plaque in both nuclear and cortical cataracts in this surgical series of infantile unilateral cataracts is remarkable. This finding can only be documented by analysis after removal of the overlying opaque lens cortex and/or nucleus.
The results of this study cannot be generalized to all eyes with monocular cataract, as IATS has excluded eyes with severe microcornea (defined as corneal diameter less than 9 mm) and eyes with severe PFV. Differences in inclusion criteria made it difficult at times to compare our study with other reports in the literature. We support the use of a careful layer-by-layer assessment and a descriptive classification of cataracts in children as we have proposed. The frequent finding of increasing cataract density in the posterior aspect of the fetal nucleus and cortex nearer the posterior capsule in association with posterior capsule plaque formation in these unilateral congenital cataracts supports a possible expansion of the spectrum of PFV, as suggested by Mullner-Eidenbock and colleagues.10
Supplementary Material
Acknowledgments
This study was supported by grants U10 EY13272 and U10 EY013287 from the National Institutes of Health and in part by an unrestricted grant to MUSC-SEI from Research to Prevent Blindness, Inc, New York, NY, and the Grady Lyman Fund.
Footnotes
Financial disclosure: M. Edward Wilson, MD: Alcon (consultant, grant), Bausch and Lomb (consultant), Springer (Royalty); Rupal H. Trivedi, MD MSCR: Alcon (grant), Bausch and Lomb (consultant), Springer (Royalty); David G. Morrison, MD: Bausch and Lomb (consultant); Scott R. Lambert, MD: None; Edward G. Buckley, MD: None; David A. Plager, MD: Alcon (consultant), Bausch and Lomb (consultant); Michael J. Lynn, MS: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parks MM, Johnson DA, Reed GW. Long-term visual results and complications in children with aphakia. A function of cataract type. Ophthalmology. 1993;100:826–40. doi: 10.1016/s0161-6420(93)31566-6. discussion 40–1. [DOI] [PubMed] [Google Scholar]
- 2.Chua BE, Mitchell P, Cumming RG. Effects of cataract type and location on visual function: The Blue Mountains Eye Study. Eye (Lond) 2004;18:765–72. doi: 10.1038/sj.eye.6701366. [DOI] [PubMed] [Google Scholar]
- 3.Lambert SR, Buckley EG, Drews-Botsch C, et al. The infant aphakia treatment study: Design and clinical measures at enrollment. Arch Ophthalmol. 2010;128:21–7. doi: 10.1001/archophthalmol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert SR, Buckley EG, Drews-Botsch C, et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: Grating acuity and adverse events at age 1 year. Arch Ophthalmol. 2010;128:810–18. doi: 10.1001/archophthalmol.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks MM. Visual results in aphakic children. Am J Ophthalmol. 1982;94:441–9. doi: 10.1016/0002-9394(82)90237-9. [DOI] [PubMed] [Google Scholar]
- 6.Forster JE, Abadi RV, Muldoon M, Lloyd IC. Grading infantile cataracts. Ophthalmic Physiol Opt. 2006;26:372–9. doi: 10.1111/j.1475-1313.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 7.Haargaard B, Wohlfahrt J, Fledelius HC, et al. A nationwide Danish study of 1027 cases of congenital/infantile cataracts: etiological and clinical classifications. Ophthalmology. 2004;111:2292–8. doi: 10.1016/j.ophtha.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Jain IS, Pillay P, Gangwar DN, et al. Congenital cataract: Etiology and morphology. J Pediatr Ophthalmol Strabismus. 1983;20:238–42. doi: 10.3928/0191-3913-19831101-06. [DOI] [PubMed] [Google Scholar]
- 9.Praveen MR, Shah SK, Vasavada AR, et al. Incidence, management and postoperative outcomes in pediatric eyes with coexisting posterior capsule plaque and cataract. J Cataract Refract Surg. 2010;36:2094–9. doi: 10.1016/j.jcrs.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Mullner-Eidenbock A, Amon M, Moser E, Klebermass N. Persistent fetal vasculature and minimal fetal vascular remnants: A frequent cause of unilateral congenital cataracts. Ophthalmology. 2004;111:906–13. doi: 10.1016/j.ophtha.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Johar K, Vasavada AR, Tatsumi K, et al. Anterior capsular plaque in congenital cataract: Occurrence, morphology, immunofluorescence, and ultrastructure. Invest Ophthalmol Vis Sci. 2007;48:4209–14. doi: 10.1167/iovs.07-0312. [DOI] [PubMed] [Google Scholar]
- 12.Pandey SK, Wilson ME. Etiology and morphology of pediatric cataracts. In: Wilson ME, Trivedi RH, Pandey SK, editors. Pediatric Cataract Surgery: Techniques, Complications, and Management. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005. pp. 6–13. [Google Scholar]
- 13.Johar SR, Savalia NK, Vasavada AR, Gupta PD. Epidemiology based etiological study of pediatric cataract in western India. Indian J Med Sci. 2004;58:115–21. [PubMed] [Google Scholar]
- 14.Wilson ME, Hennig A, Trivedi RH, et al. Clinical characteristics and early post-operative outcomes of pediatric cataract surgery with IOL implantation from Lahan, Nepal. Epub Sep 22, 2010. J Pediatr Ophthalmol Strabismus. 2010;22:1–6. doi: 10.3928/01913913-20100920-03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.