Abstract
Neurons of the geniculate ganglion innervate taste buds located in two spatially distinct targets, the tongue and palate. About 50% of these neurons die in Bdnf−/− mice and Ntf4/5−/− mice. Bdnf−/−/Ntf4/5−/− double mutants lose 90-95% of geniculate ganglion neurons. To determine whether different subpopulations are differentially influenced by neurotrophins, we quantified neurons from two ganglion subpopulations separately and remaining taste buds at birth within each target field in wild-type, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/− mice. In wild-type mice the same number of neurons innervated the anterior tongue and soft palate and each target contained the same number of taste buds. Compared to wild-type mice, Bdnf−/− mice showed a 50% reduction in geniculate neurons innervating the tongue and a 28% loss in neurons innervating the soft palate. Ntf4/5−/− mice lost 58% of the neurons innervating the tongue and 41% of the neurons innervating the soft palate. Taste bud loss was not as profound in the NT-4 null mice compared to BDNF-null mice. Tongues of Bdnf−/−/Ntf4/5−/− mice were innervated by 0 to 4 gustatory neurons and contained 3 to 16 taste buds at birth, indicating that some taste buds remain even when all innervation is lost. Thus, gustatory neurons are equally dependent on BDNF and NT-4 expression for survival, regardless of what peripheral target they innervate. However, taste buds are more sensitive to BDNF than NT-4 removal.
Indexing Terms: taste buds, neurotrophins, chorda tympani, greater superficial petrosal
The geniculate ganglion contains primary sensory neurons that convey the sense of taste. During development, the neurotrophins BDNF and NT-4 influence the total number of geniculate ganglion neurons found within the ganglion at birth. Specifically, 50% of the geniculate ganglion neurons are lost in BDNF null mice (Bdnf−/−) as well as in NT-4 null mice (Ntf4/5−/−) (Liu et al., 1995). Bdnf−/−/Ntf4/5−/− double mutant mice lose 90-95% of geniculate ganglion neurons (Conover et al., 1995; Liu et al., 1995). The loss of almost all the geniculate neurons in the double mutant mice indicates that most of these neurons depend on one or both of these two neurotrophins for continued development.
Sensory neurons of the geniculate ganglion innervate three major spatially distinct peripheral targets: 1) taste buds housed in the front two-thirds of the tongue (fungiform papillae) and some taste buds in tongue foliate papillae; 2) taste buds on the primary and soft palate; and 3) skin of the external ear. Previous studies have only examined the influence of BDNF and NT-4 on the total number of remaining geniculate neurons, without regard to the targets they innervate. Thus, the relationship between neurotrophin dependency and geniculate neuron target is unknown. A few possibilities can be postulated. Neurons innervating each gustatory region could be dependent on a specific neurotrophin. For example, the neurons that innervate the tongue could be BDNF-dependent and those innervating the palate could be NT-4-dependent. Based on available data, this possibility seems likely because BDNF, but not NT4, has been shown to be expressed in fungiform placodes in the developing tongue (Nosrat and Olson, 1995; Nosrat et al., 1996). Where and when geniculate neurons are exposed to NT-4 is not clear, but the source could be the palate. Alternatively, BDNF and NT-4 could both support lingual and palatal afferents.
BDNF and NT-4 also influence the development and survival of taste buds. Fungiform papillae and taste buds are lost in both Bdnf−/− and Ntf4/5−/− mice (Nosrat et al., 1997; Liebl et al., 1999; Mistretta et al., 1999). While it is possible that neurotrophins function directly to maintain taste buds, it is more likely that taste buds are lost in mice lacking neurotrophins because of the loss of innervation by geniculate neurons. It has been well established that when gustatory nerves are severed during development, taste buds are lost (Guth, 1957; Naga et al., 1970; Hosley et al., 1987; Nagato et al., 1995; Sollars, 2005), demonstrating that innervation is required for taste bud growth and maintenance. In addition, during normal development the size of a taste bud is related to and may be controlled by the number of neurons that innervate it (Krimm and Hill, 1998). Thus, there is a relationship between the developing gustatory neurons and the taste buds they innervate, which is disrupted by functional deletion of BDNF and NT-4.
This study examines the neurotrophin dependencies of the two largest subpopulations of geniculate neurons based on target innervated (Miller and Spangler, 1982; Krimm et al., 2001; Zhang et al., 2008). Specifically, we focused on those neurons innervating fungiform papillae at the front of the tongue and those innervating the soft palate. In addition, we examined the relationship between the number of neurons lost and the number of taste buds lost in the targets they innervate. We found that gustatory neurons were equally dependent on BDNF and NT-4 for survival regardless of the target they innervated. However, neurons innervating the tongue are more BDNF/NT-4-dependent than those innervating the soft palate. Taste bud development depends more on BDNF than on NT-4, such that the number of taste buds remaining in a specific peripheral target field cannot be predicted from the number of neurons innervating it.
Materials and Methods
Animals
Heterozygous B6.129S4-Bdnftm1Jae/J (Bdnf−/+) mice were acquired from Jackson Laboratories (Bar Harbor, ME; Stock #002266) and are on a C57BL/6J background. In these mice most of the exon 5 Bdnf coding region was replaced by a neomycin cassette, resulting in no detectable Bdnf transcripts from this allele (Ernfors et al., 1994). Bdnf+/− and Bdnf−/− have a wild-type Ntf4/5 gene. Heterozygous 129S4/SvJae-Ntf5tm1Jae/J (Ntf4/5−/+) mice are on a 129S4/SvJae background and were also obtained from Jackson Laboratories (Stock #002497). In these mice the entire coding region of the Ntf4/5 gene was replaced with a neomycin cassette (Liu et al., 1995), Ntf4/5−/+ and Ntf4/5−/− and they have a wild-type Bdnf gene. Heterozygous Bdnf+/− and Ntf4/5+/− mice were bred with one another to get Bdnf+/−/Ntf4/5+/− mice. Pairs of Bdnf+/−/Ntf4/5+/− were bred with one another to provide all the breeders for the experimental mice. Heterozygous Bdnf+/− mice resulting from the Bdnf+/−/Ntf4/5+/− pairing were bred to get wild-type, Bdnf+/−, and Bdnr−/− mice, while Bdnf+/−/Ntf4/5−/− littermates were bred to provide Ntf4/5−/− and Bdnf−/−/Ntf4/5−/− double knockout mice. Thus, all the mice used in this study were on a mixed C57BL/6J X 129S4/SvJae background. Animals were genotyped by polymerase chain reaction (PCR), as described in protocols provided by Jackson Laboratory (http://jaxmice.jax//index.html). Embryonic mice were obtained from timed breeding of females that were examined for plugs the following morning. The day a plug was identified was designated embryonic day 0.5 (E0.5). Animals were used at E18.5 for DiI-labeling and at birth for immunohistochemistry. Tail clippings were used for genotyping each embryo using PCR. Animals were cared for and used in accordance with the guidelines of the U.S Public Health Service Policy on Humane Care and Use of Laboratory Animals and the NIH Guide for the Care and Use of Laboratory Animals.
