Summary
The abscopal effect is a phenomenon in which local radiotherapy is associated with the regression of metastatic cancer at a distance from the irradiated site. The abscopal effect may be mediated by activation of the immune system. Ipilimumab is a monoclonal antibody that inhibits an immunologic checkpoint on T cells, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). We report a case of the abscopal effect in a patient with melanoma treated with ipilimumab and radiotherapy. Temporal associations were noted: tumor shrinkage with antibody responses to the cancer–testis antigen NY-ESO-1, changes in peripheral-blood immune cells, and increases in antibody responses to other antigens after radiotherapy. (Funded by the National Institutes of Health and others.)
The abscopal effect refers to a rare phenomenon of tumor regression at a site distant from the primary site of radiotherapy.1 Localized radiotherapy has been shown to induce abscopal effects in several types of cancer, including melanoma, lymphoma, and renal-cell carcinoma.2–4 The biologic characteristics underlying this effect are not completely understood, but it may be mediated by immunologic mechanisms.5
NY-ESO-1 is an antigen expressed in 30 to 40% of patients with advanced melanoma but not present in normal adult tissues except testicular germ cells and placenta.6 Ipilimumab (Bristol-Myers Squibb) has been shown to enhance immunity to NY-ESO-1, and patients with preexisting NY-ESO-1 antibodies have an increased likelihood of benefiting from ipilimumab.7 We describe a patient with metastatic melanoma in whom we measured changes in NY-ESO-1 titers before and during the observed abscopal effect.
Inducible costimulator (ICOS) is a marker of activated T cells. Increases in CD4+ ICOShigh cells have been associated with clinical benefit from ipilimumab.8 We assessed the frequency of this cell population in the patient's peripheral blood. We also measured interferon-γ–producing CD8+ and CD4+ T cells and myeloid-derived suppressor cells (defined as CD14+ HLA-DRlow),9 which contribute to tumor-induced immunosuppression, perhaps by limiting activated T-cell entry into the tumor site.10 Finally, we investigated changes in humoral immune responses before and after radiotherapy to a panel of antigens to discover additional antigenic targets potentially relevant to antitumor immunity, a process referred to as seromics.11
Case Report
A female patient received a diagnosis of cutaneous melanoma in April 2004 at 33 years of age. Biopsy of a mole on her upper back revealed melanoma, nonulcerated, with a Breslow thickness of 1.53 mm. She underwent a wide local excision of her primary lesion and biopsy of a left axillary sentinel lymph node. There was no residual melanoma at the primary site, and the five axillary lymph nodes removed were not found to be involved.
She remained disease-free until 2008, when routine chest radiography revealed a new pulmonary nodule, 2.0 cm in diameter, in her left lower lobe. The nodule was hypermetabolic on positronemission tomography, with a standard uptake value of 5.9. There were no additional sites of hypermetabolic foci. Cytologic findings from a computed tomography (CT)–guided percutaneous biopsy of the pulmonary nodule revealed metastatic melanoma. Mass-spectrometry genotyping (Sequenom) revealed no known mutations that affect the gene encoding serine–threonine protein kinase BRAF (e.g., the BRAF V600E mutation).
Standard cisplatin, vinblastine, and temozolomide (CVT) chemotherapy was initiated, and after two cycles, a CT scan showed stability of her pulmonary nodule and no evidence of additional metastases. The solitary pulmonary nodule was resected by means of a left lower lobectomy in February 2009, with pathological confirmation of metastatic melanoma.
