Abstract
Introduction
Sigmoid volvulus is a common surgical emergency in many regions of the world, with significant morbidity and mortality. The aims of this study were to (a) summarize outcomes and (b) define a treatment algorithm for sigmoid volvulus in our setting.
Experimental
Five year (2003–2008) retrospective review of sigmoid volvulus cases at Kamuzu Central Hospital, in Lilongwe, Malawi.
Results and Discussion
There were 239 cases of sigmoid volvulus identified. Cases were mostly seen in males (91.7%), with a median age of 50 (range 18–86). Gangrene was noted in 36.7% of cases. Mesosigmoidopexy (36%), Hartmann’s procedure (33%), and resection and anastomosis (23%) were the most common procedures. There was seasonal variation with more cases seen in the harvest months of March and April. The major complications noted were recurrence (5 of 6 recurrences after mesosigmoidopexy / plasty) and anastomotic leakage after resection and anastomosis (2 in gangrenous, and 2 in non-gangrenous sigmoid volvulus).
Conclusions
Gangrenous sigmoid volvulus is best managed with Hartmann’s procedure. Non-gangrenous sigmoid volvulus is best managed with resection and anastomosis, unless there are risk factors for anastomotic leakage, in which case the surgeon should consider mesosigmoidopexy with non-absorbable suture.
Keywords: Sigmoid Colon, Intestinal Volvulus, Mesosigmoidopexy, Malawi, Africa, Developing Countries
1. Introduction
Sigmoid volvulus is a life-threatening condition that is common in many regions of the world. Though rare in the United States and Europe, it is common throughout Africa, India, Iran, and Russia [1, 2]. In endemic regions, sigmoid volvulus accounts for 20–50% of all bowel obstructions [3, 4]. The mortality rate associated with sigmoid volvulus is high, estimated in some series to be around 20% depending on treatment modality and the case severity [5].
The high mortality rate from sigmoid volvulus is in part due to bowel gangrene and its sequelae. Anywhere from 8–45% of cases of sigmoid volvulus have been reported to be gangrenous [5, 6]. Successful management of sigmoid volvulus demands that the surgeon recognize two distinct disease processes—gangrenous sigmoid volvulus and non-gangrenous sigmoid volvulus.
There are several surgical options that are considered acceptable treatment for sigmoid volvulus; resection and primary anastomosis, Hartmann’s procedure, and mesosigmoidopexy. Other treatment options that are acceptable, but not possible in our setting due to resource limitations, include endoscopic sigmoidopexy [7]. It is generally thought that detorsion or decompression alone is not acceptable therapy [8].
The aims of this retrospective study were to (a) retrospectively review management choices and postoperative outcomes for sigmoid volvulus, and (b) compare our findings to the literature in order to develop an evidence-based treatment algorithm for management of sigmoid volvulus.
2. Experimental
The operative records at Kamuzu Central Hospital, in Lilongwe, Malawi, were reviewed for the five year period spanning August 2003 to August 2008. Kamuzu Central Hospital is the tertiary government hospital for the central region of Malawi. Operations done for sigmoid volvulus were recorded, including patient age, gender, diagnosis (gangrenous versus non-gangrenous; sigmoid volvulus, recurrent sigmoid volvulus, or ileosigmoid knotting), and operative procedure performed. Cases for this study were defined as patients with an intra-operative diagnosis of sigmoid volvulus. Pre-operative diagnosis was made using the history and physical examination, and plain radiographs on request of the clinician. All cases were operated emergently, and pre-operative decompression was not used. Consultant surgeons performed or immediately supervised all cases.
The technique of mesosigmoidopexy involved division of the lateral sigmoid serosa to the white line of Toldt, which was divided up to the splenic flexure and down to the pelvic brim; the cephalad serosal leaf on the sigmoid was then sutured to the serosa of the white line of Toldt extending to the splenic flexure, whilst the caudad serosal leaf on the sigmoid colon was sutured to the serosa of the white line of Toldt extending to the pelvic brim. Mesosigmoidoplasty was performed by completely dividing the sigmoid mesentery transversely, then suturing the two leaves together horizontally.
Statistical analyses were performed using Stata version 10. For comparison of the harvest month to the non-harvest month incidence of sigmoid volvulus, chi-squared analysis was used.
3. Results
Over the five year period, 239 cases for sigmoid volvulus were performed. There were more males (n=200, 91.7%) than females (n=18, 8.3%). Overall, the median age was 50 (range 18–86 years). The median age for males was 50 (range 18–86 years) and for females 49 (range 20–66 years). Gangrene was noted in 36.7% of cases.