Tongue and soft palate labeling using 1,1*-Dioctadecyl-3,3,3*,3*tetramethylindo-carbo-cyanine perchlorate (DiI)
Embryos were fixed with ice-cold 4% paraformaldehyde (PFA). The next day tongues or soft palates were labeled with DiI crystals (Molecular Probes, Eugene, OR). DiI was chosen for this labeling procedure because it has two unique features as a neuronal tracer. First, it spreads out from the site of application and fills the entire target region (illustrated in Fig. 1A), which ensures that all innervating neurons are labeled. Second, it is transported along axons in fixed tissues and is ideal for labeling embryonic neurons. For the tongue, we compared 1) placing DiI crystals in a small pocket in the base of the tongue; 2) transecting the front two-thirds of the tongue and placing DiI crystals across the entire cut surface; and 3) inserting DiI-saturated filter paper into the base of the tongue. Comparing the number of neurons labeled and the amount of background observed, placing the crystals onto the transected tongue (protocol 2) was most successful. To label the soft palate, DiI crystals were inserted underneath the soft palate and multiple incubation times were tested. We found that an incubation time of 2–4 weeks worked better than the 6–8 weeks we used for the tongue.
Figure 1.
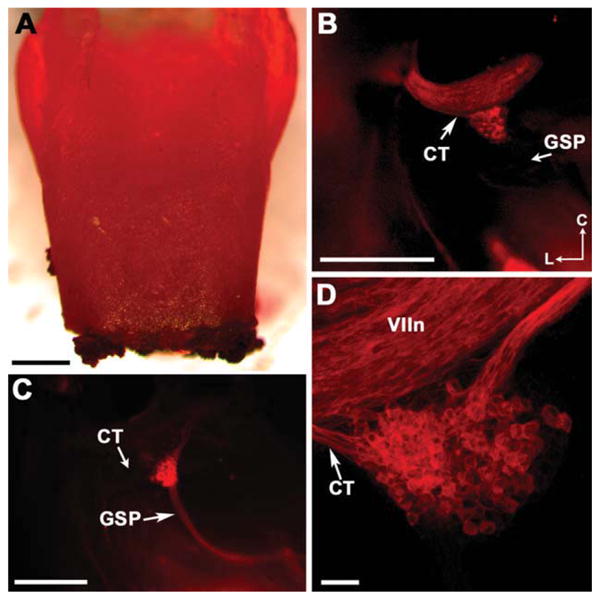
DiI is an effective retrograde label for neurons of the geniculate ganglion during embryonic development. A: Image of a mouse embryo tongue with DiI crystals placed onto the front of the tongue to label the geniculate neurons that innervate the tongue. This tongue was dissected out at the same time as the geniculate ganglia, after incubation of the embryo for 6–8 weeks in 4% PFA. Note that the entire tongue is stained red because DiI filled the entire tongue. B: A geniculate ganglion as seen through a fluorescent dissecting microscope before its removal from the embryo. A geniculate ganglion following DiI labeling of the tongue shows brightly labeled geniculate neurons and labeled CT nerve, but no labeling of the GSP nerve. C: A bright-red geniculate ganglion as seen through a fluorescent dissecting microscope after the DiI had traveled from the palate back to the geniculate ganglion. Here the GSP was labeled but the CT was not. D: A composite image of a confocal stack from a wild-type geniculate ganglion following tongue labeling. Note that positively labeled neurons have a clear dark nucleus, making them easy to count through each section of the confocal stack. VII = seventh (facial) cranial nerve. The directional arrows in B indicate caudal (C) and lateral (L) for the embryo and apply to B–D. Scale bars = 500 μm in A–C; 50 μm in D. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
For labeling the tongue the front third of the tongue was transected and the DiI crystals were placed onto the exposed cross-sectioned nerves traversing the tongue (Fig. 1A). A wedge of parafilm was placed between the tongue and the soft palate to avoid cross-contamination of the soft palate by loose crystals. For soft palate labeling, an incision was made into the epithelium between the hard and soft palates and DiI crystals were inserted under the entire soft palate. Embryos were placed between two buffer-soaked towels for 0.5–1 hours, then returned to 4% PFA for incubation at 37°C for 6-8 weeks for tongues and 2-4 weeks for the soft palate, allowing retrograde transport of the DiI.
Dissection and analysis of the geniculate ganglion
After incubation the geniculate ganglion was dissected using a Leica MZFL fluorescent dissecting microscope. Once the brain and trigeminal ganglion were removed the geniculate ganglion was easily recognized as a 200-μm bright red fluorescent structure (Fig. 1B,C). The ganglion was removed and placed into a drop of glycerol contained within a self-adhesive reinforcement label (Avery, #5721) on a slide to avoid damage to the ganglion from the pressure of the coverslip. Serial optical sections (1.5 μm) of the ganglia were captured as 100-200-μm-thick image stacks using an Olympus confocal microscope. Image stacks were collected from geniculate ganglia following DiI labeling of the tongue of wild-type (n = 12), Bdnr− +/− (n = 9), Bdnr−/− (n = 5), Ntf4/5−/− (n = 9), and Bdnf−/−/Ntf4/5−/− (n = 8) mice and the soft palate of wild-type (n = 11), Bdnf− +/− (n = 3), Bdnr−/− (n = 6), Ntf4/5−/− (n = 7), and Bdnr−/−/Ntf4/5−/− (n = 5) mice. These image stacks were quantified using Stereoinvestigator (MicroBrightField, Colchester, VT), so that each cell could be followed in a complete serial reconstruction of the ganglion and thus counted only once. Every labeled neuron was counted so the actual number of neurons was quantified without any method of estimation. Cell soma area (μm2) was measured for 100 neurons in each ganglion for comparison of cell size for the tongue-labeled ganglia in wild-type (n = 4), Bdnf−/− (n = 4), and Ntf4/5−/− (n = 3) mice, and soft palate-labeled, wild-type (n = 3), Bdnf−/− (n = 3), and Ntf4/5−/− (n = 3) mice. Neurons were sampled beginning at the center of the ganglion and cells were measured in sections flanking the middle section by 10 μm until 100 cells were measured. Cell diameters were calculated from measures of area.
Immunofluorescent labeling of taste buds
Animals were perfused transcardially at birth with ice-cold 4% PFA. The tongue and soft palate were dissected out and immersion fixed for another 2 hours in 4% PFA, followed by cryoprotection consisting of an overnight incubation in 30% sucrose/phosphate buffer. The following day the tissue was embedded in Tissue Tek O.C.T. compound (Sakura Finetek, Torrance, CA; #4583). Sagittal serial sections of the tongue and coronal serial sections of the soft palate were cut at a thickness of 16 μm and collected onto Superfrost plus slides. Sections were heat-dried overnight, rehydrated, placed into citrate buffer (pH 6.0), heated for 15 minutes in a boiling water bath, and incubated for 10 minutes at room temperature (RT) for antigen retrieval. Slides were washed in PBS (pH = 7.4) and incubated overnight at RT in anti-cytokeratin-8 in PBS (Table 1). Following incubation with the anti-cytokeratin-8 antibody the slides were rinsed in PBS and the fluorescently labeled secondary antibody antirat Alexa-555 (1:200; Molecular Probes) was applied for 2 hours. After washing with PBS, sections were blocked in 5% normal goat serum / 0.25% Triton X-100 / PBS, followed by incubation in a polyclonal rabbit anti-GAP43. Following an overnight incubation with anti-GAP-43 the slides were rinsed in PBS (3 × 5 min) and the tissue was incubated in secondary antibody anti-rabbit Alexa-488 (Molecular Probes) for 2 hours at RT. After washing in PBS (3 × 5 min) the slides were dehydrated, cleared in Citrisolv (a xylene substitute), and coverslipped using DPX mounting medium (Fluka, Buchs, Switzerland). Control tissues in which the primary antibody was eliminated showed no specific staining of the secondary antibodies.