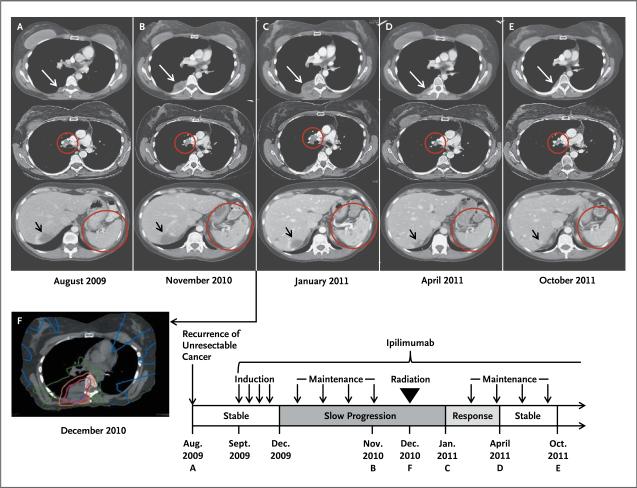
In August 2009, a surveillance CT scan detected recurrent disease with a new pleural-based paraspinal mass and right hilar lymphadenopathy (Fig. 1A). In September 2009, the patient enrolled in a clinical trial at our institution (CA184-087; ClinicalTrials.gov number, NCT00920907): a randomized, open-label trial comparing the safety and pharmacokinetics of ipilimumab manufactured by means of two distinct processes. She received ipilimumab at a dose of 10 mg per kilogram of body weight every 3 weeks, for a total of four doses, as part of induction therapy. A follow-up CT scan in December 2009 (12 weeks after ipilimumab initiation) showed overall stable disease with slight enlargement of the pleural mass (not shown). Responses to ipilimumab are not always seen on the initial CT scan 12 weeks after treatment initiation,12 and she was permitted to continue with ipilimumab as maintenance therapy, with a dose given every 12 weeks.
Figure 1. Results of Diagnostic and Radiotherapy Simulation Imaging throughout the Disease Course.
Axial CT images are shown, corresponding to the timeline showing therapy and disease status. White arrows indicate the paraspinal mass, red circles indicate the right hilar lymphadenopathy and spleen, and black arrows indicate an incidental hepatic hemangioma. Panel A (top) represents the status before treatment with ipilimumab. Panel B shows enlargement of the paraspinal mass (top), stable right hilar lymphadenopathy (middle), and new splenic lesions (bottom). Panel C shows images 1 month after radiotherapy, when the response to radiotherapy had not yet occurred, with apparent continued worsening disease at all three sites. Several months after radiotherapy, the targeted paraspinal mass showed a response (Panel D, top). Furthermore, disease response outside of the radiation field was seen with decreased right hilar lymphadenopathy (middle) and resolution of splenic lesions (bottom). The response was durable, as shown in Panel E. Panel F shows the CT simulation image for radiotherapy planning, with the target volume (indicated in purple) encompassing the right paraspinal metastatic mass. The isodose lines represent total doses of 2850 cGy (pink), 2000 cGy (orange), 1000 cGy (green), and 200 cGy (blue). Disease regression was confirmed by means of three-dimensional volumetric assessment (Table 2 in the Supplementary Appendix).
Over the course of 2010, there was slight radiographic evidence of worsening disease, but treatment was continued with maintenance ipilimumab, since the patient was clinically well. Mild, asymptomatic hypothyroidism developed that required thyroid hormone supplementation, but she had no other clinically significant treatment-related toxicity. By November 2010, however, she had progressive enlargement of the pleural-based paraspinal mass and new splenic lesions (Fig. 1B). To treat right-sided back pain caused by the paraspinal mass, palliative radiotherapy was initiated. In December 2010, a total of 2850 cGy was administered in three fractions over a period of 7 days to the paraspinal mass with 6-MV photons by means of a coplanar six-field intensity-modulated, image-guided technique (Fig. 1F). This regimen is within the range of acceptable, commonly used dose-fractionation schemes.
One month after radiotherapy, in January 2011, a CT scan had not yet shown a response at the primary irradiated site, the right hilar lymph node, and the spleen (Fig. 1C). The patient was given one additional dose of ipilimumab in February 2011. By April 2011, her targeted paraspinal lesion had regressed significantly (Fig. 1D). Remarkably, lesions in areas not targeted by radiotherapy had also regressed (right hilar lymphadenopathy and splenic lesions [Fig. 1D]). A subsequent CT scan obtained in October 2011 (10 months after radiotherapy) showed stability, with the continued presence of minimal disease (Fig. 1E).