The most common procedure was mesosigmoidopexy (36%), followed by Hartmann’s procedure (33%), primary anastomosis (23%), mesosigmoidoplasty (3%), and detorsion (3%). All mesosigmoidopexies were done in nongangrenous cases, whilst all Hartmann’s procedures but one were done in gangrenous cases, and resection and anastomosis was done more often for non-gangrenous than for gangrenous cases (Table 1).
Table 1.
Procedures done based on presense or absence of gangrene.
| Gangrene | Mesosig-moidopexy | Hartmann’s | Resection and Anastomosis | Mesosig-moidoplasty | Detorsion |
|---|---|---|---|---|---|
| Yes | 0 | 61 | 4 | 0 | 0 |
| No | 83 | 1 | 12 | 7 | 7 |
| Unspecified | 0 | 14 | 37 | 1 | 0 |
| Overall | 83 | 76 | 53 | 8 | 7 |
The incidence of sigmoid volvulus was noted to be higher during the harvest months of March and April. In total during the harvest months, there were 57 cases of sigmoid volvulus, and during the non-harvest months there were 157 cases. This corresponds to 1.51 cases per week during the harvest months, and 0.72 cases per week during non-harvest months (P<0.001).
There were two types of complications noted in the series: recurrence after surgical procedure (13 patients), and anastomotic leak with subsequent death (4 patients).
Of the 4 case of anstomotic leak, 2 were noted in patients who underwent resection and anastomosis for gangrenous sigmoid volvulus, and 2 had undergone resection and anastomosis for non-gangrenous sigmoid volvulus. Overall, 2 of these 4 patients with leaks died.
Of the 13 recurrences, 7 were in patients whose initial surgery was done before the study period. Of the remaining 6 recurrences, all were in patients who had their primary operation during the study period and had nongangrenous sigmoid volvulus; four of these recurrences were after mesosigmoidopexy, 1 after mesosigmoidoplasty, and 1 after Hartmann’s procedure. In the cases that recurred, absorbable sutures were used in the initial mesosigmoidopexies and the mesosigmoidoplasty. These recurrences were treated with Hartmann’s procedure (n=3), resection with primary anastomosis (n=2), and mesosigmoidopexy (n=1). There were no observed fatalities in patients who originally had a mesosigmoidopexy or mesosigmoidoplasty.
4. Discussion
In this 5 year retrospective review of sigmoid volvulus, we found that the two most common major complications were recurrence and anastomotic leak. We also noted that sigmoid volvulus was more common in harvest months (March and April), which is consistent with prior observations that intestinal volvulus follows seasonal patterns [7, 9, 10].
There are several limitations to our study. First, though all cases of sigmoid volvulus are operated emergently at our hospital, it is likely that some cases of sigmoid volvulus were missed during the study; we were unable to confirm the number of patients that died prior to surgery as there is no central registry which includes cause of death, nor are autopsies performed. Therefore the total number of cases was assumed to be the number identified from operative records. A second limitation of the study was that for many of the operative cases, data was not available as to whether the colon was gangrenous or non-gangrenous. Lastly, information related to the chronicity and extent of symptoms was not available, nor was information available on any cases which might have previously been managed non-operatively.
During the study period, there were 6 recurrences which occurred in patients who had their primary procedure also during the study period. All of these patients with recurrences were treated for non-gangrenous sigmoid volvulus. Five of these 6 patients were initially treated with either mesosigmoidopexy (n=4) or mesosigmoidoplasty (n=1); furthermore, in these 5 patients, a review of the surgical technique found that absorbable sutures were used for the repairs. Of patients who underwent resection and anastomosis, there were no recurrences. Therefore, our data suggests that among patients with non-gangrenous sigmoid volvulus, resection and anastomosis minimizes recurrence of sigmoid volvulus when compared to mesosigmoidopexy or mesosigmoidoplasty.
The other major complication we observed was anastomotic leak, which occurred in 4 patients who had undergone resection with primary anastomosis (2 gangrenous, 2 nongangrenous). Two of these 4 patients died. Among the patients with gangrenous sigmoid volvulus who were treated with Hartmann’s procedure, there were no reported deaths. From this, our data suggests that among patients with gangrenous sigmoid volvulus, Hartmann’s procedure avoids the complication of anastomotic leak which was observed with resection and anastomosis.