TABLE 1. Primary Antibodies Used in the Study.
| Antigen | Dilution | Host species | Source | Catalog no. | Immunogen |
|---|---|---|---|---|---|
| Cytokeratin-8 | 1:100 of concentrate | Rat | Developmental Studies Hybridoma Bank | TROMA-1 | Trophoblastoma cells |
| GAP-43 | 1:1,000 | Rabbit | Chemicon | AB5220 | Recombinant rat GAP-43 (complete sequence) |
The TROMA 1 (cytokeratin-8) antiserum was characterized with Western blot analysis that stained a single band of 55 kD molecular weight (manufacturer's technical information) from preimplanted embryos (Brulet et al., 1980). In adults this antibody was found to label simple epithelia (Kemler et al., 1981). The cDNA clone which encodes the TROMA 1 protein was later isolated and the protein was determined to be synonymous with cytokeratin-8, which is present in most simple epithelia (Brulet and Jacob, 1982; Magin et al., 1986). The mature cells of taste buds are columnar in shape and extend from the basal lamina to the free surface and so they are classified as simple epithelia. Consistent with their morphology they label with a variety of cytokeratin antibodies that label simple epithelia including antibodies against cytokeratin-8 (Toh et al., 1993; Knapp et al., 1995), while the surrounding stratified epithelia does not. As a result, anti-cytokeratin-8 antibodies were used to help identify taste buds in situations where their normal morphology may be compromised by either nerve section or growth factor manipulation (Liebl et al., 1999; Sun and Oakley, 2002; Guagliardo and Hill, 2007). We have used the cytokeratin-8 antibody to label both developing and adult tongues and have observed the same cellular pattern and distribution as was observed in previous studies (Toh et al., 1993; Knapp et al., 1995; Liebl et al., 1999; Sun and Oakley, 2002; Guagliardo and Hill, 2007).
Gap-43 (growth-associated protein 43) is a well-established marker of growth cones and developing and/or remodeling axons (Zuber et al., 1989; De la Monte et al., 1989; Fishman and Valenzuela, 1991; Strittmatter et al., 1994; Fishman, 1996). Anti-Gap-43 has been used previously as a marker for developing gustatory innervation (Mbiene and Mistretta, 1997; Ito and Nosrat, 2009; Ito et al., 2010) and our labeling showed the same cellular distribution and pattern as the previous studies. The GAP-43 antibody was characterized with Western blot analysis (1:500) which detected a single 43 kD molecular weight band from mouse brain lysates (manufacturer's technical information) indicating that it does not label proteins that are not 43 kD.
Quantification and volume analysis of taste buds
Taste buds were counted in 16-μm serial sections of immunofluorescently labeled tongue and soft palate tissues using a fluorescence enabled Leica DMLB microscope (n = 3 each for wild-type, Bdnr+/−, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/−). For volume measurement, 10-15 taste buds per animal were analyzed by collecting 1-μm optical serial sections through the entire taste bud using an Olympus confocal microscope. The area was measured in each 1-μm image using Stereoin-vestigator-7 software (MicroBrightField) and summed to obtain the volume for each taste bud.
Data analysis
Geniculate ganglion cell and taste bud numbers were quantified as described above. Total DiI-labeled neuron numbers were compared between target (tongue and soft palate) for wild-type, Bdnf+/−, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/−, using two-way analysis of variance (ANOVA). Total taste bud number and total volume also were compared between targets for wild-type, Bdnf+/−, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/−, using two-way ANOVA. Bonferroni's post-hoc test was used to compare individual means as needed. The significance level was set at P < 0.05 for all statistical comparisons.
Figures containing images were constructed in Adobe Photoshop (San Jose, CA) and color levels were adjusted on the images to maximize their distribution and the contrast was enhanced on the black-and-white images.
Results
Most of the geniculate neurons that are part of the gustatory system innervate two spatially distinct targets, the anterior tongue and the soft palate. To understand the influence of the neurotrophins BDNF and NT-4 on the individual geniculate subpopulations that innervate the tongue versus the soft palate, we quantified these sub-populations in wild-type, Bdnf+/−, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/− double knockout mice at E18.5. The specific subpopulations were identified by labeling each of these two peripheral targets with the lipophilic tracer DiI, followed by quantification of the geniculate ganglion for DiI-labeled neurons. During the incubation period the DiI label spread throughout the entire tongue (Fig. 1A) and soft palate. The chorda tympani nerves (CT) were labeled red following DiI placement on the tongue (Fig. 1B), and the greater superficial petrosal (GSP) nerves were labeled red following DiI placement on the palate (Fig. 1C). Positively labeled neurons were visible as bright-red fluorescent cells with a dark nucleus (Fig. 1D). When the tongue was labeled the neurons tended to be clustered toward the facial nerve, whereas the neurons were clustered around the GSP following labeling of the palate. Both Bdnf−/− (Fig. 2B,F) and Ntf4/5−/− (Fig. 2C,G) mice had noticeably fewer DiI-labeled neurons in the geniculate ganglion than wild-type mice (Fig. 2A,E) following both palate and tongue labeling. Few, if any, neurons remained in Bdnf−/−/Ntf4/5−/− double knockout mice following tongue labeling (Fig. 2D), while there were always some neurons innervating the soft palate (Fig. 2H). Since 3D information concerning the geniculate ganglia was retained following optical sectioning (see Supporting Movie 1), each neuron could be followed through serial optical sections (see Supporting Movie 2), allowing accurate quantification of the total number of labeled neurons.
Figure 2.