Results
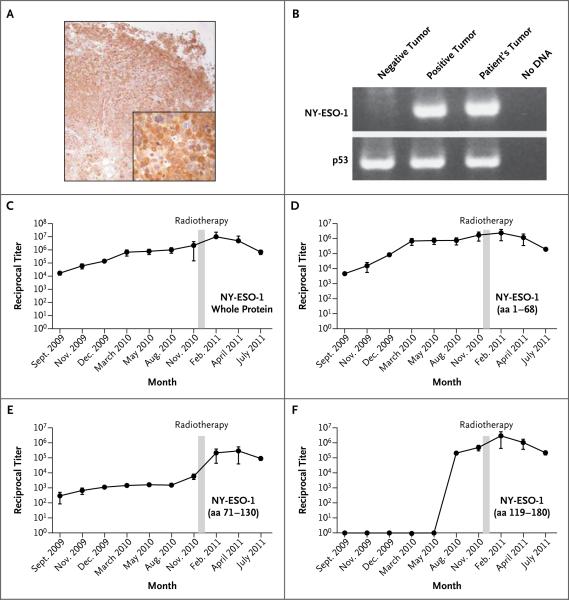
In the only metastatic lesion available for analysis, a pulmonary nodule removed before ipilimumab treatment, NY-ESO-1 expression was confirmed by means of both immunohistochemical analysis, showing homogeneously strong positivity (Fig. 2A), and a reverse-transcriptase–polymerase-chain-reaction assay (Fig. 2B) performed according to previously described methods.13
Figure 2. NY-ESO-1 Expression and Antibody Response to Ipilimumab and Radiotherapy.
Immunohistochemical analysis of NY-ESO-1 expression in the pulmonary metastatic melanoma nodule is shown with the use of monoclonal antibody E978 (Panel A, with the inset showing a portion of the image magnified by a factor of 4) and polymerase-chain-reaction (PCR) assay (Panel B). For the PCR results, p53 was used as a reference standard, and the positive tumor specimen in lane 2 is the NY-ESO-1-positive melanoma cell line SK-Mel-37. Titers of antibody against the whole NY-ESO-1 protein and the N-terminal portion (amino acids [aa] 1–68) rose as the disease progressed and ipilimumab therapy was administered and diminished with the disease response after radiotherapy (Panels C and D). After radiotherapy, there was an increase by a factor of more than 30 in the titer of antibodies against an epitope or epitopes within the central portion of NY-ESO-1 (aa 71–130), which corresponded to the period of disease resolution (Panel E). Seroconversion to an epitope or epitopes in the C-terminal portion of NYESO-1 (aa 119–180) occurred with disease progression before radiotherapy (Panel F). Panels C through F show the means from an average of nine independent determinations, and the I bars indicate standard deviations. Reciprocal antibody titers of more than 100 are considered to be significant.
Using serum samples collected before the first ipilimumab treatment and before and after radiotherapy, we measured the antibody titers against the whole NY-ESO-1 recombinant protein and against specific synthetic portions of the NY-ESO-1 protein by means of an enzyme-linked immunosorbent assay, as previously described.14 Before all therapy, the patient was seropositive for whole NY-ESO-1 protein, with reactivity confined primarily to an epitope or epitopes contained within the N-terminal portion (amino acids 1–68) (Fig. 2C and 2D). During ipilimumab treatment, and in parallel with an increasing disease burden, titers of antibodies against the whole NY-ESO-1 protein and against the N-terminal portion increased. Titers remained elevated, though there was a trend toward lower titers as the disease burden decreased.