These findings are supported by a prior randomized controlled trial conducted in Guinea by Bagarani et al. [11]. In this study, 31 consecutive patients presenting with sigmoid volvulus were randomized according to nongangrenous (group A) or gangrenous (group B) intraoperative findings. Group A was randomized intraoperatively to either receive a mesosigmoidopexy or resection and primary anastomosis. Group B was randomized to receive either resection and anastomosis or Hartmann’s procedure. The authors found 5 major complications; in group A, 2 of 7 patients who underwent mesosigmoidopexy had a recurrence, whereas there were no noted complications in group A subjects who underwent resection and anastomosis. In group B, 3 of 7 patients who underwent resection and anastomosis had leaks (2 of which died), whereas there were no complications in patients undergoing Hartmann’s procedure (additionally, bowel continuity was restored in all of these patients but one who was lost to follow-up). From this, the authors conclude that non-gangrenous sigmoid volvulus is best managed with resection and anastomosis, whereas gangrenous sigmoid volvulus is best managed with Hartmann’s procedure.
It is interesting to note that, in our study, all recurrences after mesosigmoidopexy or mesosigmoidoplasty occurred when absorbable sutures were used. Notable as well, the protocol used by Bagarani et al. also called for absorbable sutures for mesosigmoidopexies 11. Because there is no prospective, randomized trial of mesosigmoidopexy using non-absorbable sutures, mesosigmoidopexy cannot be universally recommended as a management strategy for non-gangrenous sigmoid volvulus. However, there is significant retrospective data in our series to support at least consideration of mesosigmoidopexy as an acceptable treatment for non-gangrenous sigmoid volvulus (we observed no deaths and no recurrences with non-absorbable suture). Additionally, at our institution absorbable sutures have been replaced by non-absorbable sutures for mesosigmoidopexy, and we have yet to observe a recurrence of sigmoid volvulus after mesosigmoidopexy using non-absorbable sutures.
Though resection and anastomosis overall appears to be the best option for treating non-gangrenous sigmoid volvulus, we did note 2 cases of anastomotic leakage in this subset of patients. Given the high mortality rate associated with leakage in our setting (50%), care should be taken to avoid this complication when performing resection and anastomosis for non-gangrenous sigmoid volvulus. In non-gangrenous sigmoid volvulus, the morbidity of leakage after resection and anastomosis must be balanced against the morbidity associated with recurrence after mesosigmoidopexy. As such, we propose that non-gangrenous sigmoid volvulus should usually be treated with resection and anastomosis. However, in the subset of patients with non-gangrenous sigmoid volvulus at high risk of anastomotic leakage, the surgeon should consider mesosigmoidopexy as an alternative.
Risk factors associated with anastomotic leak include poor surgical technique, infection, low anastomoses, inadequate patient nutrition, and concomitant pulmonary or cardiovascular disease [12–15]. In patients with such risk factors and non-gangrenous sigmoid volvulus, the surgeon who is comfortable with the technique of mesosigmoidopxy should consider this as an attractive alternative to resection and anastomosis. Based on our experience, we recommend that non-absorbable sutures be used in such cases.
5. Conclusions
We recommend Hartmann’s procedure for all cases of gangrenous sigmoid volvulus. For non-gangrenous cases, we recommend either resection and anastomosis or mesosigmoidopexy based on patient factors (Figure 1). Further research should be conducted on mesosigmoidopexy before this technique can be universally recommended for management of non-gangrenous sigmoid volvulus. Additionally, such a study should utilize non-absorbable sutures for the repair. Lastly, surgeons should be aware of and monitor for recurrence after mesosigmoidopexy and leak after resection and anastomosis, the two most common major complications associated with surgical treatment of sigmoid volvulus.
Figure 1.