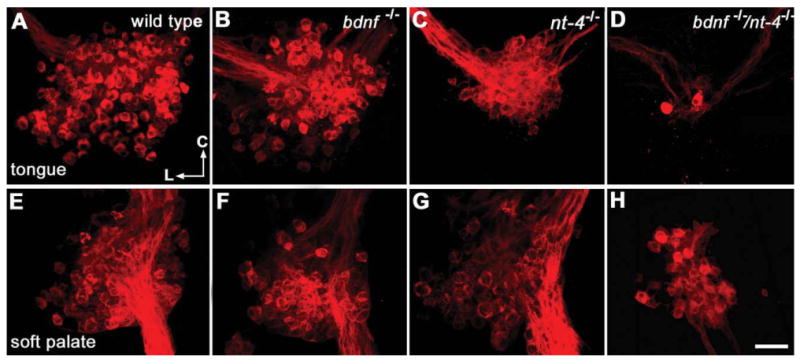
Composite images of E18.5 geniculate ganglia labeled with DiI from wild-type, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/− mice illustrate neuron losses that occur following manipulation of neurotrophic factors. A-D: Composite images of geniculate ganglia following DiI application to the tongue show labeling of neurons in the chorda tympani nerve. E-H: Images of geniculate ganglia following DiI application to the soft palate show positively labeled neurons in the GSP nerve. Note that even in these composite images the individual neurons are identifiable with bright-red cytoplasm and a dark nucleus. Following tongue and soft palate labeling the geniculate ganglia had fewer labeled cells in Bdnf−/− or Ntf4/5−/− mice as compared to wild-type ganglia. A profound reduction in neuron number and ganglion size is observed in the Bdnf−/−/Ntf4/5−/− ganglia with both tongue and soft palate labeling (D,H). The directional arrows in H indicate caudal (C) and lateral (L) for the embryo. Scale bar = 50 μm (applies to all). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We quantified labeled neurons in serial optical sections and found that the same number of geniculate neurons innervated the tongue (230.5 ± 14.36) as the soft palate (231.9 ± 14.13; P = 0.941; Fig. 3) in wild-type mice. In addition, no difference was observed between Bdnf+/− and wild-type mice in the number of neurons innervating the tongue (P = 0.541) or soft palate (P = 1.0, Fig. 3). Therefore, there was no gene dosage effect for the Bdnf gene on gustatory neuron number. Ganglia from Bdnf−/− knockout mice showed a 50% (P < 0.001) reduction in the number of neurons innervating the tongue and a 28% reduction (P = 0.038) in the number of neurons innervating the soft palate, as compared to wild-type ganglia (Fig. 3). The ganglia from Ntf4/5−/− mice lost 58% (P < 0.001) of the neurons that innervate the tongue and 41% (P < 0.003) of the neurons that innervate the soft palate (Fig. 3). Thus, removal of BDNF resulted in the loss of comparatively more neurons that innervate the tongue than the soft palate (P < 0.05; Fig. 3), while deletion of NT-4 resulted in the loss of similar numbers of neurons that innervate the tongue vs. the soft palate (P = 0.122; Fig. 3). There were no differences in the number of neurons innervating the tongue between Bdnf−/− and Ntf4/5−/− mice (P = 0.96), and there were no differences in the numbers of neurons remaining to innervate the palate between Bdnf−/− and Ntf4/5−/− mice (P = 0.78). Therefore, BDNF and NT-4 are equally important for maintaining gustatory neuron numbers for both tongue innervating and soft palate innervating neurons in the geniculate ganglion.
Figure 3.
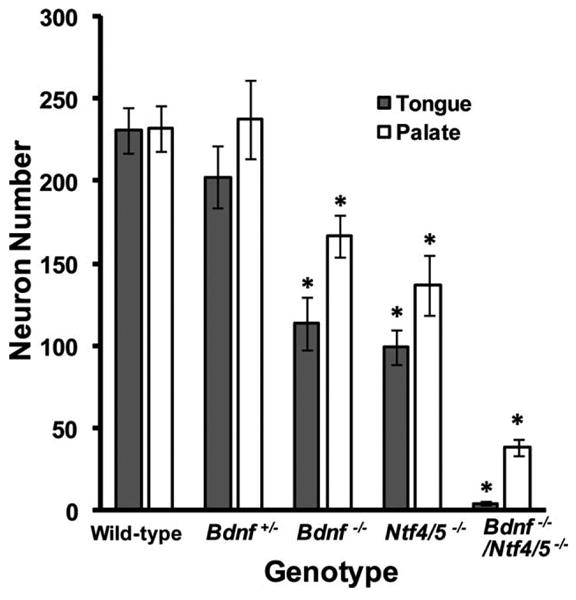
BDNF and NT-4 support equal numbers of neurons in both the tongue and the soft palate. Total neuron numbers were plotted following labeling of the tongue and palate with DiI in wild-type, Bdnf+/−, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/− mice. The same number of neurons innervated the tongue and the soft palate in wild-type mice. Compared to wild-type mice, Bdnf−/− and Ntf4/5−/− ganglia showed significantly reduced numbers of neurons innervating the tongue and soft palate. There was no difference in the number of neurons lost between Bdnf−/− and Ntf4/5−/− mice. Bdnf−/−/Ntf4/5−/− mice lost almost all of the neurons that innervate the tongue, but a few neurons still innervated the soft palate. More of the neurons that innervate the tongue than the soft palate were lost following removal of neurotrophic factors, indicating that the neurons innervating the tongue are more TrkB-ligand-dependent than neurons innervating the palate. *P < 0.05 versus wild-type.
Removal of both BDNF and NT-4 had a greater impact on the neurons that innervate the tongue as compared to the soft palate. Of the eight Bdnf−/−/Ntf4/5−/− embryos with tongue labeling, four had no ganglion cells that contained DiI. An average of only 3.3 neurons per ganglion was observed for these animals (Fig. 3). All embryos examined following soft palate labeling had at least a few neuronal cells containing DiI. The soft palate was innervated by 83% fewer (P < 0.001) neurons in Bdnf−/−/Ntf4/5−/− mice as compared to wild-type mice (Fig. 3). These findings indicate that the neurons innervating the tongue are more dependent on BDNF/NT-4 signaling than the subpopulation of neurons that innervate the soft palate. More of the neurons innervating the palate may be dependent on NT-3 or may simply be nonneurotrophic factor-dependent.
The development of taste buds is thought to be supported by gustatory neurons (Guth, 1957; Hosley et al., 1987; Oakley et al., 1990; Nagato et al., 1995; Sollars et al., 2002; Guagliardo and Hill, 2007). To understand the impact of neuronal loss on taste buds in neurotrophin knockout mice, we quantified the developing taste buds at birth for wild-type, Bdnf+/−, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/− mice. Taste buds were visualized using an antibody against cytokeratin-8 (Fig. 4A-D). We observed that developing taste buds from wild-type and Ntf4/5−/− mice positively labeled with anti-cytokeratin-8 could also be easily recognized using a hematoxylin and eosin (H&E) stain. In H&E-stained tissue the developing taste buds appeared as round balls of cells within fungiform papillae, which is typical of immature taste buds at birth (Fig. 4E). However, there were anti-cytokeratin-8-positive clusters in Bdnf−/− and Bdnf−/−/Ntf4/5−/− double knockout mice that were too small to be identified with H&E (Fig. 4F,H) staining. Since these clusters were located in fungiform papillae (Fig. 4J,L) they likely are immature taste buds whose development has been stunted by the lack of neurotrophins. We did not observe any anti-cytokeratin-8-positive clusters that were located outside of fungiform papillae. Thus, quantification of both the number and size of anti-cytokeratin-8-labeled clusters was used as an indicator of immature taste bud number and size.
Figure 4.