After completing radiotherapy, the patient had an increase by a factor of more than 30 in the titer of antibodies against an epitope or epitopes within the central portion of the protein (amino acids 71–130), correlating with the time of disease resolution (Fig. 2E). She also had seroconversion against an epitope or epitopes in the C-terminal portion (amino acids 119–180) (Fig. 2F) during treatment with ipilimumab before radiotherapy, which corresponded to the time of increasing disease. Results were considered immunologically significant if the titers changed by more than a factor of 5 between two time points. Seroreactivity against dihydrofolate reductase was considered to be a negative control (see Fig. 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
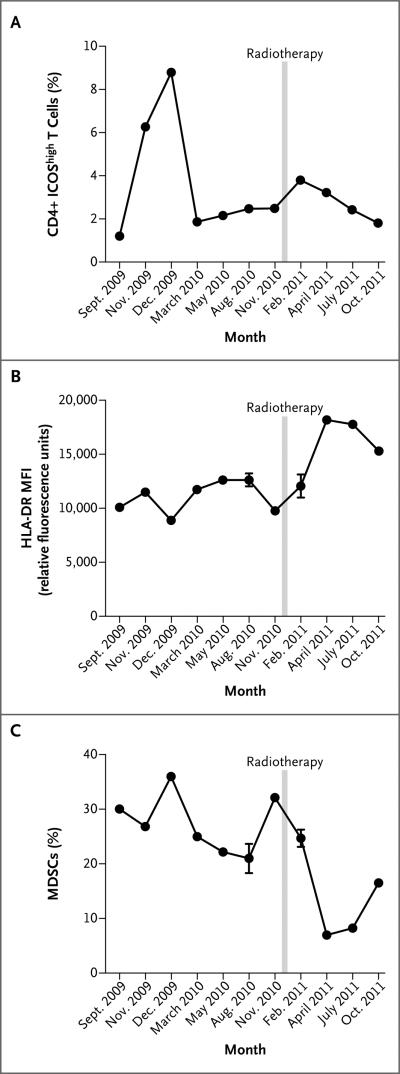
Measurement of CD4+ T-cell activation based on ICOS expression (CD4+ ICOShigh) and myeloid-lineage activation based on the quantity of myeloid-derived suppressor cells and HLA-DR expression on CD14+ monocytes was performed by means of flow cytometry in peripheral-blood mononuclear cells at serial time points coincident with measurements of NY-ESO-1 titers. Before radiotherapy, CD4+ ICOShigh T cells (Fig. 3A) increased during ipilimumab induction and then decreased. A clear trend in the quantity of myeloid-derived suppressor cells was not apparent during this time (Fig. 3C). After radiotherapy, there was a second increase in CD4+ ICOShigh cells and an increase in HLA-DR expression on CD14+ monocytes (Fig. 3B) with a reciprocal, marked decline in the quantity of myeloid-derived suppressor cells that preceded radiographic disease regression, which was not evident until April 2011.
Figure 3. Results of Flow Cytometry of Peripheral-Blood Mononuclear Cells.
Panel A shows that levels of CD4+ ICOShigh cells increased during ipilimumab induction but decreased before radiotherapy; after radiotherapy, there was a second increase in the levels. Panel B shows an increase in HLA-DR expression on monocytes, expressed as mean fluorescence intensity (MFI), after radiotherapy. Panel C shows a decline in levels of myeloid-derived suppressor cells (MDSCs) (CD14+ HLA-DRlow)9 after radiotherapy. Data in Panel A are a representative sample from two independent determinations; data in Panels B and C are the means from two determinations. I bars indicate standard deviations.
We evaluated frequencies of interferon-γ–producing CD8+ and CD4+ T cells. Though there was no appreciable change in NY-ESO-1–specific interferon-γ–producing CD8+ cells, with ongoing ipilimumab therapy and disease progression, the frequency of NY-ESO-1–specific interferon-γ–producing CD4+ cells increased (Fig. 2 in the Supplementary Appendix). Despite this increase, tumor regression was seen only after radiotherapy, suggesting that the tumor was resistant to T-cell–mediated antitumor effects until radiotherapy occurred.
Serum specimens obtained before and after radiotherapy were also used to assess, with the use of seromic analysis, the binding of IgG antibodies against a panel of more than 9000 human antigens (ProtoArray microarray, version 5.0; Invitrogen) by means of previously described methods.11 Seromic analysis identified 10 antigenic targets that had increased antibody responses (and 2 with decreased responses) after radiotherapy (Table 1 in the Supplementary Appendix). These differences were considered to be immunologically significant because they represented changes from pre-radiotherapy values that were greater than a factor of 5.