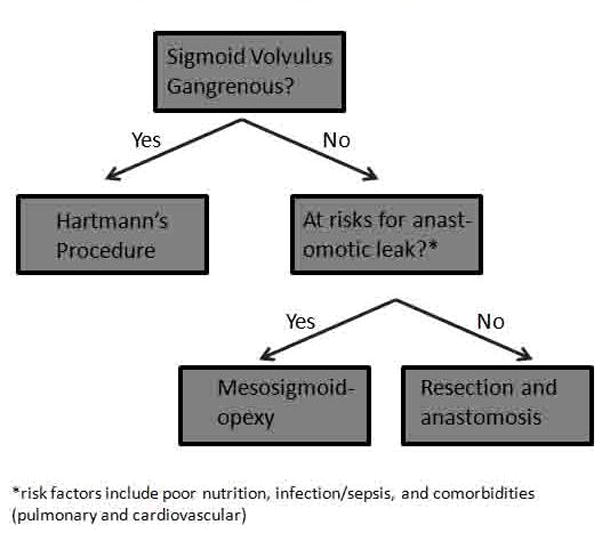
Treatment algorithm for sigmoid volvulus in our setting
Acknowledgments
Funding was provided by the Doris Duke Charitable Foundation, the NC Jaycee Burn Center at the University of North Carolina, and by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental & Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases Health, and National Institutes of Health Office of Women’s Health and Research through the International Clinical Research Fellows Program at Vanderbilt University (R24 TW007988), and by the University of North Carolina Center for AIDS Research Developmental Award (P30 AI50410).
Footnotes
Author contributions were as follows: Conception and design (JCS, AA, NM, AGC); acquisition of data (JCS, AA, NM); analysis and interpretation of data (JCS); drafting of the manuscript (JCS); critical revision of the manuscript (BAC, APM, AGC).
References
- 1.Lal S, Morgenstern R, Vinjirayer E, Matin A. Sigmoid volvulus an update. Gastrointestinal endoscopy clinics of North America. 2006;16:175–187. doi: 10.1016/j.giec.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne G, Brandner M, Beart R, Ilstrup D. Volvulus of the colon. Incidence and mortality. Annals of Surgery. 1985;202:83–91. doi: 10.1097/00000658-198507000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udezue N. Sigmoid volvulus in Kaduna, Nigeria. Diseases of the Colon and Rectum. 1990;33:647–649. doi: 10.1007/BF02150738. [DOI] [PubMed] [Google Scholar]
- 4.SchagenVanLeeuwen J. Sigmoid volvulus in a West African population. Diseases of the Colon and Rectum. 1985;28:712–716. doi: 10.1007/BF02560280. [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne G. Review of sigmoid volvulus: history and results of treatment. Diseases of the Colon and Rectum. 1982;25:494–501. doi: 10.1007/BF02553666. [DOI] [PubMed] [Google Scholar]
- 6.Raveenthiran V. Restorative resection of unprepared left-colon in gangrenous versus viable sigmoid volvulus. International Journal of Colorectal Diseases. 2004;19:258–263. doi: 10.1007/s00384-003-0536-6. [DOI] [PubMed] [Google Scholar]
- 7.Muyco A, Kushner A, Mvula C. Management of sigmoid volvulus at a tertiary care hospital in Africa. East and Central African Journal of Surgery. 2005;10:105. [Google Scholar]
- 8.Mangiante E, Croce M, Fabian T. Sigmoid volvulus: a four-decade experience. American Surgeon. 1989;55:41–44. [PubMed] [Google Scholar]
- 9.Vaez-Zadeh K, Kutz W, Nowrooz-Zadeh M. Volvulus of the small intestine in adults. A study of predisposing factors. Annals of Surgery. 1969;169:265–271. doi: 10.1097/00000658-196902000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demissie M. Small intestinal volvulus in southern Ethiopia. East African Medical Journal. 2001;78:208–211. doi: 10.4314/eamj.v78i4.9065. [DOI] [PubMed] [Google Scholar]
- 11.Bagarani M, Conde A, Longo R, Italiano A, Terenzi A, Venuto G. Sigmoid volvulus in West Africa: A prospective study on surgical treatments. Diseases of the Colon and Rectum. 1993;36:186–190. doi: 10.1007/BF02051177. [DOI] [PubMed] [Google Scholar]
- 12.Chambers W, Mortensen N. Postoperative leakage and abscess formation after colorectal surgery. Best Practice and Research Clinical Gastroenterology. 2004;18:865–880. doi: 10.1016/j.bpg.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Golub R, Golub R, Cantu R, Stein H. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. Journal of the American College of Surgeons. 1997;184:364–372. [PubMed] [Google Scholar]
- 14.Virginali A, Fazio W, Lavery I, Milsom J, Church J, Hull T, Strong S, Oakley J. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. Journal of the American College of Surgeons. 1997;185:105–113. doi: 10.1016/s1072-7515(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 15.Mileski W, Joehl R, Rege R, Narhwold D. Treatment of anastomotic leakage following low anterior colon resection. Archives of Surgery. 1988;123:968–971. doi: 10.1001/archsurg.1988.01400320054011. [DOI] [PubMed] [Google Scholar]