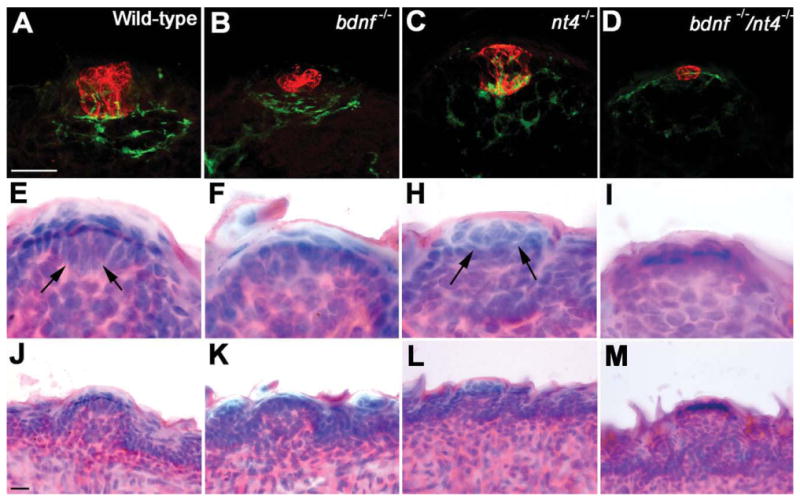
The morphology of the remaining taste buds is more sensitive to deletion of BDNF than NT-4. Images of taste buds in the tongue fungiform papillae in mice at birth were visualized using double immunolabeling for anti-cytokeratin-8 (developing taste bud; red) and anti-GAP-43 (innervation; green) (A-D). Compared to wild-type taste buds, the developing taste buds in Bdnf−/− (B) and Bdnf−/−/Ntf4/5−/− (D) mice show a drastic reduction in overall size. The taste buds in Ntf4/5−/− (C) mice are normal (similar to the wild-type taste buds in A in appearance). The morphology of some taste buds was visualized using H&E staining (E–I). Note that the taste buds in wild-type (E) and Ntf4/5−/− (H) mice are recognizable based on their morphology in the H&E-stained images (arrows). However, anti-cyto-keratin-8-positive clusters in Bdnf−/− (B) and Bdnf−/−/Ntf4/5−/− (D) mice do not morphologically resemble taste buds in H&E-stained tissue (F,I). All taste buds are located in clear fungiform papillae (J-M). A magenta-green copy of this image is provided as Supporting Figure 1. Scale bars = 10 μm in A,J.
Wild-type mice had the same number of developing taste buds in the tongue (128.3 ± 4.3) as in the soft palate (142.6 ± 10.4; P = 0.085; Fig. 5). Bdnf−/− mice showed a loss of 59% (P < 0.001) in the total number of developing taste buds from the tongue and a 69% (P < 0.001) loss of developing taste buds from the soft palate. However, removal of NT-4 resulted in only an 18% (P < 0.015; Fig. 5) reduction in the number of taste buds on the tongue, and no loss in palatal developing taste buds (P = 0.436; Fig. 5). Tongues from Bdnf−/−/Ntf4/5r−/− double knockout mice had only a few taste buds remaining on the tongue (7.6 ± 4.2). The soft palate of Bdnf−/−/Ntf4/5−/− mice had more developing taste buds remaining (33 ± 1.3; Fig. 5; P < 0.005) than in the tongue, and the same number of developing taste buds as the soft palate of Bdnf−/− mice (P = 1.0), which is consistent with the above observation that there was no effect of NT-4 removal on palatal taste buds. These results demonstrate that unlike gustatory neurons, which are equally dependent on BDNF and NT-4, more taste buds in the tongue are lost in Bdnf−/− mice than in Ntf4/5−/− mice, and taste buds on the palate are not lost in Ntf4/5−/− mice at all.
Figure 5.
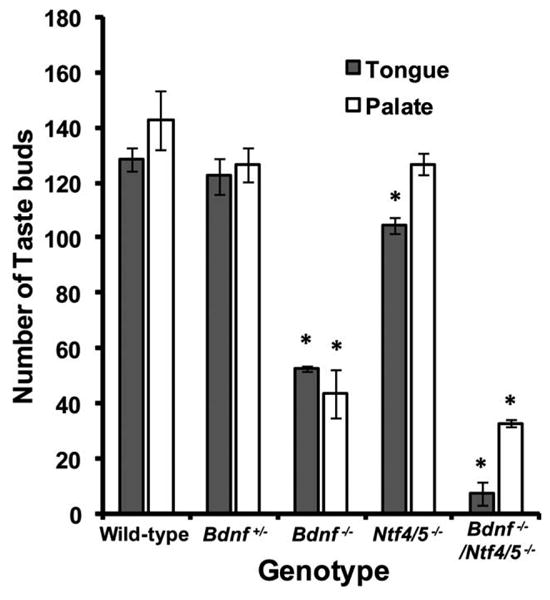
Removal of BDNF has a greater influence on taste bud number than removal of NT-4. The total number of taste buds per tongue was plotted in wild-type, Bdnf+/−, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/− mice. In wild-type mice, no significant difference was observed in the number of taste buds in the tongue and the soft palate. Compared to wild-type mice, Bdnf−/− mice had a significant reduction in the total number of taste buds in the tongue and soft palate. Ntf4/5−/− mice lost only a small number of taste buds from the tongue and none from the soft palate. Only a few taste buds remained in the tongue of Bdnf−/−/Ntf4/5−/− double knockout mice, but more taste buds remained in the soft palate. Thus, on average, two-thirds of the taste buds in the tongue and palate are dependent on BDNF for survival by birth, whereas only about one-sixteenth of tongue and one-ninth of palatal taste buds are dependent on NT-4 for surviva by birth. *P < 0.05 versus wild-type.
The loss in neurotrophic factors and geniculate neurons was reflected in the size (volume, μm3) of the remaining taste buds in Bdnf−/− as well as Bdnf−/−/Ntf4/5−/− double knockout mice (Fig. 6). There was a 59% decrease in the volume of taste buds in the tongues of Bdnf−/− mice (P = 0.002) and an 80% decrease in the volume of taste buds in the tongues of Bdnf−/−/Ntf4/5−/− double knockout mice (P < 0.001) as compared to wild-type mice. No significant difference was observed in the volume of tongue taste buds for Ntf4/5−/− (1,233 ± 53 μm3) as compared to wild-type mice (1,414.2 ± 164 μm3; P = 0.63; Fig. 6). Thus, taste buds present in the tongue at birth retain their size in the absence of NT-4, but not in the absence of BDNF.
Figure 6.
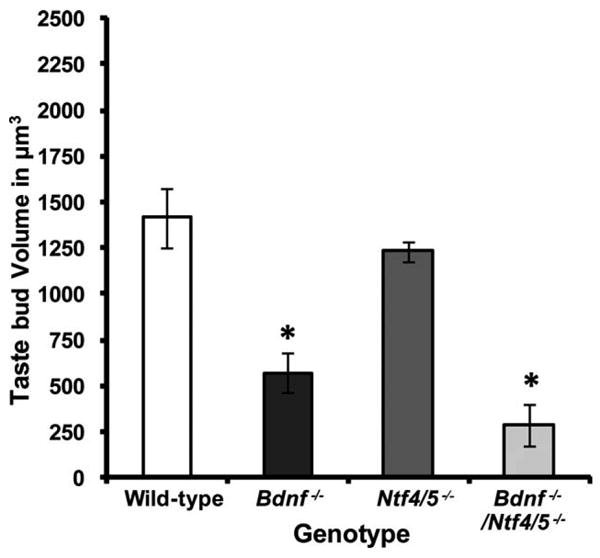
In the absence of BDNF, taste buds are significantly smaller in size. Mean taste bud volumes for taste buds located in the tongue were plotted for wild-type, Bdnf−/−, Ntf4/5−/−, and Bdnf−/−/Ntf4/5−/− mice. Deletion of BDNF resulted in a drastic reduction in taste bud volume as compared to wild-type volumes. However, in the absence of NT-4, the surviving taste buds retained their normal size. Bdnf−/−/Ntf4/5−/− mice had very small taste buds, which were even smaller than those in BDNF mutant mice, indicating that in the absence of BDNF, the presence or absence of NT-4 does influence taste bud size. *P < 0.05 versus wild-type.
