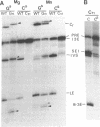
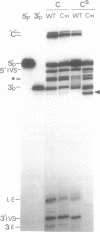
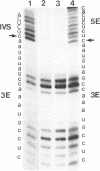
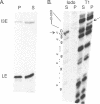
Abstract
RNA polymerases can synthesize RNA containing phosphorothioate linkages in which a sulfur replaces one of the nonbridging oxygens. Only the Rp isomer is generated during transcription. A Rp phosphorothioate at the 5' splice-site of the Tetrahymena group I intron does not inhibit splicing (McSwiggen, J.A. and Cech, T.R. (1989) Science 244, 679). Transcription of mutants in which the first base of the 3' exon, U+1, was mutated to C or G, in the presence, respectively, of either cytosine or guanosine thiotriphosphate, introduced a phosphorothioate at the 3' splice-site. In both cases exon ligation was blocked. In the phosphorothioate substituted U+1G mutant, a new 3' splice-site was selected one base downstream of the correct site; despite the fact that the correct site was selected with very high fidelity in unsubstituted RNA. In contrast, the exon ligation reaction was successfully performed in reverse using unsubstituted intron RNA and ligated exons containing an Rp phosphorothioate at the exon junction site. Chirality was reversed during transesterification as in 5' splice-site cleavage (vide supra). This suggests that one non-bridging oxygen is particularly crucial for both splicing reactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Been M. D., Perrotta A. T. Group I intron self-splicing with adenosine: evidence for a single nucleoside-binding site. Science. 1991 Apr 19;252(5004):434–437. doi: 10.1126/science.2017681. [DOI] [PubMed] [Google Scholar]
- Buzayan J. M., Feldstein P. A., Segrelles C., Bruening G. Autolytic processing of a phosphorothioate diester bond. Nucleic Acids Res. 1988 May 11;16(9):4009–4023. doi: 10.1093/nar/16.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. The chemistry of self-splicing RNA and RNA enzymes. Science. 1987 Jun 19;236(4808):1532–1539. doi: 10.1126/science.2438771. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Zaug A. J., Grabowski P. J. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell. 1981 Dec;27(3 Pt 2):487–496. doi: 10.1016/0092-8674(81)90390-1. [DOI] [PubMed] [Google Scholar]
- Chowrira B. M., Burke J. M. Binding and cleavage of nucleic acids by the "hairpin" ribozyme. Biochemistry. 1991 Sep 3;30(35):8518–8522. doi: 10.1021/bi00099a003. [DOI] [PubMed] [Google Scholar]
- Conway L., Wickens M. Analysis of mRNA 3' end formation by modification interference: the only modifications which prevent processing lie in AAUAAA and the poly(A) site. EMBO J. 1987 Dec 20;6(13):4177–4184. doi: 10.1002/j.1460-2075.1987.tb02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm S. C., Uhlenbeck O. C. Role of divalent metal ions in the hammerhead RNA cleavage reaction. Biochemistry. 1991 Oct 1;30(39):9464–9469. doi: 10.1021/bi00103a011. [DOI] [PubMed] [Google Scholar]
- Deeney C. M., Eperon I. C., Potter B. V. Self-splicing of Tetrahymena rRNA can proceed with phosphorothioate substitution at the splice sites. Nucleic Acids Symp Ser. 1987;(18):277–280. [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Gish G., Eckstein F. DNA and RNA sequence determination based on phosphorothioate chemistry. Science. 1988 Jun 10;240(4858):1520–1522. doi: 10.1126/science.2453926. [DOI] [PubMed] [Google Scholar]
- Griffiths A. D., Potter B. V., Eperon I. C. Stereospecificity of nucleases towards phosphorothioate-substituted RNA: stereochemistry of transcription by T7 RNA polymerase. Nucleic Acids Res. 1987 May 26;15(10):4145–4162. doi: 10.1093/nar/15.10.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschlag D., Cech T. R. Catalysis of RNA cleavage by the Tetrahymena thermophila ribozyme. 2. Kinetic description of the reaction of an RNA substrate that forms a mismatch at the active site. Biochemistry. 1990 Nov 6;29(44):10172–10180. doi: 10.1021/bi00496a004. [DOI] [PubMed] [Google Scholar]