Discussion
Ipilimumab has shown an overall survival benefit in two randomized, phase 3 trials involving patients with advanced melanoma, yet response rates remain modest, between 10% and 15%.15,16 Increasing the response rate to ipilimumab by administering it in combination with targeted therapy, chemotherapy, other immunotherapy, or radiotherapy are areas of active investigation. Our patient had a systemic response to localized radiotherapy after having had disease progression while receiving ipilimumab. The right hilar lymph-node mass and spleen, which were not the target of radiotherapy, received only low, nontherapeutic doses of radiation (133 cGy and 2.3 cGy, respectively), further supporting the notion that disease regression at these distant sites was due to an enhanced systemic response. Delayed responses occurring 18 to 20 weeks after ipilimumab treatment are well known, yet in our opinion, the 19-month interval between the start of ipilimumab and disease response, with radiotherapy having been administered in the interim, is more supportive of an abscopal effect.
Our clinical observation is consistent with pre-clinical evidence characterizing the role of the immune system in the abscopal effect. In mouse models, Chakravarty and colleagues17 and Demaria and colleagues18 reported that the abscopal effect was dependent on a functional immune system. Additional work in a murine breast-cancer model showed decreased pulmonary metastases and improved survival only for mice treated with radiotherapy in combination with CTLA-4 blockade.19 The schedule of radiotherapy may be important, since the abscopal effect was seen in one study20 only when hypofractionated radiotherapy was delivered with CTLA-4 blockade.
Dunn and colleagues21 referred to the three Es of immunoediting — equilibrium, escape, and elimination — when describing the complex relationship between tumors and the immune system. We assessed antibody responses to NY-ESO-1 as a surrogate marker of the antitumor immune response. Antibody titers against NY-ESO-1 have been shown to increase with progressive disease (escape) and decrease with disease regression (elimination).22 We found a similar trend with increasing antibody titers during tumor progression but ultimately a decrease with disease response. After completing radiotherapy, a marked increase in titers of antibodies against an epitope or epitopes within the central portion of NY-ESO-1 correlated with a response detectable on radiography. The precise significance of antibody-titer increases against specific portions of the NY-ESO-1 protein is not clear, as titers against the C-terminal portion of NY-ESO-1 increased before radiotherapy, possibly as a result of autoimmunization from enlarging tumor deposits.
Since patients who had sustained elevation of CD4+ ICOShigh cells after ipilimumab therapy were found to have improved clinical benefit and overall survival,8 we chose to focus on this particular T-cell subgroup as a surrogate T-cell marker of the antitumor immune response. The marked increase in CD4+ ICOShigh cells during ipilimumab induction (weeks 1 to 12) was expected. More interesting was the observed modest increase after radiotherapy, suggesting that radiotherapy may have played an immunomodulatory role in expanding this activated T-cell population.
Radiotherapy has been shown to increase the presentation of antigen by myeloid cells within the tumor stroma and thereby enhance T-cell killing of tumor cells.23 Analysis of the peripheral-blood CD14+ cellular compartment revealed several signs of myeloid-lineage activation, including increased HLA-DR expression and a reduction in the quantity of myeloid-derived suppressor cells after radiotherapy. We hypothesize that since these changes preceded the reduction in tumor burden seen on a CT scan, they may represent early signals of a shift in immune phenotype — from immune escape toward immune-mediated tumor elimination.
We are intrigued by the results of seromic analysis, which detected 10 antigenic targets with enhanced antibody responses after radiotherapy. Two antigens, focal adhesion kinase 1 (PTK2) and mediator complex subunit 6 (MED6), also known as NY-REN-28, have been described in melanoma24 and renal-cell cancer, respectively.25 Though the precise significance of the enhanced humoral immune response against these specific antigenic targets is not clear, our study highlights the potential for seromics as a strategy to define the diverse repertoire of the humoral immune response in a patient with cancer.