Loss or gain of neurotrophins can affect neuron cell soma size as well as number. To determine if geniculate ganglion neuronal subpopulations varied in size based on neurotrophin dependency or on the specific peripheral target innervated, we measured cell soma sizes of the tongue and palate neurons in wild-type, Ntf4/5−/−, and Bdnf−/− ganglia. Wild-type ganglia did not show any difference between cell soma diameter of tongue neurons (19.6 ± 0.7 μm) compared to palatal neurons (18.3 ± 0.3 μm, P = 0.066; Fig. 7). In addition, neurons that innervate the soft palate were the same size in wild-type mice, Bdnf−/− (P = 1.0), and Ntf4/5−/− mice (P = 1.0). On the other hand, neurons that innervate the tongue were smaller in Bdnf−/− mice (16.4 ±0.6 μm, P = 0.002; Fig. 7) than in wild-type mice. Neurons innervating the tongue in Ntf4/5−/− mice (19.5 ± 0.8 μm, P = 1.0) were the same size as in wild-type mice (Fig. 7). Thus, either the larger neurons innervating the tongue are more likely to be dependent on BDNF for survival or BDNF regulates the development of gustatory neuron size for neurons innervating the tongue but not the palate.
Figure 7.
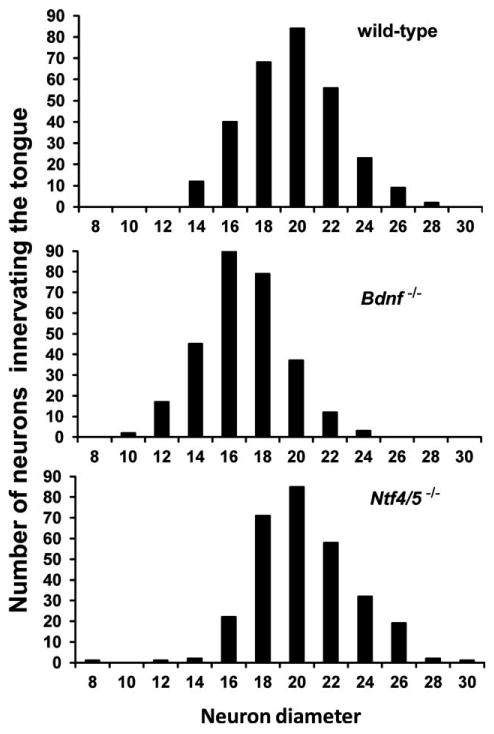
Soma diameters of neurons innervating the tongue are smaller in the absence of BDNF. Neuron size (diameter, μm) histograms were plotted for wild-type (top), Bdnf−/− (middle) and Ntf4/5−/− (bottom) mice. Note that there are more moderately smaller neurons (between 16-18 μm in diameter) in Bdnf−/− mice compared to wild-type and Ntf4/5−/− mice (18-22 μm). It is possible that BDNF is required to support normal cell size for neurons innervating the tongue.
Discussion
About half of the primary sensory neurons of the geniculate ganglion are dependent on BDNF or NT-4 for survival (Liu et al., 1995). Likewise, about half the gustatory portion of this ganglion projects to the tongue and half to the palate. Thus, the aim of this study was to decipher the relationship between the location of the target that a group of geniculate neurons innervates (tongue vs. soft palate) and their neurotrophin dependencies (BDNF vs. NT-4). To accomplish this goal we focused on the two target fields innervated by the most gustatory neurons in the geniculate ganglion, those innervating the fungiform taste buds in the front two-thirds of the tongue and those innervating the soft palate. One possibility was that each gustatory neuron subpopulation would depend on a different neurotrophic factor. However, this was not the case. Instead, neurons innervating the tongue and the soft palate were equally dependent on BDNF and NT-4 for survival. Unlike gustatory neurons, mice lacking BDNF had fewer remaining taste buds in both the fungiform papillae and the soft palate than mice lacking NT-4. Therefore, there is not a proportional relationship between number of neurons and the number of remaining taste buds.
Since BDNF and NT-4 both support the same number of neurons innervating each gustatory target, it is possible that they support the same neurons. While each target may be innervated by a subpopulation of BDNF-dependent neurons as well as a subpopulation of NT-4-dependent neurons, it is equally likely that some neurons survive when either neurotrophin is expressed, while others may require both neurotrophins in order to survive. If this scenario is the case, then geniculate neurons can best be described as TrkB ligand-sensitive, TrkB ligand-less sensitive, TrkB-ligand-insensitive. Consistent with the notion that cell number in the geniculate is regulated by the overall level of neurotrophins, NT-4 can effectively substitute for BDNF to maintain geniculate neuron number (Fan et al., 2000). Although this is not how we are used to thinking about neurotrophin sensitivities, it is the most likely scenario for geniculate neurons given relative equal dependence on BDNF and NT4 of both tongue projecting and palatal projecting neurons.
It is well established that taste buds are supported by the neuronal fibers that innervate them. For example, when the chorda tympani nerve is severed during development, taste buds and the fungiform papillae in which they are located are lost (Nagato et al., 1995; Sollars et al., 2002; Sollars, 2005). In mice lacking either BDNF or NT-4, roughly half the neurons that innervate the tongue are lost. If these neurons are required to support taste buds, why is the taste bud loss in mice lacking NT-4 so modest? Each taste bud in the adult mouse tongue is innervated by 4-5 neurons, and each palatal taste bud is innervated by 5-8 neurons (Zaidi and Whitehead, 2006), making the relationship between the loss of neurons and the loss of target more complicated than a simple 1:1 ratio. In addition, some geniculate neurons can innervate more than one taste bud (Miller, 1974; Zaidi et al., 2007). Consistent with the finding that taste buds are innervated by multiple neurons, we observed a ratio of three-to-four neurons per taste bud for both the palate and tongue in wild-type mice. Therefore, each taste bud should be innervated by at least three or four neurons even if chorda tympani axons do not branch and innervate more than one taste bud. However, a small amount of branching does occur in the adult mouse (Zaidi and Whitehead, 2006), and at birth, there is probably more branching than in adults (Mistretta et al., 1988; Nagai et al., 1988). If we simplify the gustatory system, then we can use a hyper-geometric distribution analysis to predict the percentage of taste buds that would not be innervated when 50% of the gustatory neurons are randomly lost. We used the mean number of wild-type taste buds at birth for this calculation. That is, half a normal mouse tongue should have 64 taste buds that are each innervated by approximately four geniculate ganglion cells (Zaidi et al., 2007); if we assume that there is no branching, then only 4 (6%) taste buds would be expected to lose all four neurons innervating them. While this calculation does not represent exactly what is happening in Ntf4/5−/− mice, it does illustrate that a fairly large number of geniculate neurons can be lost without a complete loss of innervation to most taste buds. The Ntf4/5−/− animals lost 58% of the neurons innervating the tongue, which means that 96 neurons still remained to innervate the 64 taste buds that should be present on half of the tongue. Thus, enough neurons still remained for most taste buds to be innervated. Similarly, 59% of the neurons remained to innervate the developing taste buds in the palate. Therefore, it is not surprising that only 18% of tongue taste buds and 10% of palatal taste buds disappeared in the absence of NT-4. While it is possible that all of the missing taste buds were completely uninnervated, it is also possible that some taste buds innervated by only one remaining neuron did not have sufficient support for continued development.