- Inoue T., Sullivan F. X., Cech T. R. New reactions of the ribosomal RNA precursor of Tetrahymena and the mechanism of self-splicing. J Mol Biol. 1986 May 5;189(1):143–165. doi: 10.1016/0022-2836(86)90387-6. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Ohtsuka E. Effects of phosphorothioate and 2-amino groups in hammerhead ribozymes on cleavage rates and Mg2+ binding. Biochemistry. 1991 May 28;30(21):5145–5150. doi: 10.1021/bi00235a005. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Ohtsuka E. Effects of phosphorothioate and 2-amino groups in hammerhead ribozymes on cleavage rates and Mg2+ binding. Biochemistry. 1991 May 28;30(21):5145–5150. doi: 10.1021/bi00235a005. [DOI] [PubMed] [Google Scholar]
- Maschhoff K. L., Padgett R. A. Phosphorothioate substitution identifies phosphate groups important for pre-mRNA splicing. Nucleic Acids Res. 1992 Apr 25;20(8):1949–1957. doi: 10.1093/nar/20.8.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwiggen J. A., Cech T. R. Stereochemistry of RNA cleavage by the Tetrahymena ribozyme and evidence that the chemical step is not rate-limiting. Science. 1989 May 12;244(4905):679–683. doi: 10.1126/science.2470150. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Rajagopal J., Doudna J. A., Szostak J. W. Stereochemical course of catalysis by the Tetrahymena ribozyme. Science. 1989 May 12;244(4905):692–694. doi: 10.1126/science.2470151. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Uhlenbeck O. C. Thiophosphate interference experiments locate phosphates important for the hammerhead RNA self-cleavage reaction. Nucleic Acids Res. 1990 Oct 25;18(20):6025–6029. doi: 10.1093/nar/18.20.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slim G., Gait M. J. Configurationally defined phosphorothioate-containing oligoribonucleotides in the study of the mechanism of cleavage of hammerhead ribozymes. Nucleic Acids Res. 1991 Mar 25;19(6):1183–1188. doi: 10.1093/nar/19.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E. R., Waring R. B. Base pairing between the 3' exon and an internal guide sequence increases 3' splice site specificity in the Tetrahymena self-splicing rRNA intron. Mol Cell Biol. 1990 Jun;10(6):2960–2965. doi: 10.1128/mcb.10.6.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner N. K., Cech T. R. Self-catalyzed cyclization of the intervening sequence RNA of Tetrahymena: inhibition by intercalating dyes. Nucleic Acids Res. 1985 Nov 11;13(21):7741–7758. doi: 10.1093/nar/13.21.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring R. B. Identification of phosphate groups important to self-splicing of the Tetrahymena rRNA intron as determined by phosphorothioate substitution. Nucleic Acids Res. 1989 Dec 25;17(24):10281–10293. doi: 10.1093/nar/17.24.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring R. B., Ray J. A., Edwards S. W., Scazzocchio C., Davies R. W. The Tetrahymena rRNA intron self-splices in E. coli: in vivo evidence for the importance of key base-paired regions of RNA for RNA enzyme function. Cell. 1985 Feb;40(2):371–380. doi: 10.1016/0092-8674(85)90151-5. [DOI] [PubMed] [Google Scholar]
- Williamson C. L., Tierney W. M., Kerker B. J., Burke J. M. Site-directed mutagenesis of core sequence elements 9R', 9L, 9R, and 2 in self-splicing Tetrahymena pre-rRNA. J Biol Chem. 1987 Oct 25;262(30):14672–14682. [PubMed] [Google Scholar]
- Woodson S. A., Cech T. R. Reverse self-splicing of the tetrahymena group I intron: implication for the directionality of splicing and for intron transposition. Cell. 1989 Apr 21;57(2):335–345. doi: 10.1016/0092-8674(89)90971-9. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Grabowski P. J., Cech T. R. Autocatalytic cyclization of an excised intervening sequence RNA is a cleavage-ligation reaction. Nature. 1983 Feb 17;301(5901):578–583. doi: 10.1038/301578a0. [DOI] [PubMed] [Google Scholar]
- van Tol H., Buzayan J. M., Feldstein P. A., Eckstein F., Bruening G. Two autolytic processing reactions of a satellite RNA proceed with inversion of configuration. Nucleic Acids Res. 1990 Apr 25;18(8):1971–1975. doi: 10.1093/nar/18.8.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]