It would be worthwhile to increase the number of patients who benefit from ipilimumab. This patient's surprising systemic response after local radiotherapy in combination with ipilimumab provides new insights and suggests new therapeutic avenues to pursue. Clinical trials to prospectively validate this approach are under way in prostate cancer (NCT00861614) and melanoma (NCT01449279).
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (RC2CA148468, to Drs. Gnjatic and Wolchok), the American Cancer Society (MRSG-11-054-01-LIB, to Dr. Lesokhin), the Melanoma Research Alliance (to Drs. Gnjatic, Lesokhin, and Wolchok), Swim Across America (to Dr. Wolchok), the Cancer Research Institute (to Drs. Gnjatic and Wolchok), the Virginia and D.K. Ludwig Fund for Cancer Research (to Dr. Allison), the Lita Annenberg Hazen Foundation (to Dr. Wolchok), and the Commonwealth Foundation for Cancer Research (to Dr. Wolchok).
We thank Dr. David Schaer for his technical assistance and artistic guidance in creating earlier versions of the figures.
Footnotes
Drs. Postow and Callahan contributed equally to this article.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Dedicated to the memory of Dr. Lloyd Old, our mentor and friend and one of the true leaders in investigational cancer medicine. His contributions to tumor immunology inspired much of this work.
References
- 1.Mole RH. Whole body irradiation: radiobiology or medicine? Br J Radiol. 1953;26:234–41. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 2.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48:863–6. doi: 10.1259/0007-1285-48-574-863. [DOI] [PubMed] [Google Scholar]
- 3.Robin HI, AuBuchon J, Varanasi VR, Weinstein AB. The abscopal effect: demonstration in lymphomatous involvement of kidneys. Med Pediatr Oncol. 1981;9:473–6. doi: 10.1002/mpo.2950090510. [DOI] [PubMed] [Google Scholar]
- 4.Wersäll PJ, Blomgren H, Pisa P, Lax I, Kälkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45:493–7. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 5.Drake C. Radiation-induced immune modulation. In: DeWeese TL, Laiho M, editors. Molecular determinants of radiation response. Springer; New York: 2011. pp. 251–63. [Google Scholar]
- 6.Jungbluth AA, Chen YT, Stockert E, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–60. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 7.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108:16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 10.Lesokhin A, Hohl TM, Kitano S, et al. Monocytic CCR2+ myeloid derived suppressor cells promote immune escape by limiting activated CD8 T cell infiltration into the tumor microenvironment. Cancer Res. 2011 Dec 15; doi: 10.1158/0008-5472.CAN-11-1792. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnjatic S, Wheeler C, Ebner M, et al. Seromic analysis of antibody responses in non-small cell lung cancer patients and healthy donors using conformational protein arrays. J Immunol Methods. 2009;341:50–8. doi: 10.1016/j.jim.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 13.Jungbluth AA, Chen YT, Stockert E, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–60. doi: 10.1002/ijc.1282. Erratum, Int J Cancer 2002;97:878. [DOI] [PubMed] [Google Scholar]
- 14.Gnjatic S, Old LJ, Chen YT. Autoantibodies against cancer antigens. Methods Mol Biol. 2009;520:11–9. doi: 10.1007/978-1-60327-811-9_2. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. Erratum, N Engl J Med 2010;363:1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–32. [PubMed] [Google Scholar]
- 18.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 20.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Jager E, Stockert E, Zidianakis Z, et al. Humoral immune responses of cancer patients against “Cancer-Testis” antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–10. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahana O, Micksche M, Witz IP, Yron I. The focal adhesion kinase (P125FAK) is constitutively active in human malignant melanoma. Oncogene. 2002;21:3969–77. doi: 10.1038/sj.onc.1205472. [DOI] [PubMed] [Google Scholar]
- 25.Scanlan MJ, Gordan JD, Williamson B, et al. Antigens recognized by autologous antibody in patients with renal-cell carcinoma. Int J Cancer. 1999;83:456–64. doi: 10.1002/(sici)1097-0215(19991112)83:4<456::aid-ijc4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.