Similar to Ntf4/5−/− mice, Bdnf−/− mice lost about half the neurons innervating the tongue and palate. Therefore, similar to Ntf4/5−/− mice, they should lose a relatively small number of taste buds. However, consistent with other laboratories (Nosrat et al., 1997; Mistretta et al., 1999), we found a substantial loss in the number of taste buds for both the palate and the tongue in the absence of BDNF. This additional loss probably occurs because of failed target innervation in Bdnf−/− mice. Epithelial-derived BDNF is required during development for the correct targeting of fibers to the taste buds (Ma et al., 2009). Thus, in the absence of BDNF, geniculate neurons fail to innervate most of the taste buds, resulting in their loss. Consistent with this idea, quantification of the number of innervated and uninnervated papillae in Bdnf−/− mice when papillae are first innervated is predictive of a large taste bud loss (Thirumangalathu et al., 2009). Furthermore, the remaining taste buds are substantially smaller in Bdnf−/− mice compared to both wild-type and Ntf4/5−/− mice, and thus the remaining taste buds may be uninnervated taste buds that are in the process of degenerating. In the absence of NT-4, the axons of the surviving neurons arrive successfully at the taste buds. Therefore, even though similar numbers of neurons are lost in Bdnf−/− and Ntf4/5−/− ganglia, there are differences in the number of taste buds that are successfully innervated, resulting in differences in the number of remaining taste buds.
The two targets examined in this study are innervated by approximately half (462) of the 800-1,000 geniculate neurons present in the mouse. While most of the additional neurons are probably somatosensory in nature, some of these neurons are also gustatory. A small number of geniculate neurons would be expected to innervate taste buds in the foliate papillae. It is not clear whether these neurons were labeled by our placement of DiI. Because DiI tends to expand to fill the labeled area it is likely that they were labeled and so there may be slightly fewer neurons/fungiform papilla than we calculated. In addition, some geniculate neurons innervate taste buds in the nasoincisive papillae, while this population of taste buds is a fairly sizable percentage of the palatal taste buds in rat (30%; Miller and Spangler, 1982), we have only observed ≈10 taste buds in the region in the mouse (Krimm et al., 2001). This subpopulation of taste buds is located a substantial distance from the soft palate and it is likely that the neurons that innervate them were not labeled in the present study. It must be kept in mind that the unlabeled neurons may have different neurotrophic factor dependencies than the labeled neurons in this study.
Deletion of both BDNF and NT-4 resulted in the loss of most gustatory neurons and taste buds. While some neurons continued to innervate the soft palate in double knockout mice, half of the total number of geniculate ganglia quantified had no neurons remaining to innervate the tongue. However, the tongue always contained a few anti-cytokeratin-8-labeled developing taste buds, suggesting that a small number of developing, noninnervated taste buds remain at birth. Although a small number of cytokeratin-8-positive cell clusters remained in the absence of innervation, the morphology of these surviving “taste buds” as observed using H&E (Fig. 4D,H) was unrecognizable in Bdnf−/−/Ntf4/5−/− mice. This observation indicates that some of the histological characteristics of taste buds are more dependent on neurotrophins and innervation than other characteristics. Since very early taste bud development likely occurs completely independent of neurotrophins (Thirumangalathu et al., 2009; Ito and Nosrat, 2009; Ito et al., 2010), it is likely that taste buds begin to form and then degenerate in Bdnf−/−/Ntf4/5−/− mice. If this is the case, these few remaining taste buds in Bdnf−/−/Ntf4/5−/− mice may be in the process of degenerating.
We examined the neuronal subpopulations of the geniculate ganglia that innervate two spatially distinct gustatory targets, the tongue and soft palate, using the lipophilic dye DiI. A critical issue was the correct placement of DiI so that all the neurons innervating each target were labeled with minimal background. Our protocol likely labeled all the neurons innervating the tongue, as our neuron counts were very similar to those obtained following labeling of the chorda tympani nerve in adult mice (Zaidi and Whitehead, 2006). Specifically, wild-type E18.5 ganglia contained an average of 230.5 geniculate neurons that innervate the tongue, while 213 ± 6.2 neurons were found in the adult geniculate ganglion after the chorda tympani was labeled with a mixture of fast DiI, DiO, and DiD (Zaidi and Whitehead, 2006). The slight discrepancy observed in total counts may be due to differences in the age of the animals, as well as the method of labeling. However, since we counted slightly more neurons than were reported following chorda tympani labeling (Zaidi and Whitehead, 2006), we believe that we successfully labeled all the neurons innervating the tongue. Data are unavailable to compare our palatal neuron counts.
In summary, our results show that both BDNF and NT-4 are required equally for the development of normal numbers of taste neurons innervating the tongue and palate. However, taste bud numbers are much more sensitive to loss of BDNF than NT-4. Taste buds are more sensitive to the loss of BDNF because BDNF regulates both the number of remaining neurons and the ability of these neurons to successfully invade their peripheral target. Both of these processes, in turn, determine whether taste buds are initially innervated and continue to develop. We also observed that neurons that innervate the tongue are more dependent on the TrkB ligands, BDNF and NT-4, than those that innervate the soft palate.
Supplementary Material
Acknowledgments
We thank Douglas J. Lorenz, from the Dept. of Bioinformatics and Biostatistics, with help conducting the hyper-geometric distribution analysis.
Grant sponsor: National Institutes of Health; Grant number: DC007176 (to R.F.K.).
Footnotes
Additional supporting information may be found in the online version of this article.
Literature Cited
- Brulet P, Jacob F. Molecular cloning of a cDNA sequence encoding a trophectoderm-specific marker during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1982;79:2328–2332. doi: 10.1073/pnas.79.7.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulet P, Babinet C, Kemler R, Jacob F. Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1980;77:4113–4117. doi: 10.1073/pnas.77.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, et al. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- De la Monte SM, Federoff HJ, Ng SC, Grabczyk E, Fishman MC. GAP-43 gene expression during development: persistence in a distinctive set of neurons in the mature central nervous system. Brain Res Dev Brain Res. 1989;46:161–168. doi: 10.1016/0165-3806(89)90279-4. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Fan G, Egles C, Sun Y, Minichiello L, Renger JJ, Klein R, Liu G, Jaenisch R. Knocking the NT4 gene into the BDNF locus rescues BDNF deficient mice and reveals distinct NT4 and BDNF activities. Nat Neurosci. 2000;3:350–357. doi: 10.1038/73921. [DOI] [PubMed] [Google Scholar]
- Fishman MC. GAP-43: putting constraints on neuronal plasticity. Perspect Dev Neurobiol. 1996;4:193–198. [PubMed] [Google Scholar]
- Fishman MC, Valenzuela D. GAP-43 and neuronal remodeling. Prog Brain Res. 1991;89:89–95. doi: 10.1016/s0079-6123(08)61717-3. [DOI] [PubMed] [Google Scholar]
- Guagliardo NA, Hill DL. Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. J Comp Neurol. 2007;504:206–216. doi: 10.1002/cne.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L. The effects of glossopharyngeal nerve transection on the circumvallate papilla of the rat. Anat Rec. 1957;128:715–731. doi: 10.1002/ar.1091280406. [DOI] [PubMed] [Google Scholar]
- Hosley MA, Hughes SE, Morton LL, Oakley B. A sensitive period for the neural induction of taste buds. J Neurosci. 1987;7:2075–2080. doi: 10.1523/JNEUROSCI.07-07-02075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Nosrat CA. Gustatory papillae and taste bud development and maintenance in the absence of TrkB ligands BDNF and NT-4. Cell Tissue Res. 2009;337:349–359. doi: 10.1007/s00441-009-0833-7. [DOI] [PubMed] [Google Scholar]
- Ito A, Nosrat IV, Nosrat CA. Taste cell formation does not require gustatory and somatosensory innervation. Neurosci Lett. 2010;471:189–194. doi: 10.1016/j.neulet.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R, Brulet P, Schnebelen MT, Gaillard J, Jacob F. Reactivity of monoclonal antibodies against intermediate filament proteins during embryonic development. J Embryol Exp Morphol. 1981;64:45–60. [PubMed] [Google Scholar]
- Knapp L, Lawton A, Oakley B, Wong L, Zhang C. Keratins as markers of differentiated taste cells of the rat. Differentiation. 1995;58:341–349. doi: 10.1046/j.1432-0436.1995.5850341.x. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol. 1998;398:13–24. [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev Biol. 2001;232:508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Mbiene JP, Parada LF. NT4/5 mutant mice have deficiency in gustatory papillae and taste bud formation. Dev Biol. 1999;213:378–389. doi: 10.1006/dbio.1999.9385. [DOI] [PubMed] [Google Scholar]
- Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Ma L, Lopez GF, Krimm RF. Epithelial-derived brain-derived neurotrophic factor is required for gustatory neuron targeting during a critical developmental period. J Neurosci. 2009;29:3354–3364. doi: 10.1523/JNEUROSCI.3970-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin TM, Jorcano JL, Franke WW. Cytokeratin expression in simple epithelia. II. cDNA cloning and sequence characteristics of bovine cytokeratin A (no. 8) Differentiation. 1986;30:254–264. doi: 10.1111/j.1432-0436.1986.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: nerves follow distinctive and spatially restricted pathways. Acta Anat (Basel) 1997;160:139–158. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- Miller IJ., Jr Branched chorda tympani neurons and interactions among taste receptors. J Comp Neurol. 1974;158:155–166. doi: 10.1002/cne.901580204. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Jr, Spangler K. Taste bud distribution and innervation on the palate of the rat. Chem Senses. 1982;7:99–108. [Google Scholar]
- Mistretta CM, Gurkan S, Bradley RM. Morphology of chorda tympani fiber receptive fields and proposed neural rearrangements during development. J Neurosci. 1988;8:73–78. doi: 10.1523/JNEUROSCI.08-01-00073.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistretta CM, Goosens KA, Farinas I, Reichardt LF. Alterations in size, number, and morphology of gustatory papillae and taste buds in BDNF null mutant mice demonstrate neural dependence of developing taste organs. J Comp Neurol. 1999;409:13–24. [PMC free article] [PubMed] [Google Scholar]
- Naga IA, Sakla FB, Girgis ZA, State FA. Denervation of taste buds in the rabbit. Am J Anat. 1970;129:53–63. doi: 10.1002/aja.1001290105. [DOI] [PubMed] [Google Scholar]
- Nagai T, Mistretta CM, Bradley RM. Developmental decrease in size of peripheral receptive fields of single chorda tympani nerve fibers and relation to increasing NaCl taste sensitivity. J Neurosci. 1988;8:64–72. doi: 10.1523/JNEUROSCI.08-01-00064.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagato T, Matsumoto K, Tanioka H, Kodama J, Toh H. Effect of denervation on morphogenesis of the rat fungi-form papilla. Acta Anat (Basel) 1995;153:301–309. doi: 10.1159/000147739. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Olson L. Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. J Comp Neurol. 1995;360:698–704. doi: 10.1002/cne.903600413. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Ebendal T, Olson L. Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. J Comp Neurol. 1996;376:587–602. doi: 10.1002/(SICI)1096-9861(19961223)376:4<587::AID-CNE7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Oakley B, Wu LH, Lawton A, deSibour C. Neural control of ectopic filiform spines in adult tongue. Neuroscience. 1990;36:831–838. doi: 10.1016/0306-4522(90)90026-z. [DOI] [PubMed] [Google Scholar]
- Sollars SI. Chorda tympani nerve transection at different developmental ages produces differential effects on taste bud volume and papillae morphology in the rat. J Neurobiol. 2005;64:310–320. doi: 10.1002/neu.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollars SI, Smith PC, Hill DL. Time course of morphological alterations of fungiform papillae and taste buds following chorda tympani transection in neonatal rats. J Neurobiol. 2002;51:223–236. doi: 10.1002/neu.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter SM, Igarashi M, Fishman MC. GAP-43 amino terminal peptides modulate growth cone morphology and neurite outgrowth. J Neurosci. 1994;14:5503–5513. doi: 10.1523/JNEUROSCI.14-09-05503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Oakley B. Development of anterior gustatory epithelia in the palate and tongue requires epidermal growth factor receptor. Dev Biol. 2002;242:31–43. doi: 10.1006/dbio.2001.0526. [DOI] [PubMed] [Google Scholar]
- Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. Fate mapping of mammalian embryonic taste bud progenitors. Development. 2009;136:1519–1528. doi: 10.1242/dev.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H, Rittman G, Mackenzie IC. Keratin expression in taste bud cells of the circumvallate and foliate papillae of adult mice. Epithelial Cell Biol. 1993;2:126–133. [PubMed] [Google Scholar]
- Zaidi FN, Whitehead MC. Discrete innervation of murine taste buds by peripheral taste neurons. J Neurosci. 2006;26:8243–8253. doi: 10.1523/JNEUROSCI.5142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi FN, Krimm RF, Whitehead MC. Exuberant neuronal convergence onto reduced taste bud targets with preservation of neural specificity in mice overexpressing neurotrophin in the tongue epithelium. J Neurosci. 2007;27:13875–13881. doi: 10.1523/JNEUROSCI.2517-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Zhang HY, Deng SP, Qin YM, Wang TH. Quantitative study of taste bud distribution within the oral cavity of the postnatal mouse. Arch Oral Biol. 2008;53:583–589. doi: 10.1016/j.archoralbio.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Goodman DW, Karns LR, Fishman MC. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science. 1989;244:1193–1195. doi: 10.1126/science.2658062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


